Abstract
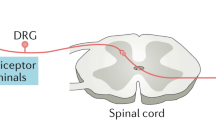
Nerve growth factor (NGF) is a neurotrophin that activates nociceptive neurons to transmit pain signals from the peripheral to the central nervous system and that exerts its effects on neurons by signalling through tyrosine kinase receptors. Antibodies that inhibit the function of NGF and small molecule inhibitors of NGF receptors have been developed and tested in clinical studies to evaluate the efficacy of NGF inhibition as a form of analgesia in chronic pain states including osteoarthritis and chronic low back pain. Clinical studies in individuals with painful knee and hip osteoarthritis have revealed that NGF inhibitors substantially reduce joint pain and improve function compared with NSAIDs for a duration of up to 8 weeks. However, the higher tested doses of NGF inhibitors also increased the risk of rapidly progressive osteoarthritis in a small percentage of those treated. This Review recaps the biology of NGF and the studies that have been performed to evaluate the efficacy of NGF inhibition for chronic musculoskeletal pain states. The adverse events associated with NGF inhibition and the current state of knowledge about the mechanisms involved in rapidly progressive osteoarthritis are also discussed and future studies proposed to improve understanding of this rare but serious adverse event.
Key points
-
Chronic pain from osteoarthritis (OA) is highly prevalent, and effective non-opioid medications are few.
-
Nerve growth factor (NGF) is an important neurotrophin that activates nociceptive neurons to transmit pain signals from the peripheral to the central nervous system.
-
Treatment with anti-NGF antibodies inhibits joint pain and improves function in individuals with moderate to severe knee and hip OA.
-
NGF inhibition is associated with rapidly progressive large joint OA; many theories exist as to why but the exact mechanisms involved remain unknown.
-
Anti-NGF antibody treatments, if approved, should reduce pain and improve quality of life for individuals with knee and hip OA; however, safety monitoring programmes will be necessary.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Gaskin, D. J. & Richard, P. The economic costs of pain in the United States. J. Pain 13, 715–724 (2012).
van den Driest, J. J. et al. Opioid prescriptions in patients with osteoarthritis: a population-based cohort study. Rheumatology 59, 2462–2470 (2020).
Hochberg, M. C. et al. When is osteonecrosis not osteonecrosis?: Adjudication of reported serious adverse joint events in the tanezumab clinical development program. Arthritis Rheumatol. 68, 382–391 (2016).
Nobel Media. The Nobel Prize in Physiology or Medicine 1906. The Nobel Prize https://www.nobelprize.org/prizes/medicine/1906/summary/ (2020).
Levi-Montalcini, R. & Hamburger, V. Selective growth stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryo. J. Exp. Zool. 116, 321–361 (1951).
Mizumura, K. & Murase, S. Role of nerve growth factor in pain. Handb. Exp. Pharmacol. 227, 57–77 (2015).
Leibrock, J. et al. Molecular cloning and expression of brain-derived neurotrophic factor. Nature 341, 149–152 (1989).
Maisonpierre, P. C. et al. Neurotrophin-3: a neurotrophic factor related to NGF and BDNF. Science 247, 1446–1451 (1990).
Berkemeier, L. R. et al. Neurotrophin-5: a novel neurotrophic factor that activates trk and trkB. Neuron 7, 857–866 (1991).
Gotz, R. et al. Neurotrophin-6 is a new member of the nerve growth factor family. Nature 372, 266–269 (1994).
Lai, K. O., Fu, W. Y., Ip, F. C. & Ip, N. Y. Cloning and expression of a novel neurotrophin, NT-7, from carp. Mol. Cell Neurosci. 11, 64–76 (1998).
Bothwell, M. Recent advances in understanding context-dependent mechanisms controlling neurotrophin signaling and function. F1000Res 8, 1658 (2019).
Ullrich, A., Gray, A., Berman, C., Coussens, L. & Dull, T. J. Sequence homology of human and mouse beta-NGF subunit genes. Cold Spring Harb. Symp. Quant. Biol. 48, 435–442 (1983).
Ruberti, F. et al. Phenotypic knockout of nerve growth factor in adult transgenic mice reveals severe deficits in basal forebrain cholinergic neurons, cell death in the spleen, and skeletal muscle dystrophy. J. Neurosci. 20, 2589–2601 (2000).
Hempstead, B. L., Martin-Zanca, D., Kaplan, D. R., Parada, L. F. & Chao, M. V. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature 350, 678–683 (1991).
Barker, P. A. & Shooter, E. M. Disruption of NGF binding to the low affinity neurotrophin receptor p75LNTR reduces NGF binding to TrkA on PC12 cells. Neuron 13, 203–215 (1994).
Hartman, D. S., McCormack, M., Schubenel, R. & Hertel, C. Multiple trkA proteins in PC12 cells bind NGF with a slow association rate. J. Biol. Chem. 267, 24516–24522 (1992).
Kaplan, D. R., Hempstead, B. L., Martin-Zanca, D., Chao, M. V. & Parada, L. F. The trk proto-oncogene product: a signal transducing receptor for nerve growth factor. Science 252, 554–558 (1991).
Wang, T. et al. NT-4 attenuates neuroinflammation via TrkB/PI3K/FoxO1 pathway after germinal matrix hemorrhage in neonatal rats. J. Neuroinflammation 17, 158 (2020).
Joo, W., Hippenmeyer, S. & Luo, L. Neurodevelopment. Dendrite morphogenesis depends on relative levels of NT-3/TrkC signaling. Science 346, 626–629 (2014).
Ehlers, M. D., Kaplan, D. R., Price, D. L. & Koliatsos, V. E. NGF-stimulated retrograde transport of trkA in the mammalian nervous system. J. Cell Biol. 130, 149–156 (1995).
Korsching, S. & Thoenen, H. Quantitative demonstration of the retrograde axonal transport of endogenous nerve growth factor. Neurosci. Lett. 39, 1–4 (1983).
Kessler, J. A. & Black, I. B. Nerve growth factor stimulates the development of substance P in sensory ganglia. Proc. Natl Acad. Sci. USA 77, 649–652 (1980).
Inaishi, Y., Kashihara, Y., Sakaguchi, M., Nawa, H. & Kuno, M. Cooperative regulation of calcitonin gene-related peptide levels in rat sensory neurons via their central and peripheral processes. J. Neurosci. 12, 518–524 (1992).
Hafstrom, I., Gyllenhammar, H., Palmblad, J. & Ringertz, B. Substance P activates and modulates neutrophil oxidative metabolism and aggregation. J. Rheumatol. 16, 1033–1037 (1989).
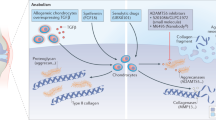
Mamet, J., Lazdunski, M. & Voilley, N. How nerve growth factor drives physiological and inflammatory expressions of acid-sensing ion channel 3 in sensory neurons. J. Biol. Chem. 278, 48907–48913 (2003).
Ji, R. R., Samad, T. A., Jin, S. X., Schmoll, R. & Woolf, C. J. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 36, 57–68 (2002).
Latremoliere, A. & Woolf, C. J. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J. Pain 10, 895–926 (2009).
McMahon, S. B., Armanini, M. P., Ling, L. H. & Phillips, H. S. Expression and coexpression of Trk receptors in subpopulations of adult primary sensory neurons projecting to identified peripheral targets. Neuron 12, 1161–1171 (1994).
Pincelli, C. et al. Expression and function of nerve growth factor and nerve growth factor receptor on cultured keratinocytes. J. Invest. Dermatol. 103, 13–18 (1994).
Manni, L. et al. Nerve growth factor release by human synovial fibroblasts prior to and following exposure to tumor necrosis factor-alpha, interleukin-1 beta and cholecystokinin-8: the possible role of NGF in the inflammatory response. Clin. Exp. Rheumatol. 21, 617–624 (2003).
Berdun, S., Rychter, J. & Vergara, P. Effects of nerve growth factor antagonist K252a on peritoneal mast cell degranulation: implications for rat postoperative ileus. Am. J. Physiol. Gastrointest. Liver Physiol. 309, G801–G806 (2015).
Welle, S., Wolf, A. M., Dernbach, C., Klarmann-Schulz, U. & Seidel, M. F. Disease activity-dependent expression of nerve growth factor TRKA and P75 receptors on elevated dendritic cells and peripheral leucocytes in patients with systemic lupus erythematosus. Lupus 29, 970–975 (2020).
Chartier, S. R., Mitchell, S. A., Majuta, L. A. & Mantyh, P. W. Immunohistochemical localization of nerve growth factor, tropomyosin receptor kinase A, and p75 in the bone and articular cartilage of the mouse femur. Mol. Pain 13, 1744806917745465 (2017).
Nencini, S. et al. Mechanisms of nerve growth factor signaling in bone nociceptors and in an animal model of inflammatory bone pain. Mol. Pain 13, 1744806917697011 (2017).
Grills, B. L. & Schuijers, J. A. Immunohistochemical localization of nerve growth factor in fractured and unfractured rat bone. Acta Orthop. Scand. 69, 415–419 (1998).
Sang, X. G. et al. Analysis of the mechanism by which nerve growth factor promotes callus formation in mice with tibial fracture. Exp. Ther. Med. 13, 1376–1380 (2017).
Chen, W. H., Mao, C. Q., Zhuo, L. L. & Ong, J. L. Beta-nerve growth factor promotes neurogenesis and angiogenesis during the repair of bone defects. Neural Regen. Res. 10, 1159–1165 (2015).
Jimenez-Andrade, J. M. et al. Nerve growth factor sequestering therapy attenuates non-malignant skeletal pain following fracture. Pain 133, 183–196 (2007).
Malfait, A. M., Miller, R. E. & Block, J. A. Targeting neurotrophic factors: novel approaches to musculoskeletal pain. Pharmacol. Ther. 211, 107553 (2020).
von Loga, I. S. et al. Active immunisation targeting nerve growth factor attenuates chronic pain behaviour in murine osteoarthritis. Ann. Rheum. Dis. 78, 672–675 (2019).
Ogura, T. et al. Differences in levels of inflammatory mediators in meniscal and synovial tissue of patients with meniscal lesions. J. Exp. Orthop. 3, 7 (2016).
Barthel, C. et al. Nerve growth factor and receptor expression in rheumatoid arthritis and spondyloarthritis. Arthritis Res. Ther. 11, R82 (2009).
Stoppiello, L. A. et al. Structural associations of symptomatic knee osteoarthritis. Arthritis Rheumatol. 66, 3018–3027 (2014).
Seidel, M. F., Fiebich, B. L., Lieb, K., Ulrich-Merzenich, G. & Koch, F. Substance P-induced nerve growth factor release is down-regulated by serotonin in serum-free cultured osteoarthritis macrophage-like synovial cells. Synergy 5, 9–12 (2017).
Walsh, D. A. et al. Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology 49, 1852–1861 (2010).
Aso, K. et al. Associations of symptomatic knee osteoarthritis with histopathologic features in subchondral bone. Arthritis Rheumatol. 71, 916–924 (2019).
Seidel, M. et al. Human lumbar spine facet joint osteoarthritis displays predominant NGF expression and signaling in capsular synovium and subchondral bone marrow tissues independent of osteoarthritis grade [abstract 482]. Ann. Rheum. Dis. 78, 532 (2019).
Shelton, D. L. & Reichardt, L. F. Studies on the expression of the beta nerve growth factor (NGF) gene in the central nervous system: level and regional distribution of NGF mRNA suggest that NGF functions as a trophic factor for several distinct populations of neurons. Proc. Natl Acad. Sci. USA 83, 2714–2718 (1986).
Chen, K. S. & Gage, F. H. Somatic gene transfer of NGF to the aged brain: behavioral and morphological amelioration. J. Neurosci. 15, 2819–2825 (1995).
Cattaneo, A. et al. Functional blockade of tyrosine kinase A in the rat basal forebrain by a novel antagonistic anti-receptor monoclonal antibody. J. Neurosci. 19, 9687–9697 (1999).
Xhima, K. et al. Focused ultrasound delivery of a selective TrkA agonist rescues cholinergic function in a mouse model of Alzheimer’s disease. Sci. Adv. 6, eaax6646 (2020).
de Bellis, A., de Bellis, M. & Aloe, L. Long-term non-invasive treatment via intranasal administration of nerve growth factor protects the human brain in frontotemporal dementia associated with corticobasal syndrome: a pilot study. J. Alzheimers Dis. Rep. 2, 67–77 (2018).
Erdo, F., Denes, L. & de Lange, E. Age-associated physiological and pathological changes at the blood-brain barrier: a review. J. Cereb. Blood Flow Metab. 37, 4–24 (2017).
Owolabi, J. B. et al. Characterization of antiallodynic actions of ALE-0540, a novel nerve growth factor receptor antagonist, in the rat. J. Pharmacol. Exp. Ther. 289, 1271–1276 (1999).
McNamee, K. E. et al. Treatment of murine osteoarthritis with TrkAd5 reveals a pivotal role for nerve growth factor in non-inflammatory joint pain. Pain 149, 386–392 (2010).
Covaceuszach, S., Cattaneo, A. & Lamba, D. Purification, crystallization and preliminary X-ray analysis of the Fab fragment from MNAC13, a novel antagonistic anti-tyrosine kinase A receptor monoclonal antibody. Acta Crystallogr. D. 57, 1307–1309 (2001).
Raychaudhuri, S. P., Sanyal, M., Weltman, H. & Kundu-Raychaudhuri, S. K252a, a high-affinity nerve growth factor receptor blocker, improves psoriasis: an in vivo study using the severe combined immunodeficient mouse-human skin model. J. Invest. Dermatol. 122, 812–819 (2004).
Djouhri, L. PG110, a humanized anti-NGF antibody, reverses established pain hypersensitivity in persistent inflammatory pain, but not peripheral neuropathic pain, rat models. Pain Med. 17, 2082–2094 (2016).
Doebele, R. C. et al. An oncogenic NTRK fusion in a patient with soft-tissue sarcoma with response to the tropomyosin-related kinase inhibitor LOXO-101. Cancer Discov. 5, 1049–1057 (2015).
Sanga, P. et al. Efficacy, safety, and tolerability of fulranumab, an anti-nerve growth factor antibody, in the treatment of patients with moderate to severe osteoarthritis pain. Pain 154, 1910–1919 (2013).
Tiseo, P. J., Ren, H. & Mellis, S. Fasinumab (REGN475), an antinerve growth factor monoclonal antibody, for the treatment of acute sciatic pain: results of a proof-of-concept study. J. Pain Res. 7, 523–530 (2014).
Watt, F. E. et al. Tropomyosin-related kinase A (TrkA) inhibition for the treatment of painful knee osteoarthritis: results from a randomized controlled phase 2a trial. Osteoarthritis Cartilage 27, 1590–1598 (2019).
Drilon, A. et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N. Engl. J. Med. 378, 731–739 (2018).
Seidel, M. F., Wise, B. L. & Lane, N. E. Nerve growth factor: an update on the science and therapy. Osteoarthritis Cartilage 21, 1223–1228 (2013).
Balanescu, A. R. et al. Efficacy and safety of tanezumab added on to diclofenac sustained release in patients with knee or hip osteoarthritis: a double-blind, placebo-controlled, parallel-group, multicentre phase III randomised clinical trial. Ann. Rheum. Dis. 73, 1665–1672 (2014).
Birbara, C. et al. Safety and efficacy of subcutaneous tanezumab in patients with knee or hip osteoarthritis. J. Pain Res. 11, 151–164 (2018).
Brown, M. T. et al. Tanezumab reduces osteoarthritic knee pain: results of a randomized, double-blind, placebo-controlled phase III trial. J. Pain 13, 790–798 (2012).
Ekman, E. F. et al. Efficacy and safety of intravenous tanezumab for the symptomatic treatment of osteoarthritis: 2 randomized controlled trials versus naproxen. J. Rheumatol. 41, 2249–2259 (2014).
Spierings, E. L. et al. A phase III placebo- and oxycodone-controlled study of tanezumab in adults with osteoarthritis pain of the hip or knee: response. Pain 155, 2432–2433 (2014).
Mayorga, A. J., Wang, S., Kelly, K. M. & Thipphawong, J. Efficacy and safety of fulranumab as monotherapy in patients with moderate to severe, chronic knee pain of primary osteoarthritis: a randomised, placebo- and active-controlled trial. Int. J. Clin. Pract. 70, 493–505 (2016).
Sanga, P. et al. Long-term safety and efficacy of fulranumab in patients with moderate-to-severe osteoarthritis pain: a phase II randomized, double-blind, placebo-controlled extension study. Arthritis Rheumatol. 69, 763–773 (2017).
Dakin, P. et al. The efficacy, tolerability, and joint safety of fasinumab in osteoarthritis pain: a phase IIb/III double-blind, placebo-controlled, randomized clinical trial. Arthritis Rheumatol. 71, 1824–1834 (2019).
Berenbaum, F. et al. Subcutaneous tanezumab for osteoarthritis of the hip or knee: efficacy and safety results from a 24-week randomised phase III study with a 24-week follow-up period. Ann. Rheum. Dis. 79, 800–810 (2020).
Kivitz, A. J. et al. Efficacy and safety of tanezumab versus naproxen in the treatment of chronic low back pain. Pain 154, 1009–1021 (2013).
Gimbel, J. S. et al. Long-term safety and effectiveness of tanezumab as treatment for chronic low back pain. Pain 155, 1793–1801 (2014).
Leite, V. F. et al. Anti-nerve growth factor in the treatment of low back pain and radiculopathy: a systematic review and a meta-analysis. Pain Physician 17, E45–E60 (2014).
Garber, K. Fate of novel painkiller mAbs hangs in balance. Nat. Biotechnol. 29, 173–174 (2011).
Sinha, G. Pfizer and Lilly shoulder novel pain-drug risks. Nat. Biotechnol. 32, 9 (2014).
Butt, M. et al. Morphologic, stereologic, and morphometric evaluation of the nervous system in young cynomolgus monkeys (Macaca fascicularis) following maternal administration of tanezumab, a monoclonal antibody to nerve growth factor. Toxicol. Sci. 142, 463–476 (2014).
Brown, M., Koltzenburg, M., Nguyen, H., West, C. & Verburg, K. Tanezumab does not cause sympathetic nervous system dysfunction in clinical osteoarthritis studies [abstract P3.303]. Neurology 84, P3.303 (2015).
Hochberg, M. C. et al. Subcutaneous tanezumab versus NSAID for the treatment of osteoarthritis: joint safety events in a randomized, double-blind, active-controlled, 80-week, phase-3 study [abstract]. Arthritis Rheumatol. 71, 2756 (2019).
Schnitzer, T. J. et al. Effect of tanezumab on joint pain, physical function, and patient global assessment of osteoarthritis among patients with osteoarthritis of the hip or knee: a randomized clinical trial. JAMA 322, 37–48 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02709486 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02528188 (2020).
Rashad, S. et al. Effect of non-steroidal anti-inflammatory drugs on the course of osteoarthritis. Lancet 2, 519–522 (1989).
Chen, M. R. & Dragoo, J. L. The effect of nonsteroidal anti-inflammatory drugs on tissue healing. Knee Surg. Sports Traumatol. Arthrosc. 21, 540–549 (2013).
Karsdal, M. A. et al. Serological biomarker profiles of rapidly progressive osteoarthritis in tanezumab-treated patients. Osteoarthritis Cartilage 27, 484–492 (2019).
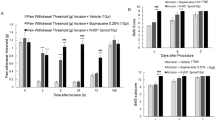
Lane, N. E. et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. N. Engl. J. Med. 363, 1521–1531 (2010).
Miller, R. E., Block, J. A. & Malfait, A. M. Nerve growth factor blockade for the management of osteoarthritis pain: what can we learn from clinical trials and preclinical models? Curr. Opin. Rheumatol. 29, 110–118 (2017).
LaBranche, T. P. et al. Nerve growth factor inhibition with tanezumab influences weight-bearing and subsequent cartilage damage in the rat medial meniscal tear model. Ann. Rheum. Dis. 76, 295–302 (2017).
Xu, L. et al. The anti-NGF antibody muMab 911 both prevents and reverses pain behaviour and subchondral osteoclast numbers in a rat model of osteoarthritis pain. Osteoarthritis Cartilage 24, 1587–1595 (2016).
Tomlinson, R. E. et al. NGF-TrkA signaling in sensory nerves is required for skeletal adaptation to mechanical loads in mice. Proc. Natl Acad. Sci. USA 114, E3632–E3641 (2017).
Ugolini, G., Marinelli, S., Covaceuszach, S., Cattaneo, A. & Pavone, F. The function neutralizing anti-TrkA antibody MNAC13 reduces inflammatory and neuropathic pain. Proc. Natl Acad. Sci. USA 104, 2985–2990 (2007).
Springer. ABT 110. Adis Insight https://adisinsight.springer.com/drugs/800021385 (2013).
Springer. Tanezumab — Eli Lilly and Company/Pfizer. Adis Insight https://adisinsight.springer.com/drugs/800019466 (2020).
Springer. Fasinumab — Mitsubishi Tanabe Pharma/Regeneron/Teva. Adis Insight https://adisinsight.springer.com/drugs/800029842 (2020).
Springer. Fulranumab. Adis Insight https://adisinsight.springer.com/drugs/800024342 (2020).
Schnitzer, T. J. et al. Efficacy and safety of tanezumab monotherapy or combined with non-steroidal anti-inflammatory drugs in the treatment of knee or hip osteoarthritis pain. Ann. Rheum. Dis. 74, 1202–1211 (2015).
Spierings, E. L. et al. A phase III placebo- and oxycodone-controlled study of tanezumab in adults with osteoarthritis pain of the hip or knee. Pain 154, 1603–1612 (2013).
Schnitzer, T. J. et al. Onset and maintenance of efficacy of subcutaneous tanezumab in patients with moderate to severe osteoarthritis of the knee or hip: a 16-week dose-titration study. Semin. Arthritis Rheum. 50, 387–393 (2020).
Schnitzer, T. J. & Marks, J. A. A systematic review of the efficacy and general safety of antibodies to NGF in the treatment of OA of the hip or knee. Osteoarthritis Cartilage 23, S8–S17 (2015).
Kan, S. L. et al. Tanezumab for patients with osteoarthritis of the knee: a meta-analysis. PLoS One 11, e0157105 (2016).
Chen, J. et al. Efficacy and safety of tanezumab on osteoarthritis knee and hip pains: a meta-analysis of randomized controlled trials. Pain Med. 18, 374–385 (2017).
Tive, L. et al. Pooled analysis of tanezumab efficacy and safety with subgroup analyses of phase III clinical trials in patients with osteoarthritis pain of the knee or hip. J. Pain Res. 12, 975–995 (2019).
Bueker, E. D. Implantation of tumors in the hind limb field of the embryonic chick and the developmental response of the lumbosacral nervous system. Anat. Rec. 102, 369–389 (1948).
Nobel Media. The Nobel Prize in Physiology or Medicine 1986. The Nobel Prize https://www.nobelprize.org/prizes/medicine/1986/summary/ (2020).
Theodosiou, M. et al. Hyperalgesia due to nerve damage: role of nerve growth factor. Pain 81, 245–255 (1999).
Abdiche, Y. N., Malashock, D. S. & Pons, J. Probing the binding mechanism and affinity of tanezumab, a recombinant humanized anti-NGF monoclonal antibody, using a repertoire of biosensors. Protein Sci. 17, 1326–1335 (2008).
Seidel, M. F., Herguijuela, M., Forkert, R. & Otten, U. Nerve growth factor in rheumatic diseases. Semin. Arthritis Rheum. 40, 109–126 (2010).
Pfizer. US FDA accepts regulatory submission for tanezumab, a potential first-in-class treatment for patients with chronic pain due to moderate-to-severe osteoarthritis. Pfizer https://www.pfizer.com/news/ (2020).
Author information
Authors and Affiliations
Contributions
M.F.S. and N.E.L. researched data for the article. All authors contributed substantially to discussions of content, wrote the article and reviewed or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
B.L.W. declares that he received a research grant from Pfizer from 2011 to 2012. M.F.S. declares that he has been a consultant for Eli Lilly and Pfizer, and that he has received educational grants from these companies. N.E.L. declares that she has performed phase II and phase III clinical trials for Pfizer (2010–2012 and 2016–2019) and has been a consultant for Pfizer (2011–2019).
Additional information
Peer review information
Nature Reviews Rheumatology thanks F. Berenbaum and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Placode
-
Ectodermal structures in embryonic development that give rise to several different sensory systems.
- Dorsal root ganglia
-
The cell bodies of sensory nerves that transmit action potentials to the spinal cord.
- Retrograde axoplasmic transport
-
A process in which signalling molecules are moved from the periphery towards the cell body of an axon.
- Antidromal transport
-
Axoplasmic transport of signalling molecules from the nucleus to nociceptors.
- Allodynia
-
Painful sensation in response to non-painful stimuli.
- Zygapophyseal joints
-
Vertebral (facet) joints that interconnect the vertebral bodies.
- Paraesthesia
-
Abnormal skin sensation without stimulation.
- Hypoaesthesia
-
Numbness of the skin with a reduction of sensations to sensory stimuli.
Rights and permissions
About this article
Cite this article
Wise, B.L., Seidel, M.F. & Lane, N.E. The evolution of nerve growth factor inhibition in clinical medicine. Nat Rev Rheumatol 17, 34–46 (2021). https://doi.org/10.1038/s41584-020-00528-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-020-00528-4
This article is cited by
-
The alterations in nerve growth factor concentration in plasma and synovial fluid before and after total knee arthroplasty
Scientific Reports (2024)
-
Nerve growth factor receptor limits inflammation to promote remodeling and repair of osteoarthritic joints
Nature Communications (2024)
-
Osteoarthritis: pathogenic signaling pathways and therapeutic targets
Signal Transduction and Targeted Therapy (2023)
-
Piezo2 expressing nociceptors mediate mechanical sensitization in experimental osteoarthritis
Nature Communications (2023)
-
Maximizing treatment efficacy through patient stratification in neuropathic pain trials
Nature Reviews Neurology (2023)