Abstract
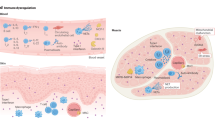
Immune-mediated necrotizing myopathy (IMNM) is a group of inflammatory myopathies that was distinguished from polymyositis in 2004. Most IMNMs are associated with anti-signal recognition particle (anti-SRP) or anti-3-hydroxy-3-methylglutaryl-coA reductase (anti-HMGCR) myositis-specific autoantibodies, although ~20% of patients with IMNM remain seronegative. These associations have led to three subclasses of IMNM: anti-SRP-positive IMNM, anti-HMGCR-positive IMNM and seronegative IMNM. IMNMs are frequently rapidly progressive and severe, displaying high serum creatine kinase levels, and failure to treat IMNMs effectively may lead to severe muscle impairment. In patients with seronegative IMNM, disease can be concomitant with cancer. Research into IMNM pathogenesis has shown that anti-SRP and anti-HMGCR autoantibodies cause weakness and myofibre necrosis in mice, suggesting that, as well as being diagnostic biomarkers of IMNM, they may play a key role in disease pathogenesis. Therapeutically, treatments such as rituximab or intravenous immunoglobulins can now be discussed for IMNM, and targeted therapies, such as anticomplement therapeutics, may be a future option for patients with refractory disease.
Key points
-
Anti-3-hydroxy-3-methylglutaryl-coA reductase (anti-HMGCR) and anti-signal recognition particle (anti-SRP) myositis-specific autoantibodies are crucial for defining immune-mediated necrotizing myopathy (IMNM) when muscle biopsy is absent but required for diagnosis.
-
IMNM can be considered a muscle-specific autoimmune disease; patients with anti-SRP-positive IMNM are at an increased risk of myocarditis and patients with seronegative IMNM are at a major risk of associated malignancy.
-
Among treatable idiopathic inflammatory myopathies, IMNM is the most severe in terms of muscle-related morbidities such as muscle atrophy, muscle fat replacement and/or disability, and is of long duration.
-
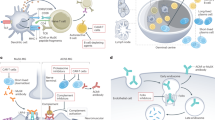
Anti-HMGCR and anti-SRP myositis-specific autoantibodies seem to play a key role in the pathophysiology of IMNM; autoantibody-induced muscle damage is complement dependent.
-
A combination of corticosteroids, immunosuppressants and intravenous immunoglobulins is frequently required to control disease activity.
-
No specific randomized clinical trial is available to define the best treatment strategy for keeping patients with IMNM in remission.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Dobloug, C. et al. Prevalence and clinical characteristics of adult polymyositis and dermatomyositis; data from a large and unselected Norwegian cohort. Ann. Rheum. Dis. 74, 1551–1556 (2015).
Bohan, A. & Peter, J. B. Polymyositis and dermatomyositis (first of two parts). N. Engl. J. Med. 292, 344–347 (1975).
Griggs, R. C. et al. Inclusion body myositis and myopathies. Ann. Neurol. 38, 705–713 (1995).
Reeves, W. H., Nigam, S. K. & Blobel, G. Human autoantibodies reactive with the signal-recognition particle. Proc. Natl Acad. Sci. USA 83, 9507–9511 (1986).
Hoogendijk, J. E. et al. 119th ENMC international workshop: trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10–12 October 2003, Naarden, The Netherlands. Neuromuscul. Disord. 14, 337–345 (2004).
Miller, T., Al-Lozi, M. T., Lopate, G. & Pestronk, A. Myopathy with antibodies to the signal recognition particle: clinical and pathological features. J. Neurol. Neurosurg. Psychiatry 73, 420–428 (2002).
Mammen, A. L. et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum. 63, 713–721 (2011).
Smith, B. Skeletal muscle necrosis associated with cainoma. J. Pathol. 97, 207–210 (1969).
Urich, H. & Wilkinson, M. Necrosis of muscle with carcinoma: myositis or myopathy? J. Neurol. Neurosurg. Psychiatry 33, 398–407 (1970).
Kole, R. et al. Alu RNA-protein complexes formed in vitro react with a novel lupus autoantibody. J. Biol. Chem. 260, 11781–11786 (1985).
Targoff, I. N., Johnson, A. E. & Miller, F. W. Antibody to signal recognition particle in polymyositis. Arthritis Rheum. 33, 1361–1370 (1990).
Christopher-Stine, L. et al. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum. 62, 2757–2766 (2010).
Allenbach, Y. et al. Anti-HMGCR autoantibodies in European patients with autoimmune necrotizing myopathies: inconstant exposure to statin. Medicine 93, 150–157 (2014).
Drouot, L. et al. Exploring necrotizing autoimmune myopathies with a novel immunoassay for anti-3-hydroxy-3-methyl-glutaryl-CoA reductase autoantibodies. Arthritis Res. Ther. 16, R39 (2014).
Mariampillai, K. et al. Development of a new classification system for idiopathic inflammatory myopathies based on clinical manifestations and myositis-specific autoantibodies. JAMA Neurol. 75, 1528–1537 (2018).
Pinal-Fernandez, I. et al. Machine learning algorithms reveal unique gene expression profiles in muscle biopsies from patients with different types of myositis. Ann. Rheum. Dis. 79, 1234–1242 (2020).
Allenbach, Y., Mammen, A. L., Benveniste, O., Stenzel, W. & Immune-Mediated Necrotizing Myopathies Working Group. 224th ENMC International Workshop: clinico-sero-pathological classification of immune-mediated necrotizing myopathies Zandvoort, The Netherlands, 14–16 October 2016. Neuromuscul. Disord. 28, 87–99 (2018).
Lundberg, I. E. et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Arthritis Rheumatol. 76, 1955–1964 (2017).
Allenbach, Y. et al. Necrosis in anti-SRP+ and anti-HMGCR+ myopathies: role of autoantibodies and complement. Neurology 90, e507–e517 (2018).
Allenbach, Y. & Benveniste, O. Acquired necrotizing myopathies. Curr. Opin. Neurol. 26, 554–560 (2013).
Walter, P. & Blobel, G. Purification of a membrane-associated protein complex required for protein translocation across the endoplasmic reticulum. Proc. Natl Acad. Sci. USA 77, 7112–7116 (1980).
Walter, P. & Blobel, G. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature 299, 691–698 (1982).
Zwieb, C. & Bhuiyan, S. Archaea signal recognition particle shows the way. Archaea 2010, 485051 (2010).
Römisch, K., Miller, F. W., Dobberstein, B. & High, S. Human autoantibodies against the 54 kDa protein of the signal recognition particle block function at multiple stages. Arthritis Res. Ther. 8, R39 (2006).
Benveniste, O. et al. Correlation of anti-signal recognition particle autoantibody levels with creatine kinase activity in patients with necrotizing myopathy. Arthritis Rheum. 63, 1961–1971 (2011).
Carapito, R. et al. Mutations in signal recognition particle SRP54 cause syndromic neutropenia with Shwachman-Diamond-like features. J. Clin. Invest. 127, 4090–4103 (2017).
Goldstein, J. L. & Brown, M. S. Regulation of the mevalonate pathway. Nature 343, 425–430 (1990).
Liscum, L. et al. Domain structure of 3-hydroxy-3-methylglutaryl coenzyme A reductase, a glycoprotein of the endoplasmic reticulum. J. Biol. Chem. 260, 522–530 (1985).
Nagashima, S. et al. Liver-specific deletion of 3-hydroxy-3-methylglutaryl coenzyme A reductase causes hepatic steatosis and death. Arterioscler. Thromb. Vasc. Biol. 32, 1824–1831 (2012).
Osaki, Y. et al. Skeletal muscle-specific HMG-CoA reductase knockout mice exhibit rhabdomyolysis: a model for statin-induced myopathy. Biochem. Biophys. Res. Commun. 466, 536–540 (2015).
Ference, B. A. et al. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N. Engl. J. Med. 375, 2144–2153 (2016).
Parker, B. A. et al. Effect of statins on skeletal muscle function. Circulation 127, 96–103 (2013).
Mammen, A. L. et al. Rarity of anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase antibodies in statin users, including those with self-limited musculoskeletal side effects. Arthritis Care Res. 64, 269–272 (2012).
Shovman, O. et al. Anti-HMGCR antibodies demonstrate high diagnostic value in the diagnosis of immune-mediated necrotizing myopathy following statin exposure. Immunol. Res. 65, 276–281 (2017).
Meyer, A. et al. Incidence and prevalence of inflammatory myopathies: a systematic review. Rheumatology 54, 50–63 (2015).
Rönnelid, J. et al. Use of a commercial line blot assay as a screening test for autoantibodies in inflammatory myopathies. Autoimmun. Rev. 9, 58–61 (2009).
Watanabe, Y. et al. Clinical features and prognosis in anti-SRP and anti-HMGCR necrotising myopathy. J. Neurol. Neurosurg. Psychiatry 87, 1038–1044 (2016).
Pinal-Fernandez, I. et al. Longitudinal course of disease in a large cohort of myositis patients with autoantibodies recognizing the signal recognition particle. Arthritis Care Res. 69, 263–270 (2017).
Kishi, T. et al. Association of anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase autoantibodies with DRB1*07:01 and severe myositis in juvenile myositis patients. Arthritis Care Res. 69, 1088–1094 (2017).
Ueki, M. et al. Myositis-specific autoantibodies in Japanese patients with juvenile idiopathic inflammatory myopathies. Mod. Rheumatol. 29, 351–356 (2019).
Suzuki, S. et al. Inflammatory myopathy with anti-signal recognition particle antibodies: case series of 100 patients. Orphanet J. Rare Dis. 10, 61 (2015).
Hengstman, G. J. D. et al. Anti-signal recognition particle autoantibodies: marker of a necrotising myopathy. Ann. Rheum. Dis. 65, 1635–1638 (2006).
Rothwell, S. et al. Focused HLA analysis in Caucasians with myositis identifies significant associations with autoantibody subgroups. Ann. Rheum. Dis. 78, 996–1002 (2019).
Ohnuki, Y. et al. HLA-DRB1 alleles in immune-mediated necrotizing myopathy. Neurology 87, 1954–1955 (2016).
Kang, E. H. et al. Novel susceptibility alleles in HLA region for myositis and myositis specific autoantibodies in Korean patients. Semin. Arthritis Rheum. 49, 283–287 (2019).
Liang, W.-C. et al. Pediatric necrotizing myopathy associated with anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase antibodies. Rheumatology 56, 287–293 (2017).
Ge, Y., Lu, X., Peng, Q., Shu, X. & Wang, G. Clinical characteristics of anti-3-hydroxy-3-methylglutaryl coenzyme A reductase antibodies in Chinese patients with idiopathic inflammatory myopathies. PLoS One 10, e0141616 (2015).
Limaye, V. et al. Clinical and genetic associations of autoantibodies to 3-hydroxy-3-methyl-glutaryl-coenzyme a reductase in patients with immune-mediated myositis and necrotizing myopathy. Muscle Nerve 52, 196–203 (2015).
Mammen, A. L. et al. Increased frequency of DRB1*11:01 in anti-hydroxymethylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Care Res. 64, 1233–1237 (2012).
Allenbach, Y. et al. High risk of cancer in autoimmune necrotizing myopathies: usefulness of myositis specific antibody. Brain J. Neurol. 139, 2131–2135 (2016).
Kadoya, M. et al. Cancer association as a risk factor for anti-HMGCR antibody-positive myopathy. Neurol. Neuroimmunol. Neuroinflamm. 3, e290 (2016).
Tiniakou, E. et al. More severe disease and slower recovery in younger patients with anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Rheumatology 56, 787–794 (2017).
Levin, M. I., Mozaffar, T., Al-Lozi, M. T. & Pestronk, A. Paraneoplastic necrotizing myopathy: clinical and pathological features. Neurology 50, 764–767 (1998).
Vu, H. J., Pham, D., Makary, R., Nguyen, T. & Shuja, S. Paraneoplastic necrotizing myopathy presenting as severe muscle weakness in a patient with small-cell lung cancer: successful response to chemoradiation therapy. Clin. Adv. Hematol. Oncol. 9, 557–566 (2011).
Lim, J. et al. Seronegative patients form a distinctive subgroup of immune-mediated necrotizing myopathy. Neurol. Neuroimmunol. Neuroinflamm. 6, e513 (2019).
Kassardjian, C. D., Lennon, V. A., Alfugham, N. B., Mahler, M. & Milone, M. Clinical features and treatment outcomes of necrotizing autoimmune myopathy. JAMA Neurol. 72, 996–1003 (2015).
Paik, J. J. et al. Spectrum of muscle histopathologic findings in forty-two scleroderma patients with weakness. Arthritis Care Res. 67, 1416–1425 (2015).
Benveniste, O., Stenzel, W. & Allenbach, Y. Advances in serological diagnostics of inflammatory myopathies. Curr. Opin. Neurol. 29, 662–673 (2016).
Wesner, N. et al. Anti-RNP antibodies delineate a subgroup of myositis: a systematic retrospective study on 46 patients. Autoimmun. Rev. 19, 102465 (2020).
Casal-Dominguez, M. et al. Muscular and extramuscular features of myositis patients with anti-U1-RNP autoantibodies. Neurology 92, e1416–e1426 (2019).
Mohassel, P. et al. Anti-HMGCR myopathy may resemble limb-girdle muscular dystrophy. Neurol. Neuroimmunol. Neuroinflamm. 6, e523 (2019).
Kao, A. H., Lacomis, D., Lucas, M., Fertig, N. & Oddis, C. V. Anti-signal recognition particle autoantibody in patients with and patients without idiopathic inflammatory myopathy. Arthritis Rheum. 50, 209–215 (2004).
Takeguchi-Kikuchi, S. et al. Anti-signal recognition particle antibody-positive necrotizing myopathy with secondary cardiomyopathy: the first myocardial biopsy- and multimodal imaging-proven case. Intern. Med. 58, 3189–3194 (2019).
Thiébaut, M. et al. Antisignal recognition particle antibodies-related cardiomyopathy. Circulation 127, e434–e436 (2013).
Pitlick, M. & Ernste, F. Anti-HMGCR myopathy presenting with acute systolic heart failure. BMJ Case Rep. 12, e230213 (2019).
Rigolet, M. et al. Distinct interferon signatures stratify inflammatory and dysimmune myopathies. RMD Open 5, e000811 (2019).
Uruha, A. et al. Diagnostic potential of sarcoplasmic myxovirus resistance protein A expression in subsets of dermatomyositis. Neuropathol. Appl. Neurobiol. 45, 513–522 (2019).
Knauss, S. et al. PD1 pathway in immune-mediated myopathies: pathogenesis of dysfunctional T cells revisited. Neurol. Neuroimmunol. Neuroinflamm. 6, e558 (2019).
Preuße, C. et al. Immune-mediated necrotizing myopathy is characterized by a specific Th1-M1 polarized immune profile. Am. J. Pathol. 181, 2161–2171 (2012).
Yin, X. et al. CD4+ cells, macrophages, MHC-I and C5b-9 involve the pathogenesis of dysferlinopathy. Int. J. Clin. Exp. Pathol. 8, 3069–3075 (2015).
Fischer, N. et al. Sequestosome-1 (p62) expression reveals chaperone-assisted selective autophagy in immune-mediated necrotizing myopathies. Brain Pathol. 30, 261–271 (2019).
Girolamo, F. et al. Autophagy markers LC3 and p62 accumulate in immune-mediated necrotizing myopathy. Muscle Nerve 60, 315–327 (2019).
Schröder, N. W. J. et al. Pipestem capillaries in necrotizing myopathy revisited. Neuromuscul. Disord. 23, 66–74 (2013).
Landon-Cardinal, O. et al. Severe axial and pelvifemoral muscle damage in immune-mediated necrotizing myopathy evaluated by whole-body MRI. Semin. Arthritis Rheum. https://doi.org/10.1016/j.semarthrit.2020.02.009 (2020).
Pimenta, E., Wolley, M. & Stowasser, M. Adverse cardiovascular outcomes of corticosteroid excess. Endocrinology 153, 5137–5142 (2012).
Marie, I. et al. Hematological malignancy associated with polymyositis and dermatomyositis. Autoimmun. Rev. 11, 615–620 (2012).
Antiochos, B. B. et al. Malignancy is associated with dermatomyositis but not polymyositis in Northern New England, USA. J. Rheumatol. 36, 2704–2710 (2009).
Yang, H. et al. Identification of multiple cancer-associated myositis-specific autoantibodies in idiopathic inflammatory myopathies: a large longitudinal cohort study. Arthritis Res. Ther. 19, 259 (2017).
Arouche-Delaperche, L. et al. Pathogenic role of anti-signal recognition protein and anti-3-Hydroxy-3-methylglutaryl-CoA reductase antibodies in necrotizing myopathies: myofiber atrophy and impairment of muscle regeneration in necrotizing autoimmune myopathies. Ann. Neurol. 81, 538–548 (2017).
Pinal-Fernandez, I. et al. Myositis autoantigen expression correlates with muscle regeneration but not autoantibody specificity. Arthritis Rheumatol. 71, 1371–1376 (2019).
Jacquemin, V., Butler-Browne, G. S., Furling, D. & Mouly, V. IL-13 mediates the recruitment of reserve cells for fusion during IGF-1-induced hypertrophy of human myotubes. J. Cell Sci. 120, 670–681 (2007).
Horsley, V., Jansen, K. M., Mills, S. T. & Pavlath, G. K. IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell 113, 483–494 (2003).
Trapani, L. et al. 3-hydroxy 3-methylglutaryl coenzyme A reductase inhibition impairs muscle regeneration. J. Cell. Biochem. 113, 2057–2063 (2012).
Bergua, C. et al. In vivo pathogenicity of IgG from patients with anti-SRP or anti-HMGCR autoantibodies in immune-mediated necrotising myopathy. Ann. Rheum. Dis. 78, 131–139 (2019).
Albazli, K., Kaminski, H. J. & Howard, J. F. Complement inhibitor therapy for Myasthenia Gravis. Front. Immunol. 11, 917 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04025632 (2020).
PubChem. Zilucoplan. PubChem https://pubchem.ncbi.nlm.nih.gov/compound/133083018 (2018).
Meyer, A. et al. Statin-induced anti-HMGCR myopathy: successful therapeutic strategies for corticosteroid-free remission in 55 patients. Arthritis Res. Ther. 22, 5 (2020).
Grable-Esposito, P. et al. Immune-mediated necrotizing myopathy associated with statins. Muscle Nerve 41, 185–190 (2010).
Ramanathan, S. et al. Clinical course and treatment of anti-HMGCR antibody-associated necrotizing autoimmune myopathy. Neurol. Neuroimmunol. Neuroinflamm. 2, e96 (2015).
Valiyil, R., Casciola-Rosen, L., Hong, G., Mammen, A. & Christopher-Stine, L. Rituximab therapy for myopathy associated with anti-signal recognition particle antibodies: a case series. Arthritis Care Res. 62, 1328–1334 (2010).
Landon-Cardinal, O. et al. Rituximab in the treatment of refractory anti-HMGCR immune-mediated necrotizing myopathy. J. Rheumatol. 46, 623–627 (2019).
Tansley, S. L. et al. Anti-HMGCR autoantibodies in juvenile idiopathic inflammatory myopathies identify a rare but clinically important subset of patients. J. Rheumatol. 44, 488–492 (2017).
Binns, E. L. et al. Effective induction therapy for anti-SRP associated myositis in childhood: a small case series and review of the literature. Pediatr. Rheumatol. 15, 77 (2017).
Kusumoto, T. et al. Development of necrotizing myopathy following interstitial lung disease with anti-signal recognition particle antibody. Intern. Med. 57, 2045–2049 (2018).
Giudizi, M. G. et al. Anti-HMGCR antibody-associated necrotizing myopathy: diagnosis and treatment illustrated using a case report. Scand. J. Rheumatol. 45, 427–429 (2016).
Mammen, A. L. & Tiniakou, E. Intravenous immune globulin for statin-triggered autoimmune myopathy. N. Engl. J. Med. 373, 1680–1682 (2015).
Tiniakou, E., Rivera, E., Mammen, A. L. & Christopher-Stine, L. Use of proprotein convertase Subtilisin/Kexin Type 9 inhibitors in statin-associated immune-mediated necrotizing myopathy: a case series. Arthritis Rheumatol. 71, 1723–1726 (2019).
Alemo Munters, L., Alexanderson, H., Crofford, L. J. & Lundberg, I. E. New insights into the benefits of exercise for muscle health in patients with idiopathic inflammatory myositis. Curr. Rheumatol. Rep. 16, 429 (2014).
Van Thillo, A., Vulsteke, J.-B., Van Assche, D., Verschueren, P. & De Langhe, E. Physical therapy in adult inflammatory myopathy patients: a systematic review. Clin. Rheumatol. 38, 2039–2051 (2019).
Surmachevska, N. & Tiwari, V. Corticosteroid Induced Myopathy (StatPearls Publishing, 2020).
Kostine, M. et al. EULAR points to consider for the diagnosis and management of rheumatic immune-related adverse events due to cancer immunotherapy with checkpoint inhibitors. Ann. Rheum. Dis. https://doi.org/10.1136/annrheumdis-2020-217139 (2020).
Pinal-Fernandez, I. et al. Thigh muscle MRI in immune-mediated necrotising myopathy: extensive oedema, early muscle damage and role of anti-SRP autoantibodies as a marker of severity. Ann. Rheum. Dis. 76, 681–687 (2017).
Acknowledgements
The authors thank the Association Française contre les Myopathies (AFM) for its support. The authors are grateful to N. Sabourin-Gibbs, Rouen University Hospital, for her help in editing the manuscript.
Author information
Authors and Affiliations
Contributions
All of the authors researched data for the article, wrote the article, made a substantial contribution to discussion of content and reviewed or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Rheumatology thanks I. Nishino and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Sarcolemma
-
The thin membrane enclosing a striated muscle fibre.
- Exocrine pancreatic insufficiency
-
A condition characterized by deficiency of the exocrine pancreatic enzymes, resulting in the inability to digest food properly or maldigestion.
- Scapular winging
-
Occurs when a shoulder blade sticks out rather than resting flat against the back of the chest wall.
- Muscle fascicles
-
A bundle of muscle fibres surrounded by perimysium (connective tissue demarcating a fascicle of skeletal muscle fibres).
- Endomysium
-
The sheath of delicate reticular fibrils that surrounds each muscle fibre.
Rights and permissions
About this article
Cite this article
Allenbach, Y., Benveniste, O., Stenzel, W. et al. Immune-mediated necrotizing myopathy: clinical features and pathogenesis. Nat Rev Rheumatol 16, 689–701 (2020). https://doi.org/10.1038/s41584-020-00515-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-020-00515-9
This article is cited by
-
Intravenous Immunoglobulins in Idiopathic Inflammatory Myopathies: Where Are We?
Current Treatment Options in Rheumatology (2024)
-
Complement and MHC patterns can provide the diagnostic framework for inflammatory neuromuscular diseases
Acta Neuropathologica (2024)
-
Immune-Mediated Necrotizing Myopathies: Current Landscape
Current Neurology and Neuroscience Reports (2024)
-
GKJR(Gesellschaft für Kinder- und Jugendrheumatologie)-Diagnose- und Therapieoptimierungsboard – neue Wege zu Diagnose und Therapie komplexer Erkrankungen
Zeitschrift für Rheumatologie (2024)
-
Value of the HFA-PEFF diagnostic algorithms for heart failure with preserved ejection fraction to the inflammatory myopathy population
Arthritis Research & Therapy (2023)