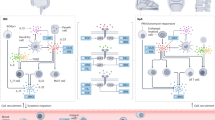
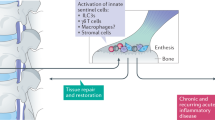
Abstract
Gut inflammation is strongly associated with spondyloarthritis (SpA), as exemplified by the high prevalence of inflammatory bowel disease (IBD) and the even higher occurrence of subclinical gut inflammation in patients with SpA. The gut–joint axis of inflammation in SpA is further reinforced by similarities in immunopathogenesis at both anatomical sites and by the clinical success of therapies blocking TNF and IL-23 in IBD and in some forms of SpA. Many genetic risk factors are shared between SpA and IBD, and changes in the composition of gut microbiota are seen in both diseases. Current dogma is that inflammation in SpA initiates in the gut and leads to joint inflammation; however, although conceptually attractive, some research does not support this causal relationship. For example, therapies targeting IL-17A are efficacious in the joint but not the gut, and interfering with gut trafficking by targeting molecules such as α4β7 in IBD can lead to onset or flares of SpA. Several important knowledge gaps remain that must be addressed in future studies. Determining the true nature of the gut–joint axis has real-world implications for the treatment of patients with co-incident IBD and SpA and for the repurposing of therapeutics from one disease to the other.
Key points
-
The majority of European patients with spondyloarthritis (SpA) have subclinical gut inflammation, and a minority have coexisting inflammatory bowel disease.
-
Many genetic risk factors for ankylosing spondylitis (AS) are shared with inflammatory bowel disease, and some of these AS risk factors cluster in pathways that are important in gut immunity.
-
Type 3 immune cytokines (for example, IL-17) are important in the gut and joint; however, their effects might be tissue specific.
-
Intestinal microbial dysbiosis occurs in SpA, but whether it is a cause, or effect, of underlying immunological alterations is unknown.
-
The failure rate of current therapies for SpA is unacceptably high; whether this failure is related to underlying gut inflammation is unknown.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
30 July 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41584-020-0486-1
References
Tito, R. Y. et al. Brief report: dialister as a microbial marker of disease activity in spondyloarthritis. Arthritis Rheumatol. 69, 114–121 (2017).
Breban, M. et al. Faecal microbiota study reveals specific dysbiosis in spondyloarthritis. Ann. Rheum. Dis. 76, 1614–1622 (2017).
Ciccia, F. et al. Dysbiosis and zonulin upregulation alter gut epithelial and vascular barriers in patients with ankylosing spondylitis. Ann. Rheum. Dis. 76, 1123–1132 (2017).
Hueber, W. et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 61, 1693–1700 (2012).
Stolwijk, C. et al. The epidemiology of extra-articular manifestations in ankylosing spondylitis: a population-based matched cohort study. Ann. Rheum. Dis. 74, 1373–1378 (2015).
Wilkinson, M. & Bywaters, E. G. Clinical features and course of ankylosing spondylitis; as seen in a follow-up of 222 hospital referred cases. Ann. Rheum. Dis. 17, 209–228 (1958).
Granfors, K. et al. Yersinia antigens in synovial-fluid cells from patients with reactive arthritis. N. Engl. J. Med. 320, 216–221 (1989).
Granfors, K. et al. Salmonella lipopolysaccharide in synovial cells from patients with reactive arthritis. Lancet 335, 685–688 (1990).
Hermann, E., Yu, D. T., Meyer zum Büschenfelde, K. H. & Fleischer, B. HLA-B27-restricted CD8 T cells derived from synovial fluids of patients with reactive arthritis and ankylosing spondylitis. Lancet 342, 646–650 (1993).
Mielants, H. et al. The evolution of spondyloarthropathies in relation to gut histology. I. Clinical aspects. J. Rheumatol. 22, 2266–2272 (1995).
Mielants, H. et al. The evolution of spondyloarthropathies in relation to gut histology. III. Relation between gut and joint. J. Rheumatol. 22, 2279–2284 (1995).
Mielants, H. et al. The evolution of spondyloarthropathies in relation to gut histology. II. Histological aspects. J. Rheumatol. 22, 2273–2278 (1995).
Leirisalo-Repo, M., Turunen, U., Stenman, S., Helenius, P. & Seppälä, K. High frequency of silent inflammatory bowel disease in spondylarthropathy. Arthritis Rheum. 37, 23–31 (1994).
De Vos, M., Mielants, H., Cuvelier, C., Elewaut, A. & Veys, E. Long-term evolution of gut inflammation in patients with spondyloarthropathy. Gastroenterology 110, 1696–703 (1996).
Varkas, G. et al. Association of inflammatory bowel disease and acute anterior uveitis, but not psoriasis, with disease duration in patients with axial spondyloarthritis. Arthritis Rheumatol. 70, 1588–1596 (2018).
Van Praet, L., Jacques, P., Van den Bosch, F. & Elewaut, D. The transition of acute to chronic bowel inflammation in spondyloarthritis. Nat. Rev. Rheumatol. 8, 288–295 (2012).
Van Praet, L. et al. Microscopic gut inflammation in axial spondyloarthritis: a multiparametric predictive model. Ann. Rheum. Dis. 72, 414–417 (2013).
Goyette, P. et al. High-density mapping of the MHC identifies a shared role for HLA-DRB1*01:03 in inflammatory bowel diseases and heterozygous advantage in ulcerative colitis. Nat. Genet. 47, 172–179 (2015).
Karreman, M. C., Luime, J. J., Hazes, J. M. W. & Weel, A. E. A. M. The prevalence and incidence of axial and peripheral spondyloarthritis in inflammatory bowel disease: a systematic review and meta-analysis. J. Crohns Colitis 11, jjw199 (2016).
Malaty, H., Lo, G. & Hou, J. Characterization and prevalence of spondyloarthritis and peripheral arthritis among patients with inflammatory bowel disease. Clin. Exp. Gastroenterol. 10, 259–263 (2017).
Ossum, A. M. et al. Ankylosing spondylitis and axial spondyloarthritis in patients with long-term inflammatory bowel disease: results from 20 years of follow-up in the IBSEN Study. J. Crohns Colitis 12, 96–104 (2018).
Hamilton, L., Macgregor, A., Warmington, V., Pinch, E. & Gaffney, K. The prevalence of inflammatory back pain in a UK primary care population. Rheumatology 53, 161–164 (2014).
Chan, J. et al. Prevalence of sacroiliitis in inflammatory bowel disease using a standardized computed tomography scoring system. Arthritis Care Res. 70, 807–810 (2018).
Michielan, A. & D’Incà, R. Intestinal permeability in inflammatory bowel disease: pathogenesis, clinical evaluation, and therapy of leaky gut. Mediators Inflamm. 2015, 628157 (2015).
Bischoff, S. C. et al. Intestinal permeability — a new target for disease prevention and therapy. BMC Gastroenterol. 14, 189 (2014).
Hollander, D. et al. Increased intestinal permeability in patients with Crohn’s disease and their relatives. Ann. Intern. Med. 105, 883 (1986).
Kevans, D. et al. Determinants of intestinal permeability in healthy first-degree relatives of individuals with Crohn’s disease. Inflamm. Bowel Dis. 21, 879–887 (2015).
Martínez-González, O., Cantero-Hinojosa, J., Paule-Sastre, P., Gómez-Magán, J. C. & Salvatierra-Ríos, D. Intestinal permeability in patients with ankylosing spondylitis and their healthy relatives. Br. J. Rheumatol. 33, 644–647 (1994).
Laurent, M. R. & Panayi, G. S. Acute-phase proteins and serum immunoglobulins in ankylosing spondylitis. Ann. Rheum. Dis. 42, 524–528 (1983).
De Winter, J. J., Paramarta, J. E., De Jong, H. M., Van De Sande, M. G. & Baeten, D. L. Peripheral disease contributes significantly to the level of disease activity in axial spondyloarthritis. RMD Open 5, e000802 (2019).
Vermeire, S., Assche, G. Van & Rutgeerts, P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut 55, 426 (2006).
Gossec, L. et al. Preliminary definitions of ‘flare’ in axial spondyloarthritis, based on pain, BASDAI and ASDAS-CRP: an ASAS initiative. Ann. Rheum. Dis. 75, 991–996 (2016).
Cypers, H. et al. Elevated calprotectin levels reveal bowel inflammation in spondyloarthritis. Ann. Rheum. Dis. 75, 1357–1362 (2016).
Walsham, N. E. & Sherwood, R. A. Fecal calprotectin in inflammatory bowel disease. Clin. Exp. Gastroenterol. 9, 21–29 (2016).
Veys, E. M. & van Leare, M. Serum IgG, IgM, and IgA levels in ankylosing spondylitis. Ann. Rheum. Dis. 32, 493–496 (1973).
Ellinghaus, D. et al. Analysis of five chronic inflammatory diseases identifies 27 new associations and highlights disease-specific patterns at shared loci. Nat. Genet. 48, 510–518 (2016).
Cortes, A. et al. Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nat. Genet. 45, 730–738 (2013).
Song, I.-H. et al. Different response to rituximab in tumor necrosis factor blocker-naive patients with active ankylosing spondylitis and in patients in whom tumor necrosis factor blockers have failed: a twenty-four-week clinical trial. Arthritis Rheum. 62, 1290–1297 (2010).
Demetter, P. et al. Increase in lymphoid follicles and leukocyte adhesion molecules emphasizes a role for the gut in spondyloarthropathy pathogenesis. J. Pathol. 198, 517–522 (2002).
Hamada, H. et al. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J. Immunol. 168, 57–64 (2002).
Bolton, P. M., James, S. L., Newcombe, R. G., Whitehead, R. H. & Hughes, L. E. The immune competence of patients with inflammatory bowel disease. Gut 15, 213–219 (1974).
Macpherson, A., Khoo, U. Y., Forgacs, I., Philpott-Howard, J. & Bjarnason, I. Mucosal antibodies in inflammatory bowel disease are directed against intestinal bacteria. Gut 38, 365–375 (1996).
Tsui, F. W. L., Tsui, H. W., Las Heras, F., Pritzker, K. P. H. & Inman, R. D. Serum levels of novel noggin and sclerostin-immune complexes are elevated in ankylosing spondylitis. Ann. Rheum. Dis. 73, 1873–1879 (2014).
Baraliakos, X., Baerlecken, N., Witte, T., Heldmann, F. & Braun, J. High prevalence of anti-CD74 antibodies specific for the HLA class II-associated invariant chain peptide (CLIP) in patients with axial spondyloarthritis. Ann. Rheum. Dis. 73, 1079–1082 (2014).
Wright, C. et al. Detection of multiple autoantibodies in patients with ankylosing spondylitis using nucleic acid programmable protein arrays. Mol. Cell. Proteom. 11, M9.00384 (2012).
Mitsuyama, K. et al. Antibody markers in the diagnosis of inflammatory bowel disease. World J. Gastroenterol. 22, 1304–1310 (2016).
Kuna, A. T. Serological markers of inflammatory bowel disease. Biochem. Med. 23, 28–42 (2013).
Wallis, D. et al. Elevated serum anti-flagellin antibodies implicate subclinical bowel inflammation in ankylosing spondylitis: an observational study. Arthritis Res. Ther. 15, R166 (2013).
Aydin, S. Z. et al. Anti-Saccharomyces cerevisiae antibodies (ASCA) in spondyloarthropathies: a reassessment. Rheumatology 47, 142–144 (2008).
Viladomiu, M. et al. IgA-coated E. coli enriched in Crohn’s disease spondyloarthritis promote TH17-dependent inflammation. Sci. Transl Med. 9, eaaf9655 (2017).
Wang, C.-R. et al. Rare occurrence of inflammatory bowel disease in a cohort of Han Chinese ankylosing spondylitis patients — a single institute study. Sci. Rep. 7, 13165 (2017).
Lai, S.-W., Kuo, Y.-H. & Liao, K.-F. Incidence of inflammatory bowel disease in patients with ankylosing spondylitis. Ann. Rheum. Dis. https://doi.org/10.1136/annrheumdis-2019-216362 (2019).
Chi, K. R. Epidemiology: rising in the east. Nature 540, S100–S102. (2016).
Sieper, J. et al. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann. Rheum. Dis. 68, ii1–ii44 (2009).
Brown, M. A. Breakthroughs in genetic studies of ankylosing spondylitis. Rheumatology 47, 132–137 (2007).
Schlosstein, L., Terasaki, P. I., Bluestone, R. & Pearson, C. M. High association of an HL-A antigen, W27, with ankylosing spondylitis. N. Engl. J. Med. 288, 704–706 (1973).
Brewerton, D. A. et al. Ankylosing spondylitis and HL-A 27. Lancet 1, 904–907 (1973).
Cortes, A. et al. Association study of genes related to bone formation and resorption and the extent of radiographic change in ankylosing spondylitis. Ann. Rheum. Dis. 74, 1387–1393 (2015).
Polachek, A. et al. The association between HLA genetic susceptibility markers and sonographic enthesitis in psoriatic arthritis. Arthritis Rheumatol. 70, 756–762 (2018).
Chandran, V., Tolusso, D. C., Cook, R. J. & Gladman, D. D. Risk factors for axial inflammatory arthritis in patients with psoriatic arthritis. J. Rheumatol. 37, 809–815 (2010).
Castillo-Gallego, C., Aydin, S. Z., Emery, P., McGonagle, D. G. & Marzo-Ortega, H. Magnetic resonance imaging assessment of axial psoriatic arthritis: extent of disease relates to HLA-B27. Arthritis Rheum. 65, 2274–2278 (2013).
Bowness, P. HLA-B27. Annu. Rev. Immunol. 33, 29–48 (2015).
Ranganathan, V., Gracey, E., Brown, M. A., Inman, R. D. & Haroon, N. Pathogenesis of ankylosing spondylitis — recent advances and future directions. Nat. Rev. Rheumatol. 13, 359–367 (2017).
Farh, K. K. H. et al. Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature 518, 337–343 (2015).
McCole, D. F. IBD candidate genes and intestinal barrier regulation. Inflamm. Bowel Dis. 20, 1829–1849 (2014).
Kellermayer, Z. et al. IL-22–independent protection from colitis in the absence of Nkx2.3 transcription factor in mice. J. Immunol. 202, 1833–1844 (2019).
Tsukahara, T. et al. G protein-coupled receptor 35 contributes to mucosal repair in mice via migration of colonic epithelial cells. Pharmacol. Res. 123, 27–39 (2017).
de Lange, K. M. et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat. Genet. 49, 256–261 (2017).
Laukens, D. et al. CARD15 gene polymorphisms in patients with spondyloarthropathies identify a specific phenotype previously related to Crohn’s disease. Ann. Rheum. Dis. 64, 930–935 (2005).
Crane, A. M. et al. Role of NOD2 variants in spondylarthritis. Arthritis Rheum. 46, 1629–1633 (2002).
Cortes, A. & Brown, M. A. Promise and pitfalls of the Immunochip. Arthritis Res. Ther. 13, 101 (2011).
Gibbs, R. A. et al. A global reference for human genetic variation. Nature 526, 68–74 (2015).
Australo-Anglo-American Spondyloarthritis Consortium (TASC). et al. Genome-wide association study of ankylosing spondylitis identifies non-MHC susceptibility loci. Nat. Genet. 42, 123–127 (2010).
Davidson, S. I. et al. Association of ERAP1, but not IL23R, with ankylosing spondylitis in a Han Chinese population. Arthritis Rheum. 60, 3263–3268 (2009).
Lee, J. S. et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity 43, 727–738 (2015).
Pickert, G. et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 206, 1465–1472 (2009).
Hainzl, E. et al. Intestinal epithelial cell tyrosine kinase 2 transduces IL-22 signals to protect from acute colitis. J. Immunol. 195, 5011–5024 (2015).
Liang, S. C. et al. Interleukin (IL)-22 and IL-17 are coexpressed by TH17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203, 2271–2279 (2006).
Turner, J.-E., Stockinger, B. & Helmby, H. IL-22 mediates goblet cell hyperplasia and worm expulsion in intestinal helminth infection. PloS Pathog. 9, e1003698 (2013).
Withers, D. R. et al. Transient inhibition of ROR-γt therapeutically limits intestinal inflammation by reducing TH17 cells and preserving group 3 innate lymphoid cells. Nat. Med. 22, 319–323 (2016).
Leppkes, M. et al. RORγ-expressing TH17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology 136, 257–267 (2009).
Gronke, K. et al. Interleukin-22 protects intestinal stem cells against genotoxic stress. Nature 566, 249–253 (2019).
Grizotte-Lake, M. et al. Commensals suppress intestinal epithelial cell retinoic acid synthesis to regulate interleukin-22 activity and prevent microbial dysbiosis. Immunity 49, 1103–1115 (2018).
Sherlock, J. P. et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+CD3+CD4−CD8− entheseal resident T cells. Nat. Med. 18, 1069–1076 (2012).
Cuthbert, R. J. et al. Brief report: group 3 innate lymphoid cells in human enthesis. Arthritis Rheumatol. 69, 1816–1822 (2017).
Ono, T. et al. IL-17-producing γδ T cells enhance bone regeneration. Nat. Commun. 7, 10928 (2016).
Ciccia, F. et al. Overexpression of interleukin-23, but not interleukin-17, as an immunologic signature of subclinical intestinal inflammation in ankylosing spondylitis. Arthritis Rheum. 60, 955–965 (2009).
Gracey, E. et al. Sexual dimorphism in the TH17 signature of ankylosing spondylitis. Arthritis Rheumatol. 68, 679–689 (2016).
Mens, L. J. J. et al. Brief report: interleukin-17 blockade with secukinumab in peripheral spondyloarthritis impacts synovial immunopathology without compromising systemic immune responses. Arthritis Rheumatol. 70, 1994–2002 (2018).
Benham, H. et al. Interleukin-23 mediates the intestinal response to microbial β-1,3-glucan and the development of spondyloarthritis pathology in SKG mice. Arthritis Rheumatol. 66, 1755–1767 (2014).
Glatigny, S. et al. Proinflammatory Th17 cells are expanded and induced by dendritic cells in spondylarthritis-prone HLA-B27-transgenic rats. Arthritis Rheum. 64, 110–120 (2012).
van Tok, M. N. et al. The initiation, but not the persistence, of experimental spondyloarthritis is dependent on interleukin-23 signaling. Front. Immunol. 9, 1550 (2018).
DeLay, M. L. et al. HLA-B27 misfolding and the unfolded protein response augment interleukin-23 production and are associated with Th17 activation in transgenic rats. Arthritis Rheum. 60, 2633–2643 (2009).
McNamee, E. N. et al. Chemokine receptor CCR7 regulates the intestinal TH1/TH17/Treg balance during Crohn’s-like murine ileitis. J. Leukoc. Biol. 97, 1011–1022 (2015).
Zwerina, K. et al. Anti IL-17A therapy inhibits bone loss in TNF-α-mediated murine arthritis by modulation of the T-cell balance. Eur. J. Immunol. 42, 413–423 (2012).
Gracey, E. et al. IL-7 primes IL-17 in mucosal-associated invariant T (MAIT) cells, which contribute to the Th17-axis in ankylosing spondylitis. Ann. Rheum. Dis. 75, 2124–2132 (2016).
Shen, H., Goodall, J. C. & Hill Gaston, J. S. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 60, 1647–1656 (2009).
Kenna, T. J. et al. Enrichment of circulating interleukin-17-secreting interleukin-23 receptor-positive γ/δ T cells in patients with active ankylosing spondylitis. Arthritis Rheum. 64, 1420–1429 (2012).
Ciccia, F. et al. Type 3 innate lymphoid cells producing IL-17 and IL-22 are expanded in the gut, in the peripheral blood, synovial fluid and bone marrow of patients with ankylosing spondylitis. Ann. Rheum. Dis. 74, 1739–1747 (2015).
Mowat, A. M. & Agace, W. W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 14, 667–685 (2014).
Venken, K. et al. RORγt inhibition selectively targets IL-17 producing iNKT and γδ-T cells enriched in spondyloarthritis patients. Nat. Commun. 10, 9 (2019).
Al-Mossawi, M. H. et al. Unique transcriptome signatures and GM-CSF expression in lymphocytes from patients with spondyloarthritis. Nat. Commun. 8, 1510 (2017).
Bowness, P. et al. Th17 cells expressing KIR3DL2+ and responsive to HLA-B27 homodimers are increased in ankylosing spondylitis. J. Immunol. 186, 2672–2680 (2011).
Menon, B. et al. Interleukin-17+CD8+T cells are enriched in the joints of patients with psoriatic arthritis and correlate with disease activity and joint damage progression. Arthritis Rheumatol. 66, 1272–1281 (2014).
Cuthbert, R. J. et al. Evidence that tissue resident human enthesis γδT-cells can produce IL-17A independently of IL-23R transcript expression. Ann. Rheum. Dis. 78, 1559–1565 (2019).
Reinhardt, A. et al. Interleukin-23-dependent γ/δ T cells produce interleukin-17 and accumulate in the enthesis, aortic valve, and ciliary body in mice. Arthritis Rheumatol. 68, 2476–2486 (2016).
Moschen, A. R., Tilg, H. & Raine, T. I. IL-12, IL-23 and IL-17 in IBD: immunobiology and therapeutic targeting. Nat. Rev. Gastroenterol. Hepatol. 16, 185–196 (2019).
Torres, J., Mehandru, S., Colombel, J. F. & Peyrin-Biroulet, L. Crohn’s disease. Lancet 389, 1741–1755 (2017).
Lutter, L., Hoytema van Konijnenburg, D. P., Brand, E. C., Oldenburg, B. & van Wijk, F. The elusive case of human intraepithelial T cells in gut homeostasis and inflammation. Nat. Rev. Gastroenterol. Hepatol. 15, 637–649 (2018).
Friedrich, M., Pohin, M. & Powrie, F. Cytokine networks in the pathophysiology of inflammatory bowel disease. Immunity 50, 992–1006 (2019).
Shevach, E. M. Foxp3+ T regulatory cells: still many unanswered questions — a perspective after 20 years of study. Front. Immunol. 9, 1048 (2018).
Sharma, A. & Rudra, D. Emerging functions of regulatory T cells in tissue homeostasis. Front. Immunol. 9, 883 (2018).
Yang, B.-H. et al. Foxp3+ T cells expressing RORγt represent a stable regulatory T-cell effector lineage with enhanced suppressive capacity during intestinal inflammation. Mucosal Immunol. 9, 444–457 (2016).
Britton, G. J. et al. Microbiotas from humans with inflammatory bowel disease alter the balance of gut Th17 and RORγt+ regulatory T cells and exacerbate colitis in mice. Immunity 50, 212–224 (2019).
Sefik, E. et al. Individual intestinal symbionts induce a distinct population of RORγ+ regulatory T cells. Science. 349, 993–997 (2015).
Guo, H. et al. Functional defects in CD4+ CD25high FoxP3+regulatory cells in ankylosing spondylitis. Sci. Rep. 6, 37559 (2016).
Wu, Y. et al. Reduced immunomodulation potential of bone marrow-derived mesenchymal stem cells induced CCR4+CCR6+Th/Treg cell subset imbalance in ankylosing spondylitis. Arthritis Res. Ther. 13, R29 (2011).
Ciccia, F. et al. Expansion of intestinal CD4+CD25high Treg cells in patients with ankylosing spondylitis: a putative role for interleukin-10 in preventing intestinal Th17 response. Arthritis Rheum. 62, 3625–3634 (2010).
Rosenzwajg, M. et al. Immunological and clinical effects of low-dose interleukin-2 across 11 autoimmune diseases in a single, open clinical trial. Ann. Rheum. Dis. 78, 209–217 (2019).
Habtezion, A., Nguyen, L. P., Hadeiba, H. & Butcher, E. C. Leukocyte trafficking to the small intestine and colon. Gastroenterology 150, 340–354 (2016).
Elewaut, D. et al. Enrichment of T cells carrying β7 integrins in inflamed synovial tissue from patients with early spondyloarthropathy, compared to rheumatoid arthritis. J. Rheumatol. 25, 1932–1937 (1998).
Salmi, M. & Jalkanen, S. Human leukocyte subpopulations from inflamed gut bind to joint vasculature using distinct sets of adhesion molecules. J. Immunol. 166, 4650–4657 (2001).
Qaiyum, Z., Gracey, E., Yao, Y. & Inman, R. D. Integrin and transcriptomic profiles identify a distinctive synovial CD8+T cell subpopulation in spondyloarthritis. Ann. Rheum. Dis. 78, 1566–1575 (2019).
Adams, D. H. & Eksteen, B. Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Nat. Rev. Immunol. 6, 244–251 (2006).
Salmi, M., Rajala, P. & Jalkanen, S. Homing of mucosal leukocytes to joints distinct endothelial ligands in synovium mediate leukocyte-subtype specific adhesion. J. Clin. Invest. 99, 2165–2172 (1997).
Norman, E., Lefferts, A. & Kuhn, K. Gut-joint T cell trafficking in a model of bacteria-driven murine IBD-SpA [abstract]. Arthritis Rheumatol. 70, 1828 (2018).
Teng, F. et al. Gut microbiota drive autoimmune arthritis by promoting differentiation and migration of Peyer’s patch T follicular helper cells. Immunity 44, 875–888 (2016).
Tajik, N. et al. Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat. Commun. 11, 1–14 (2020).
Hirata, I., Berrebi, G., Austin, L. L., Keren, D. F. & Dobbins, W. O. Immunohistological characterization of intraepithelial and lamina propria lymphocytes in control ileum and colon and in inflammatory bowel disease. Dig. Dis. Sci. 31, 593–603 (1986).
Park, C. O. & Kupper, T. S. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat. Med. 21, 688–697 (2015).
Edelblum, K. L. et al. γδ Intraepithelial lymphocyte migration limits transepithelial pathogen invasion and systemic disease in mice. Gastroenterology 148, 1417–1426 (2015).
Hoytema van Konijnenburg DP. et al. Intestinal epithelial and intraepithelial T cell crosstalk mediates a dynamic response to infection. Cell 171, 783–794 (2017).
Regner, E. H. et al. Functional intraepithelial lymphocyte changes in inflammatory bowel disease and spondyloarthritis have disease specific correlations with intestinal microbiota. Arthritis Res. Ther. 20, 149 (2018).
Watad, A. et al. Normal human enthesis harbours conventional CD4+ and CD8+ T cells with regulatory features and inducible IL-17A and TNF expression. Ann. Rheum. Dis. https://doi.org/10.1136/annrheumdis-2020-217309 (2020).
Gracey, E. et al. Altered cytotoxicity profile of CD 8+ T cells in ankylosing spondylitis. Arthritis Rheumatol. 72, 428–434 (2020).
Dendrou, C. A. et al. Resolving TYK2 locus genotype-to-phenotype differences in autoimmunity. Sci. Transl Med. 8, 363ra149 (2016).
Gracey, E. et al. TYK2 inhibition reduces type 3 immunity and modifies disease progression in murine spondyloarthritis. J. Clin. Invest. 130, 1863–1878 (2020).
Di Meglio, P. et al. The IL23R R381Q gene variant protects against immune-mediated diseases by impairing IL-23-induced TH17 effector response in humans. PloS One 6, e17160 (2011).
Vecellio, M. et al. The genetic association of RUNX3 with ankylosing spondylitis can be explained by allele-specific effects on IRF4 recruitment that alter gene expression. Ann. Rheum. Dis. 75, 1534–1540 (2016).
Brenner, O. et al. Loss of Runx3 function in leukocytes is associated with spontaneously developed colitis and gastric mucosal hyperplasia. Proc. Natl Acad. Sci. USA 101, 16016–16021 (2004).
Lau, M. C. et al. Genetic association of ankylosing spondylitis with TBX21 influences T-bet and pro-inflammatory cytokine expression in humans and SKG mice as a model of spondyloarthritis. Ann. Rheum. Dis. 76, 261–269 (2017).
Neurath, M. F. et al. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn’s disease. J. Exp. Med. 195, 1129–1143 (2002).
Ruscher, R., Kummer, R. L., Lee, Y. J., Jameson, S. C. & Hogquist, K. A. CD8αα intraepithelial lymphocytes arise from two main thymic precursors. Nat. Immunol. 18, 771–779 (2017).
Reis, B. S., Hoytema van Konijnenburg, D. P., Grivennikov, S. I. & Mucida, D. Transcription factor T-bet regulates intraepithelial lymphocyte functional maturation. Immunity 41, 244–256 (2014).
Mackay, L. K. et al. T-box transcription factors combine with the cytokines TGF-β and IL-15 to control tissue-resident memory T cell fate. Immunity 43, 1101–1111 (2015).
Milner, J. J. et al. Runx3 programs CD8+ T cell residency in non-lymphoid tissues and tumours. Nature 552, 253–257 (2017).
Best, J. A. et al. Transcriptional insights into the CD8+ T cell response to infection and memory T cell formation. Nat. Immunol. 14, 404–412 (2013).
Liu, Z. et al. The increased expression of IL-23 in inflammatory bowel disease promotes intraepithelial and lamina propria lymphocyte inflammatory responses and cytotoxicity. J. Leukoc. Biol. 89, 597–606 (2011).
Kamanaka, M. et al. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity 25, 941–952 (2006).
Herndler-Brandstetter, D. et al. KLRG1+ effector CD8+ T cells lose KLRG1, differentiate into all memory T cell lineages, and convey enhanced protective immunity. Immunity 48, 716–729 (2018).
De Wilde, K. et al. A20 inhibition of STAT1 expression in myeloid cells: a novel endogenous regulatory mechanism preventing development of enthesitis. Ann. Rheum. Dis. 76, 585–592 (2017).
Matmati, M. et al. A20 (TNFAIP3) deficiency in myeloid cells triggers erosive polyarthritis resembling rheumatoid arthritis. Nat. Genet. 43, 908–912 (2011).
Hammer, G. E. et al. Expression of A20 by dendritic cells preserves immune homeostasis and prevents colitis and spondyloarthritis. Nat. Immunol. 12, 1184–1193 (2011).
Bassler, K., Schulte-Schrepping, J., Warnat-Herresthal, S., Aschenbrenner, A. C. & Schultze, J. L. The myeloid cell compartment — cell by cell. Annu. Rev. Immunol. 37, 269–293 (2019).
Bridgewood, C. et al. Identification of myeloid cells in the human enthesis as the main source of local IL-23 production. Ann. Rheum. Dis. 78, 929–933 (2019).
Cambré, I. et al. Mechanical strain determines the site-specific localization of inflammation and tissue damage in arthritis. Nat. Commun. 9, 4613 (2018).
Chen, S. et al. Histologic evidence that mast cells contribute to local tissue inflammation in peripheral spondyloarthritis by regulating interleukin-17A content. Rheumatology 58, 617–627 (2019).
Noordenbos, T. et al. Interleukin-17-positive mast cells contribute to synovial inflammation in spondylarthritis. Arthritis Rheum. 64, 99–109 (2012).
Appel, H. et al. Analysis of IL-17+ cells in facet joints of patients with spondyloarthritis suggests that the innate immune pathway might be of greater relevance than the Th17-mediated adaptive immune response. Arthritis Res. Ther. 13, R95 (2011).
Noordenbos, T. et al. Human mast cells capture, store, and release bioactive, exogenous IL-17A. J. Leukoc. Biol. 100, 453–462 (2016).
Appel, H. et al. In situ analysis of interleukin-23- and interleukin-12-positive cells in the spine of patients with ankylosing spondylitis. Arthritis Rheum. 65, 1522–1529 (2013).
Ambarus, C. A., Noordenbos, T., de Hair, M. J. H., Tak, P. P. & Baeten, D. L. P. Intimal lining layer macrophages but not synovial sublining macrophages display an IL-10 polarized-like phenotype in chronic synovitis. Arthritis Res. Ther. 14, R74 (2012).
Demetter, P. et al. Colon mucosa of patients both with spondyloarthritis and Crohn’s disease is enriched with macrophages expressing the scavenger receptor CD163. Ann. Rheum. Dis. 64, 321–324 (2005).
Ciccia, F. et al. Macrophage phenotype in the subclinical gut inflammation of patients with ankylosing spondylitis. Rheumatology 53, 104–113 (2014).
Ciccia, F. et al. Proinflammatory CX3CR1+CD59+tumor necrosis factor–like molecule 1A+interleukin-23+ monocytes are expanded in patients with ankylosing spondylitis and modulate innate lymphoid cell 3 immune functions. Arthritis Rheumatol. 70, 2003–2013 (2018).
Ermoza, K. et al. Tolerogenic XCR1+ dendritic cell population is dysregulated in HLA-B27 transgenic rat model of spondyloarthritis. Arthritis Res. Ther. 21, 46 (2019).
Adamopoulos, I. E. et al. IL-23 is critical for induction of arthritis, osteoclast formation, and maintenance of bone mass. J. Immunol. 187, 951–959 (2011).
Jo, S. et al. IL-17A induces osteoblast differentiation by activating JAK2/STAT3 in ankylosing spondylitis. Arthritis Res. Ther. 20, 115 (2018).
Adamopoulos, I. E. et al. Interleukin-17A upregulates receptor activator of NF-κB on osteoclast precursors. Arthritis Res. Ther. 12, R29 (2010).
Croes, M. et al. Proinflammatory T cells and IL-17 stimulate osteoblast differentiation. Bone 84, 262–270 (2016).
Millar, N. L. et al. IL-17A mediates inflammatory and tissue remodelling events in early human tendinopathy. Sci. Rep. 6, 27149 (2016).
Rehaume, L. M. et al. ZAP-70 genotype disrupts the relationship between microbiota and host, leading to spondyloarthritis and ileitis in SKG mice. Arthritis Rheumatol. 66, 2780–2792 (2014).
McInnes, I. B. et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet 382, 780–789 (2013).
Ritchlin, C. et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann. Rheum. Dis. 73, 990–999 (2014).
Deodhar, A. et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet 391, 2213–2224 (2018).
Deodhar, A. et al. Three multicenter, randomized, double-blind, placebo-controlled studies evaluating the efficacy and safety of ustekinumab in axial spondyloarthritis. Arthritis Rheumatol. 71, 258–270 (2019).
Baeten, D. et al. Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: results of a randomised, double-blind, placebo-controlled, proof-of-concept, dose-finding phase 2 study. Ann. Rheum. Dis. 77, 1295–1302 (2018).
Feagan, B. G. et al. Risankizumab in patients with moderate to severe Crohn’s disease: an open-label extension study. Lancet Gastroenterol. Hepatol. 3, 671–680 (2018).
Mease, P. J. et al. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N. Engl. J. Med. 373, 1329–1339 (2015).
McInnes, I. B. et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 386, 1137–1146 (2015).
Mease, P. J. et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann. Rheum. Dis. 76, 79–87 (2017).
Nash, P. et al. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet 389, 2317–2327 (2017).
van der Heijde, D. et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet 392, 2441–2451 (2018).
Rosenbaum, J. T. & Asquith, M. The microbiome and HLA-B27-associated acute anterior uveitis. Nat. Rev. Rheumatol. 14, 704–713 (2018).
Gill, T., Asquith, M., Rosenbaum, J. T. & Colbert, R. A. The intestinal microbiome in spondyloarthritis. Curr. Opin. Rheumatol. 27, 319 (2015).
Turpin, W. et al. Association of host genome with intestinal microbial composition in a large healthy cohort. Nat. Genet. 48, 1413–1417 (2016).
Rothschild, D. et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 555, 210–215 (2018).
Costello, M.-E. et al. Brief report: intestinal dysbiosis in ankylosing spondylitis. Arthritis Rheumatol. 67, 686–691 (2015).
Manasson, J. et al. Gut microbiota perturbations in reactive arthritis and postinfectious spondyloarthritis. Arthritis Rheumatol. 70, 242–254 (2018).
Asquith, M. et al. HLA alleles associated with risk of ankylosing spondylitis and rheumatoid arthritis influence the gut microbiome. Arthritis Rheumatol. 71, 1642–1650 (2019).
Colmegna, I., Cuchacovich, R. & Espinoza, L. R. HLA-B27-associated reactive arthritis: pathogenetic and clinical considerations. Clin. Microbiol. Rev. 17, 348–369 (2004).
Berthelot J.-M. & Wendling D. Translocation of dead or alive bacteria from mucosa to joints and epiphyseal bone-marrow: facts and hypotheses. Joint Bone Spine (2020).
Manfredo Vieira, S. et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science 359, 1156–1161 (2018).
Ni, J., Wu, G. D., Albenberg, L. & Tomov, V. T. Gut microbiota and IBD: causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 14, 573–584 (2017).
Vieira-Silva, S. et al. Quantitative microbiome profiling disentangles inflammation- and bile duct obstruction-associated microbiota alterations across PSC/IBD diagnoses. Nat. Microbiol. 4, 1826–1831 (2019).
Halfvarson, J. et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat. Microbiol. 2, 17004 (2017).
Limon, J. J., Skalski, J. H. & Underhill, D. M. Commensal fungi in health and disease. Cell Host Microbe 22, 156–165 (2017).
Richard, M. L. & Sokol, H. The gut mycobiota: insights into analysis, environmental interactions and role in gastrointestinal diseases. Nat. Rev. Gastroenterol. Hepatol. 16, 331–345 (2019).
Neil, J. A. et al. IFN-I and IL-22 mediate protective effects of intestinal viral infection. Nat. Microbiol. 4, 1737–1749 (2019).
Rooks, M. G. & Garrett, W. S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 16, 341–352 (2016).
Taurog, J. D. et al. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J. Exp. Med. 180, 2359–2364 (1994).
Roulis, M. et al. Host and microbiota interactions are critical for development of murine Crohn’s-like ileitis. Mucosal Immunol. 9, 787–797 (2016).
Schaubeck, M. et al. Dysbiotic gut microbiota causes transmissible Crohn’s disease-like ileitis independent of failure in antimicrobial defence. Gut 65, 225–337 (2016).
Lin, P. et al. HLA-B27 and human β2-microglobulin affect the gut microbiota of transgenic rats. PLoS One 9, e105684 (2014).
Gill, T., Asquith, M., Brooks, S. R., Rosenbaum, J. T. & Colbert, R. A. Effects of HLA-B27 on gut microbiota in experimental spondyloarthritis implicate an ecological model of dysbiosis. Arthritis Rheumatol. 70, 555–565 (2018).
Rehaume, L. M. et al. IL-23 favours outgrowth of spondyloarthritis-associated pathobionts and suppresses host support for homeostatic microbiota. Ann. Rheum. Dis. 78, 494–503 (2019).
Maeda, Y. et al. Dysbiosis contributes to arthritis development via activation of autoreactive T cells in the intestine. Arthritis Rheumatol. 68, 2646–2661 (2016).
Targan, S. R. et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor α for Crohn’s Disease. N. Engl. J. Med. 337, 1029–1036 (1997).
Rutgeerts, P. et al. Efficacy and safety of retreatment with anti-tumor necrosis factor antibody (infliximab) to maintain remission in Crohn’s disease. Gastroenterology 117, 761–769 (1999).
Present, D. H. et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N. Engl. J. Med. 340, 1398–1405 (1999).
Van den Bosch, F., Kruithof, E., De Vos, M., De Keyser, F. & Mielants, H. Crohn’s disease associated with spondyloarthropathy: effect of TNF-α blockade with infliximab on articular symptoms. Lancet 356, 1821–1822 (2000).
Van den Bosch, F. et al. Effects of a loading dose regimen of three infusions of chimeric monoclonal antibody to tumour necrosis factor α (infliximab) in spondyloarthropathy: an open pilot study. Ann. Rheum. Dis. 59, 428–433 (2000).
Gladman, D. et al. Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N. Engl. J. Med. 377, 1525–1536 (2017).
Mease, P. et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N. Engl. J. Med. 377, 1537–1550 (2017).
Mease, P. et al. Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active psoriatic arthritis (EQUATOR): results from a randomised, placebo-controlled, phase 2 trial. Lancet 392, 2367–2377 (2018).
Edwards, C. J. et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3). Ann. Rheum. Dis. 75, 1065–1073 (2016).
Celgene. Study of apremilast to treat subjects with active ankylosing spondylitis (POSTURE) https://clinicaltrials.gov/ct2/show/NCT01583374 (2020)
van der Heijde, D. et al. Tofacitinib in patients with ankylosing spondylitis: a phase II, 16-week, randomised, placebo-controlled, dose-ranging study. Ann. Rheum. Dis. 76, 1340–1347 (2017).
van der Heijde, D. et al. Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active ankylosing spondylitis (TORTUGA): results from a randomised, placebo-controlled, phase 2 trial. Lancet 392, 2378–2387 (2018).
Antoni, C. et al. Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann. Rheum. Dis. 64, 1150–1157 (2005).
Mease, P. J. et al. Secukinumab in the treatment of psoriatic arthritis: efficacy and safety results through 3 years from the year 1 extension of the randomised phase III FUTURE 1 trial. RMD Open 4, e000723 (2018).
van der Heijde, D. et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheum. 52, 582–591 (2005).
Davis, J. C. et al. Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheum. 48, 3230–3236 (2003).
Braun, J. et al. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann. Rheum. Dis. 76, 1070–1077 (2017).
Maneiro, J. R., Souto, A., Salgado, E., Mera, A. & Gomez-Reino, J. J. Predictors of response to TNF antagonists in patients with ankylosing spondylitis and psoriatic arthritis: systematic review and meta-analysis. RMD Open 1, e000017 (2015).
van der Heijde, D. et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 54, 2136–2146 (2006).
Landewé, R. et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled phase 3 study. Ann. Rheum. Dis. 73, 39–47 (2014).
Inman, R. D. et al. Efficacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum. 58, 3402–3412 (2008).
Mease, P. J. et al. Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheum. 50, 2264–2272 (2004).
Mease, P. J. et al. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 52, 3279–3289 (2005).
Kavanaugh, A. et al. Golimumab, a new human tumor necrosis factor α antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: twenty-four-week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum. 60, 976–986 (2009).
Mease, P. J. et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann. Rheum. Dis. 73, 48–55 (2014).
Hanauer, S. B. et al. Human anti–tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology 130, 323–333 (2006).
Sandborn, W. J. et al. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut 56, 1232–1239 (2007).
Sandborn, W. J. et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 146, 85–95 (2014).
Sandborn, W. J. et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology 146, 96–109 (2014).
Sandborn, W. J. et al. Certolizumab pegol for the treatment of Crohn’s disease. N. Engl. J. Med. 357, 228–238 (2007).
Schreiber, S. et al. Maintenance therapy with certolizumab pegol for Crohn’s disease. N. Engl. J. Med. 357, 239–250 (2007).
Marzo-Ortega, H., McGonagle, D., O’Connor, P. & Emery, P. Efficacy of etanercept for treatment of Crohn’s related spondyloarthritis but not colitis. Ann. Rheum. Dis. 62, 74–76 (2003).
Sandborn, W. J. et al. Etanercept for active Crohn’s disease: a randomized, double-blind, placebo-controlled trial. Gastroenterology 121, 1088–1094 (2001).
Siebert, S., Millar, N. L. & McInnes, I. B. Why did IL-23p19 inhibition fail in AS: a tale of tissues, trials or translation? Ann. Rheum. Dis. 78, 1015–1018 (2018).
Panés, J. et al. Tofacitinib for induction and maintenance therapy of Crohn’s disease: results of two phase IIb randomised placebo-controlled trials. Gut 66, 1049–1059 (2017).
Sandborn, W. J. et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 376, 1723–1736 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02914561 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02914522 (2020).
Vermeire, S. et al. Long-term efficacy of vedolizumab for Crohn’s disease. J. Crohns Colitis 11, jjw176 (2016).
Loftus, E. V. et al. Long-term efficacy of vedolizumab for ulcerative colitis. J. Crohns Colitis 11, jjw177 (2016).
Targan, S. R. et al. Natalizumab for the treatment of active Crohn’s disease: results of the ENCORE Trial. Gastroenterology 132, 1672–1683 (2007).
Ciccia, F. et al. Clinical efficacy of α4 integrin block with natalizumab in ankylosing spondylitis. Ann. Rheum. Dis. 75, 2053–2054 (2016).
Varkas, G. et al. An induction or flare of arthritis and/or sacroiliitis by vedolizumab in inflammatory bowel disease: a case series. Ann. Rheum. Dis. 76, 878–881 (2017).
Dubash, S. et al. Emergence of severe spondyloarthropathy-related entheseal pathology following successful vedolizumab therapy for inflammatory bowel disease. Rheumatology 58, 963–968 (2019).
Orlando, A. et al. Clinical benefit of vedolizumab on articular manifestations in patients with active spondyloarthritis associated with inflammatory bowel disease. Ann. Rheum. Dis. 76, e31 (2017).
Varkas, G., Van den Bosch, F. & Elewaut, D. Response to: ‘Clinical benefit of vedolizumab on articular manifestations in patients with active spondyloarthritis associated with inflammatory bowel disease’ by Orlando et al. Ann. Rheum. Dis. 76, e32 (2017).
Feagan, B. G. et al. Incidence of arthritis/arthralgia in inflammatory bowel disease with long-term vedolizumab treatment: post hoc analyses of the GEMINI trials. J. Crohns Colitis 13, 50–57 (2019).
Tadbiri, S. et al. Impact of vedolizumab therapy on extra-intestinal manifestations in patients with inflammatory bowel disease: a multicentre cohort study nested in the OBSERV-IBD cohort. Aliment. Pharmacol. Ther. 47, 485–493 (2018).
Fischer, A. et al. Differential effects of α4β7 and GPR15 on homing of effector and regulatory T cells from patients with UC to the inflamed gut in vivo. Gut 65, 1642–1664 (2016).
Appel, H. et al. Synovial and peripheral blood CD4+FoxP3+ T cells in spondyloarthritis. J. Rheumatol. 38, 2445–2451 (2011).
Lee, J. C. et al. Genome-wide association study identifies distinct genetic contributions to prognosis and susceptibility in Crohn’s disease. Nat. Genet. 49, 262–268 (2017).
van der Linden, S., Valkenburg, H. A. & Cats, A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 27, 361–368 (1984).
Khan, M. A., van Der Linden, S. M., Kushner, I., Valkenburg, H. A. & Cats, A. Spondylitic disease without radiologic evidence of sacroiliitis in relatives of HLA-B27 positive ankylosing spondylitis patients. Arthritis Rheum. 28, 40–43 (1985).
Rudwaleit, M. et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann. Rheum. Dis. 68, 770–776 (2009).
Taylor, W. et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 54, 2665–2673 (2006).
Rudwaleit, M. et al. The Assessment of SpondyloArthritis international Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann. Rheum. Dis. 70, 25–31 (2011).
Furey, T. S., Sethupathy, P. & Sheikh, S. Z. Redefining the IBDs using genome-scale molecular phenotyping. Nat. Rev. Gastroenterol. Hepatol. 16, 296–311 (2019).
Sandborn, W. J. et al. Safety of celecoxib in patients with ulcerative colitis in remission: a randomized, placebo-controlled, pilot study. Clin. Gastroenterol. Hepatol. 4, 203–211 (2006).
El Miedany, Y., Youssef, S., Ahmed, I. & El Gaafary, M. The gastrointestinal safety and effect on disease activity of etoricoxib, a selective COX-2 inhibitor in inflammatory bowel diseases. Am. J. Gastroenterol. 101, 311–317 (2006).
Kvasnovsky, C. L., Aujla, U. & Bjarnason, I. Nonsteroidal anti-inflammatory drugs and exacerbations of inflammatory bowel disease. Scand. J. Gastroenterol. 50, 255–263 (2014).
Ash, Z. et al. A systematic literature review of drug therapies for the treatment of psoriatic arthritis: current evidence and meta-analysis informing the EULAR recommendations for the management of psoriatic arthritis. Ann. Rheum. Dis. 71, 319–326 (2012).
Feagan, B. G. et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 375, 1946–1960 (2016).
Sands, B. E. et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N. Engl. J. Med. 350, 876–885 (2004).
Rutgeerts, P. et al. Adalimumab induces and maintains mucosal healing in patients with Crohn’s disease: data from the EXTEND trial. Gastroenterology 142, 1102–1111 (2012).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02289417 (2020).
Danese, S. et al. Effects of apremilast, an oral inhibitor of phosphodiesterase 4, in a randomized trial of patients with active ulcerative colitis. Clin. Gastroenterol. Hepatol. https://doi.org/10.1016/j.cgh.2019.12.032 (2020).
Haibel, H. et al. Efficacy of oral prednisolone in active ankylosing spondylitis: results of a double-blind, randomised, placebo-controlled short-term trial. Ann. Rheum. Dis. 73, 243–246 (2014).
Acknowledgements
The authors thank A. Hoorens (UZ Gent) for providing the histology pictures in Fig. 1.
Author information
Authors and Affiliations
Contributions
E.G. researched data for the article; E.G., L.V., D.M., G.S., F.V.d.B. and D.E. made substantial contributions to discussions of the content; E.G., M.F., F.V.d.B. and D.E. wrote the article and E.G., M.F., G.S., S.D., M.D.V., F.V.d.B. and D.E. reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
F.V.d.B. declares that he has received speaker’s and/or consultancy fees from Abbvie, Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, Galapagos, Merck, Pfizer, Novartis, Sanofi and UCB. D.E. declares that his work is supported by grants from the Fund for Scientific Research Flanders, the Research Council of Ghent University and an Excellence of Science (EOS) grant from the Fund for Scientific Research Flanders. The other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Rheumatology thanks F. Ciccia, H. Xu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gracey, E., Vereecke, L., McGovern, D. et al. Revisiting the gut–joint axis: links between gut inflammation and spondyloarthritis. Nat Rev Rheumatol 16, 415–433 (2020). https://doi.org/10.1038/s41584-020-0454-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-020-0454-9
This article is cited by
-
Clinical features and fecal microbiota characteristics of patients with both ulcerative colitis and axial spondyloarthritis
BMC Gastroenterology (2024)
-
HLA-B27 did not protect against COVID-19 in patients with axial spondyloarthritis – data from the ReumaCov-Brasil Registry
Advances in Rheumatology (2023)
-
The bone marrow side of axial spondyloarthritis
Nature Reviews Rheumatology (2023)
-
Spondyloarthritis with inflammatory bowel disease: the latest on biologic and targeted therapies
Nature Reviews Rheumatology (2023)
-
Have Therapeutics Enhanced Our Knowledge of Axial Spondyloarthritis?
Current Rheumatology Reports (2023)