Abstract
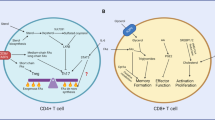
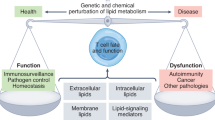
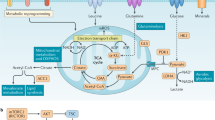
T cell subsets are critically involved in the development of systemic autoimmunity and organ inflammation in systemic lupus erythematosus (SLE). Each T cell subset function (such as effector, helper, memory or regulatory function) is dictated by distinct metabolic pathways requiring the availability of specific nutrients and intracellular enzymes. The activity of these enzymes or nutrient transporters influences the differentiation and function of T cells in autoimmune responses. Data are increasingly emerging on how metabolic processes control the function of various T cell subsets and how these metabolic processes are altered in SLE. Specifically, aberrant glycolysis, glutaminolysis, fatty acid and glycosphingolipid metabolism, mitochondrial hyperpolarization, oxidative stress and mTOR signalling underwrite the known function of T cell subsets in patients with SLE. A number of medications that are used in the care of patients with SLE affect cell metabolism, and the development of novel therapeutic approaches to control the activity of metabolic enzymes in T cell subsets represents a promising endeavour in the search for effective treatment of systemic autoimmune diseases.
Key points
-
The fate of T cell differentiation can be determined by the activity of various metabolic pathways in the cell, reflecting the central role of metabolism in controlling T cell plasticity.
-
In systemic lupus erythematosus (SLE), T cells are chronically active as a result of T cell receptor rewiring, hypomethylation of genes related to cell activation, an increase in lipid raft formation and multifactorial mTORC1 activation.
-
Manipulating metabolic pathways in T cells is a promising strategy in SLE and could enable the inhibition of pathogenic effector T cell activation and differentiation and the promotion of regulatory T cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kasper, I. R., Apostolidis, S. A., Sharabi, A. & Tsokos, G. C. Empowering regulatory T cells in autoimmunity. Trends Mol. Med. 22, 784–797 (2016).
Katsuyama, T., Tsokos, G. C. & Moulton, V. R. Aberrant T cell signaling and subsets in systemic lupus erythematosus. Front. Immunol. 9, 1088 (2018).
Suárez-Fueyo, A., Bradley, S. J., Klatzmann, D. & Tsokos, G. C. T cells and autoimmune kidney disease. Nat. Rev. Nephrol. 16, 329–343 (2017).
Perl, A. Activation of mTOR (mechanistic target of rapamycin) in rheumatic diseases. Nat. Rev. Rheumatol. 12, 169–182 (2016).
Morel, L. Immunometabolism in systemic lupus erythematosus. Nat. Rev. Rheumatol. 13, 280–290 (2017).
Buck, M. D., Sowell, R. T., Kaech, S. M. & Pearce, E. L. Metabolic instruction of immunity. Cell 169, 570–586 (2017).
Chi, H. Regulation and function of mTOR signalling in T cell fate decisions. Nat. Rev. Immunol. 12, 325–338 (2012).
Delgoffe, G. M. et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat. Immunol. 12, 295–303 (2011).
Finlay, D. & Cantrell, D. A. Metabolism, migration and memory in cytotoxic T cells. Nat. Rev. Immunol. 11, 109–117 (2011).
Wang, R. et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35, 871–882 (2011).
Frauwirth, K. A. et al. The CD28 signaling pathway regulates glucose metabolism. Immunity 16, 769–777 (2002).
MacIver, N. J., Michalek, R. D. & Rathmell, J. C. Metabolic regulation of T lymphocytes. Annu. Rev. Immunol. 31, 259–283 (2013).
Verbist, K. C. et al. Metabolic maintenance of cell asymmetry following division in activated T lymphocytes. Nature 532, 389–393 (2016).
D’Souza, A. D., Parikh, N., Kaech, S. M. & Shadel, G. S. Convergence of multiple signaling pathways is required to coordinately up-regulate mtDNA and mitochondrial biogenesis during T cell activation. Mitochondrion 7, 374–385 (2007).
Sena, L. A. et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 38, 225–236 (2013).
Taniuchi, I. CD4 Helper and CD8 Cytotoxic T Cell Differentiation. Annu. Rev. Immunol. 36, 579–601 (2018).
Guma, M., Tiziani, S. & Firestein, G. S. Metabolomics in rheumatic diseases: desperately seeking biomarkers. Nat. Rev. Rheumatol. 12, 269–281 (2016).
Zeng, H. et al. mTORC1 and mTORC2 kinase signaling and glucose metabolism drive follicular helper T cell differentiation. Immunity 45, 540–554 (2016).
Oestreich, K. J. et al. Bcl-6 directly represses the gene program of the glycolysis pathway. Nat. Immunol. 15, 957–964 (2014).
Man, K. et al. The transcription factor IRF4 is essential for TCR affinity-mediated metabolic programming and clonal expansion of T cells. Nat. Immunol. 14, 1155–1165 (2013).
Shi, L. Z. et al. HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med. 208, 1367–1376 (2011).
Shehade, H., Acolty, V., Moser, M. & Oldenhove, G. Cutting edge: hypoxia-inducible factor 1 negatively regulates Th1 function. J. Immunol. 195, 1372–1376 (2015).
Jacobs, S. R. et al. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and indepndent pathways. J. Immunol. 180, 4476–4486 (2008).
Macintyre, A. N. et al. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 20, 61–72 (2014).
Yang, J. Q. et al. RhoA orchestrates glycolysis for TH2 cell differentiation and allergic airway inflammation. J. Allergy Clin. Immunol. 137, 231–245 (2016).
Kono, M. et al. Pyruvate dehydrogenase phosphatase catalytic subunit 2 limits Th17 differentiation. Proc. Natl Acad. Sci. USA 115, 9288–9293 (2018).
Yoshida, N. et al. ICER is requisite for Th17 differentiation. Nat. Commun. 7, 12993 (2016).
Gerriets, V. A. et al. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J. Clin. Invest. 125, 194–207 (2015).
Yin, Y. et al. Normalization of CD4+ T cell metabolism reverses lupus. Sci. Transl. Med. 7, 274ra18 (2015).
Yin, Y. et al. Glucose oxidation is critical for CD4+T cell activation in a mouse model of systemic lupus erythematosus. J. Immunol. 196, 80–90 (2016).
Hasegawa, H. et al. Pioglitazone, a peroxisome proliferator-activated receptor γ activator, ameliorates experimental autoimmune myocarditis by modulating Th1/Th2 balance. J. Mol. Cell. Cardiol. 38, 257–265 (2005).
Sinclair, L. V. et al. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat. Immunol. 14, 500–508 (2013).
Wolfson, R. L. et al. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 351, 43–48 (2016).
Nakajima, H. & Kunimoto, H. TET2 as an epigenetic master regulator for normal and malignant hematopoiesis. Cancer Sci. 105, 1093–1099 (2014).
Xu, T. et al. Metabolic control of TH17 and induced Treg cell balance by an epigenetic mechanism. Nature 548, 228–233 (2017).
Metallo, C. M. et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 481, 380–384 (2011).
Sundrud, M. S. et al. Halofuginone inhibits TH17 cell differentiation by activating the amino acid starvation response. Science 324, 1334–1338 (2009).
Nakaya, M. et al. Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity 40, 692olde5 (2014).
Kono, M., Yoshida, N., Maeda, K. & Tsokos, G. C. Transcriptional factor ICER promotes glutaminolysis and the generation of Th17 cells. Proc. Natl Acad. Sci. USA 115, 2478–2483 (2018).
Johnson, M. O. et al. Distinct regulation of Th17 and Th1 cell differentiation by glutaminase-dependent metabolism. Cell 175, 1780–1795 (2018).
Raposo, B., Vaartjes, D., Ahlqvist, E., Nandakumar, K. S. & Holmdahl, R. System A amino acid transporters regulate glutamine uptake and attenuate antibody-mediated arthritis. Immunology 146, 607–617 (2015).
Klysz, D. et al. Glutamine-dependent α-ketoglutarate production regulates the balance between T helper 1 cell and regulatory T cell generation. Sci. Signal. 8, ra97 (2015).
Ma, E. H. et al. Serine is an essential metabolite for effector T cell expansion. Cell Metab. 25, 345–357 (2017).
Huennekens, F. M. The methotrexate story: a paradigm for development of cancer chemotherapeutic agents. Adv. Enzyme Regul. 34, 397–419 (1994).
Hardie, D. G. AMP-activated protein kinase: a cellular energy sensor with a key role in metabolic disorders and in cancer. Biochem. Soc. Trans. 39, 1–13 (2011).
Berod, L. et al. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat. Med. 20, 1327–1333 (2014).
Haghikia, A. et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 43, 817–829 (2015).
Mangalam, A. et al. Profile of circulatory metabolites in a relapsing-remitting animal model of multiple sclerosis using global metabolomics. J. Clin. Cell Immunol. https://doi.org/10.4172/2155-9899.1000150 (2013).
Zhang, X., Tao, Y., Troiani, L. & Markovic-Plese, S. Simvastatin inhibits IFN regulatory factor 4 expression and Th17 cell differentiation in CD4+ T cells derived from patients with multiple sclerosis. J. Immunol. 187, 3431–3437 (2011).
Youssef, S. et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature 420, 78–84 (2002).
Mueller, S. N. & Mackay, L. K. Tissue-resident memory T cells: local specialists in immune defence. Nat. Rev. Immunol. 16, 79–89 (2016).
Bantug, G. R., Galluzzi, L., Kroemer, G. & Hess, C. The spectrum of T cell metabolism in health and disease. Nat. Rev. Immunol. 18, 4 (2018).
Wherry, E. J. et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4, 225–234 (2003).
O’Sullivan, D. et al. Memory CD8+ T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity 41, 75–88 (2014).
Pan, Y. et al. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature 543, 252–256 (2017).
Pearce, E. L., Poffenberger, M. C., Chang, C. H. & Jones, R. G. Fueling immunity: insights into metabolism and lymphocyte function. Science 342, 1242425 (2013).
van der Windt, G. J. et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36, 68–78 (2012).
Gubser, P. M. et al. Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch. Nat. Immunol. 14, 1064–1072 (2013).
Phan., A. T. et al. Constitutive glycolytic metabolism supports CD8+ T cell effector memory differentiation during viral infection. Immunity 45, 1024–1037 (2016).
Balmer, M. L. et al. Memory CD8+ T cells require increased concentrations of acetate induced by stress for optimal function. Immunity 44, 1312–1324 (2016).
Michalek, R. D. et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 186, 3299–3303 (2011).
Boothby, M. Signaling in T cells—is anything the m(a)TOR with the picture(s)? F1000Res. https://doi.org/10.12688/f1000research.7027.1 (2016).
Zeng, H. & Chi, H. Metabolic control of regulatory T cell development and function. Trends Immunol. 36, 3–12 (2015).
De Rosa, V. et al. Glycolysis controls the induction of human regulatory T cells by modulating the expression of FOXP3 exon 2 splicing variants. Nat. Immunol. 16, 1174–1184 (2015).
Gerriets, V. A. et al. Foxp3 and Toll-like receptor signaling balance Treg cell anabolic metabolism for suppression. Nat. Immunol. 17, 1459–1466 (2016).
Angelin, A. et al. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 25, 1282–1293 (2017).
Pacella, I. et al. Fatty acid metabolism complements glycolysis in the selective regulatory T cell expansion during tumor growth. Proc. Natl Acad. Sci. USA 115, E6546–E6555 (2018).
Burzyn, D., Benoist, C. & Mathis, D. Regulatory T cells in nonlymphoid tissues. Nat. Immunol. 14, 1007–1013 (2013).
Procaccini, C., Galgani, M., De Rosa, V. & Matarese, G. Intracellular metabolic pathways control immune tolerance. Trends Immunol. 33, 1–7 (2012).
Feuerer, M. et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 15, 930–939 (2009).
Smith, P. M. et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573 (2013).
Furusawa, Y. et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450 (2013).
Sharabi, A. et al. Regulatory T cells in the treatment of disease. Nat. Rev. Drug Discov. 17, 823–844 (2018).
Mezrich, J. D. et al. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 185, 3190–3198 (2010).
Buck, M. D., O’Sullivan, D. & Pearce, E. L. T cell metabolism drives immunity. J. Exp. Med. 212, 1345–1360 (2015).
Cobbold, S. P. et al. Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc. Natl Acad. Sci. USA 106, 12055–12060 (2009).
Apostolidis, S. A. et al. Phosphatase PP2A is requisite for the function of regulatory T cells. Nat. Immunol. 17, 556–564 (2016).
Zeng, H. et al. mTORC1 couples immune signals and metabolic programming to establish Treg-cell function. Nature 499, 485–490 (2013).
Wu, D. et al. Lkb1 maintains Treg cell lineage identity. Nat. Commun. 8, 15876 (2017).
Yang, K. et al. Homeostatic control of metabolic and functional fitness of Treg cells by LKB1 signalling. Nature 548, 602–606 (2017).
He, N. et al. Metabolic control of regulatory T cell (Treg) survival and function by Lkb1. Proc. Natl Acad. Sci. USA 114, 12542–12547 (2017).
Wahl, D. R. et al. Characterization of the metabolic phenotype of chronically activated lymphocytes. Lupus 19, 1492–1501 (2010).
Gergely, P. Jr. et al. Mitochondrial hyperpolarization and ATP depletion in patients with systemic lupus erythematosus. Arthritis Rheum. 46, 175–190 (2002).
Perl, A. et al. Comprehensive metabolome analyses reveal N-acetylcysteine-responsive accumulation of kynurenine in systemic lupus erythematosus: implications for activation of the mechanistic target of rapamycin. Metabolomics 11, 1157–1174 (2015).
Juang, Y. T. et al. PP2A dephosphorylates Elf-1 and determines the expression of CD3ζ and FcRγ in human systemic lupus erythematosus T cells. J. Immunol. 181, 3658–3664 (2008).
Krishnan, S., Farber, D. L. & Tsokos, G. C. T cell rewiring in differentiation and disease. J. Immunol. 171, 3325–3331 (2003).
Tsokos, G. C. Systemic lupus erythematosus. N. Engl. J. Med. 365, 2110–2121 (2011).
Ichinose, K. et al. Lupus nephritis IgG induction of calcium/calmodulin-dependent protein kinase IV expression in podocytes and alteration of their function. Arthritis Rheumatol. 68, 944–952 (2016).
Li, Y., Gorelik, G., Strickland, F. M. & Richardson, B. C. Oxidative stress, T cell DNA methylation, and lupus. Arthritis Rheumatol. 66, 1574–1582 (2014).
Sunahori, K. et al. The catalytic subunit of protein phosphatase 2A (PP2Ac) promotes DNA hypomethylation by suppressing the phosphorylated mitogen-activated protein kinase/extracellular signal-regulated kinase (ERK) kinase (MEK)/phosphorylated ERK/DNMT1 protein pathway in T-cells from controls and systemic lupus erythematosus patients. J. Biol. Chem. 288, 21936–21944 (2013).
Li, H. et al. Precision DNA demethylation ameliorates disease in lupus-prone mice. JCI Insight https://doi.org/10.1172/jci.insight.120880 (2018).
Perry, D. J. et al. Murine lupus susceptibility locus Sle1c2 mediates CD4+ T cell activation and maps to estrogen-related receptor γ. J. Immunol. 189, 793–803 (2012).
Morel, L., Blenman, K. R., Croker, B. P. & Wakeland, E. K. The major murine systemic lupus erythematosus susceptibility locus, Sle1, is a cluster of functionally related genes. Proc. Natl Acad. Sci. USA 98, 1787–1792 (2001).
Vyshkina, T. et al. Association of common mitochondrial DNA variants with multiple sclerosis and systemic lupus erythematosus. Clin. Immunol. 129, 31–35 (2008).
McDonald, G. et al. Normalizing glycosphingolipids restores function in CD4+ T cells from lupus patients. J. Clin. Invest. 124, 712–724 (2014).
Krishnan, S. et al. Alterations in lipid raft composition and dynamics contribute to abnormal T cell responses in systemic lupus erythematosus. J. Immunol. 172, 7821–7831 (2004).
Waddington, K. E., Jury, E. C. & Pineda-Torra, I. Liver X receptors in immune cell function in humans. Biochem. Soc. Trans. 43, 752–757 (2015).
Anderson, M. K., Hernandez-Hoyos, G., Diamond, R. A. & Rothenberg, E. V. Precise developmental regulation of Ets family transcription factors during specification and commitment to the T cell lineage. Development 126, 3131–3148 (1999).
Zhang, L. et al. An immunological renal disease in transgenic mice that overexpress Fli-1, a member of the ets family of transcription factor genes. Mol. Cell. Biol. 15, 6961–6970 (1995).
Zhang, X. K. et al. Decreased expression of the Ets family transcription factor Fli-1 markedly prolongs survival and significantly reduces renal disease in MRL/lpr mice. J. Immunol. 173, 6481–6489 (2004).
Mathenia, J. et al. Impact of Fli-1 transcription factor on autoantibody and lupus nephritis in NZM2410 mice. Clin. Exp. Immunol. 162, 362–371 (2010).
Richard, E. M. et al. Reducing FLI1 levels in the MRL/lpr lupus mouse model impacts T cell function by modulating glycosphingolipid metabolism. PLOS ONE 8, e75175 (2013).
Sundararaj, K. P. et al. FLI1 levels impact CXCR3 expression and renal infiltration of T cells and renal glycosphingolipid metabolism in the MRL/lpr lupus mouse strain. J. Immunol. 195, 5551–5560 (2015).
Morris, E. E. et al. A GA microsatellite in the Fli1 promoter modulates gene expression and is associated with systemic lupus erythematosus patients without nephritis. Arthritis Res. Ther. 12, R212 (2010).
Nowling, T. K. et al. Renal glycosphingolipid metabolism is dysfunctional in lupus nephritis. J. Am. Soc. Nephrol. 26, 1402–1413 (2015).
Perl, A. Oxidative stress in the pathology and treatment of systemic lupus erythematosus. Nat. Rev. Rheumatol. 9, 674–686 (2013).
Delgoffe, G. M. et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 30, 832–844 (2009).
Lai, Z. W. et al. N-acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 64, 2937–2946 (2012).
Lai, Z. W. et al. Mechanistic target of rapamycin activation triggers IL-4 production and necrotic death of double-negative T cells in patients with systemic lupus erythematosus. J. Immunol. 191, 2236–2246 (2013).
Lee, S. Y. et al. Metformin suppresses systemic autoimmunity in Roquin san/san mice through inhibiting B cell differentiation into plasma cells via regulation of AMPK/mTOR/STAT3. J. Immunol. 198, 2661–2670 (2017).
Koga, T. et al. CaMK4-dependent activation of AKT/mTOR and CREM-α underlies autoimmunity-associated Th17 imbalance. J. Clin. Invest. 124, 2234–2245 (2014).
Katsuyama, T., Li, H., Comte, D., Tsokos, G. C. & Moulton, V. R. Splicing factor SRSF1 controls T cell hyperactivity and systemic autoimmunity. J. Clin. Invest. https://doi.org/10.1172/JCI127949 (2019).
Psarelis, S. & Nikiphorou, E. Coexistence of SLE, tuberous sclerosis and aggressive natural killer-cell leukaemia: coincidence or correlated? Lupus 26, 107–108 (2017).
Olde Bekkink, M., Ahmed-Ousenkova, Y. M., Netea, M. G., van der Velden, W. J. & Berden, J. H. Coexistence of systemic lupus erythematosus, tuberous sclerosis and aggressive natural killer-cell leukaemia: coincidence or correlated? Lupus 25, 766–771 (2016).
Carrasco Cubero, C., Bejarano Moguel, V., Fernández Gil, M. Á. & Álvarez Vega, J. L. Coincidence of tuberous sclerosis and systemic lupus erythematosus—a case report. Reumatol. Clin. 12, 219–222 (2016).
Singh, N., Birkenbach, M., Caza, T., Perl, A. & Cohen, P. L. Tuberous sclerosis and fulminant lupus in a young woman. J. Clin. Rheumatol. 19, 134–137 (2013).
Koga, T. et al. Promotion of calcium/calmodulin-dependent protein kinase 4 by GLUT1-dependent glycolysis in systemic lupus erythematosus. Arthritis Rheumatol. 71, 766–772 (2019).
Kono, M. et al. Glutaminase 1 inhibition reduces glycolysis and ameliorates lupus-like disease in MRL/lpr mice and experimental autoimmune encephalomyelitis. Arthritis Rheumatol. 71, 1869–1878 (2019).
Huang, N. & Perl, A. Metabolism as a target for modulation in autoimmune diseases. Trends Immunol. 39, 562–576 (2018).
Moreno-Aurioles, V. R. & Sobrino, F. Glucocorticoids inhibit fructose 2,6-bisphosphate synthesis in rat thymocytes. Opposite effect of cycloheximide. Biochim. Biophys. Acta 1091, 96–100 (1991).
Domhan, S. et al. Molecular mechanisms of the antiangiogenic and antitumor effects of mycophenolic acid. Mol. Cancer Ther. 7, 1656–1668 (2008).
He, X. et al. Mycophenolic acid-mediated suppression of human CD4+ T cells: more than mere guanine nucleotide deprivation. Am. J. Transplant. 11, 439–449 (2011).
Shuvalov, O. et al. One-carbon metabolism and nucleotide biosynthesis as attractive targets for anticancer therapy. Oncotarget 8, 23955–23977 (2017).
Fernández-Ramos, A. A., Poindessous, V., Marchetti-Laurent, C., Pallet, N. & Loriot, M. A. The effect of immunosuppressive molecules on T-cell metabolic reprogramming. Biochimie 127, 23–36 (2016).
Furst, D. E. The rational use of methotrexate in rheumatoid arthritis and other rheumatic diseases. Br. J. Rheumatol. 36, 1196–1204 (1997).
Fernandez, D., Bonilla, E., Mirza, N., Niland, B. & Perl, A. Rapamycin reduces disease activity and normalizes T cell activation-induced calcium fluxing in patients with systemic lupus erythematosus. Arthritis Rheum. 54, 2983–2988 (2006).
Lai, Z. W. et al. Sirolimus in patients with clinically active systemic lupus erythematosus resistant to, or intolerant of, conventional medications: a single-arm, open-label, phase 1/2 trial. Lancet 391, 1186–1196 (2018).
Choi, S. C. et al. Inhibition of glucose metabolism selectively targets autoreactive follicular helper T cells. Nat. Commun. 9, 4369 (2018).
Wang, H., Li, T., Chen, S., Gu, Y. & Ye, S. Neutrophil extracellular trap mitochondrial DNA and its autoantibody in systemic lupus erythematosus and a proof-of-concept trial of metformin. Arthritis Rheumatol. 67, 3190–3200 (2015).
Zhao, W. et al. The peroxisome-proliferator activated receptor-γ agonist pioglitazone modulates aberrant T cell responses in systemic lupus erythematosus. Clin. Immunol. 149, 119–132 (2013).
Johnson, K. M. et al. Identification and validation of the mitochondrial F1F0-ATPase as the molecular target of the immunomodulatory benzodiazepine Bz-423. Chem. Biol. 12, 485–496 (2005).
Blatt, N. B. et al. Benzodiazepine-induced superoxide signals B cell apoptosis: mechanistic insight and potential therapeutic utility. J. Clin. Invest. 110, 1123–1132 (2002).
Bednarski, J. J. et al. Attenuation of autoimmune disease in Fas-deficient mice by treatment with a cytotoxic benzodiazepine. Arthritis Rheum. 48, 757–766 (2003).
Bengtsson, A. A. et al. Metabolic profiling of systemic lupus erythematosus and comparison with primary Sjögren’s syndrome and systemic sclerosis. PLOS ONE 11, e0159384 (2016).
Actelion Press Release August. Zavesca approved —first oral treatment option for type 1 Gaucher disease. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021348s010lbl.pdf (2003).
Oya, Y. et al. Lack of B and T lymphocyte attenuator exacerbates autoimmune disorders and induces Fas-independent liver injury in MRL-lpr/lpr mice. Int. Immunol. 23, 335–344 (2011).
Kashiwakuma, D. et al. B and T lymphocyte attenuator suppresses IL-21 production from follicular Th cells and subsequent humoral immune responses. J. Immunol. 185, 2730–2736 (2010).
Sawaf, M. et al. Defective BTLA functionality is rescued by restoring lipid metabolism in lupus CD4+ T cells. JCI Insight https://doi.org/10.1172/jci.insight.99711 (2018).
Hansen, A. L. et al. Nitro-fatty acids are formed in response to virus infection and are potent inhibitors of STING palmitoylation and signaling. Proc. Natl Acad. Sci. USA 115, E7768–E7775 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02460146 (2016).
Israelsen, W. J. & Vander Heiden, M. G. Pyruvate kinase: function, regulation and role in cancer. Semin. Cell Dev. Biol. 43, 43–51 (2015).
Van Bruggen, M. C., Walgreen, B., Rijke, T. P. & Berden, J. H. Attenuation of murine lupus nephritis by mycophenolate mofetil. J. Am. Soc. Nephrol. 9, 1407–1415 (1998).
Dörner, T. & Furie, R. Novel paradigms in systemic lupus erythematosus. Lancet 393, 2344–2358 (2019).
Segal, R., Dayan, M., Zinger, H. & Mozes, E. Methotrexate treatment in murine experimental systemic lupus erythematosus (SLE); clinical benefits associated with cytokine manipulation. Clin. Exp. Immunol. 101, 66–72 (1995).
Lui, S. L. et al. Rapamycin attenuates the severity of established nephritis in lupus-prone NZB/W F1 mice. Nephrol. Dial. Transplant. 23, 2768–2776 (2008).
Jones, D. D. et al. mTOR has distinct functions in generating versus sustaining humoral immunity. J. Clin. Invest. 126, 4250–4261 (2016).
Zhao, W. et al. The peroxisome proliferator-activated receptor γ agonist pioglitazone improves cardiometabolic risk and renal inflammation in murine lupus. J. Immunol. 183, 2729–2740 (2009).
Suwannaroj, S., Lagoo, A., Keisler, D. & McMurray, R. W. Antioxidants suppress mortality in the female NZB × NZW F1 mouse model of systemic lupus erythematosus (SLE). Lupus 10, 258–265 (2001).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Nonessential amino acids
-
Six amino acids that can be synthesized in sufficient quantities in the body (alanine, aspartic acid, asparagine, glutamic acid, serine and selenocysteine), unlike essential amino acids that must be supplied in the diet.
- Anabolic metabolism phenotype
-
A phenotype characterized by rapid biosynthesis of molecules through various metabolic pathways.
- Mitochondrial biogenesis
-
The increase in mitochondrial mass within a cell as a result of cellular stress or prolonged activation. This process includes an increase in metabolic enzymes involved in glycolysis and oxidative phosphorylation and is regulated by AMPK and transcriptional modifications.
- Malate-aspartate shuttle
-
A cytoplasmic–mitochondrial network in which reducing equivalents (such as NADH) in the cytoplasm are carried by malate across the inner membrane of the mitochondria to drive oxidative phosphorylation.
- One-carbon metabolism
-
A cytoplasmic network of interlinking metabolic pathways, including the folate and the methionine cycles, that transfer carbon units (from amino acids) to metabolic pathways related to cell proliferation and growth.
- Mevalonate pathway
-
Also known as the HMG-CoA reductase pathway, this pathway uses acetyl-CoA to generate two five-carbon structures called isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) that are used in macromolecules called isoprenoids, such as cholesterol, vitamin K and steroids.
- Pentose phosphate pathway
-
One of three glucose metabolism pathways that branch from the glycolysis pathway. This pathway converts glucose-6-phosphate to ribose-5-phosphate to generate reducing equivalents, including NADPH, for the synthesis of nucleic acids and amino acids during cell activation.
Rights and permissions
About this article
Cite this article
Sharabi, A., Tsokos, G.C. T cell metabolism: new insights in systemic lupus erythematosus pathogenesis and therapy. Nat Rev Rheumatol 16, 100–112 (2020). https://doi.org/10.1038/s41584-019-0356-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-019-0356-x
This article is cited by
-
Efficacy and safety of gut microbiota-based therapies in autoimmune and rheumatic diseases: a systematic review and meta-analysis of 80 randomized controlled trials
BMC Medicine (2024)
-
Longitudinal variation of serum PCSK9 in ulcerative colitis: association with disease activity, T helper 1/2/17 cells, and clinical response of tumor necrosis factor inhibitor
Irish Journal of Medical Science (1971 -) (2024)
-
Identification of EPSTI1 as a new potential biomarker for SLE based on GEO database
Clinical Rheumatology (2024)
-
CircPTPN22 modulates T-cell activation by sponging miR-4689 to regulate S1PR1 expression in patients with systemic lupus erythematosus
Arthritis Research & Therapy (2023)
-
TP53 and p21 (CDKN1A) polymorphisms and the risk of systemic lupus erythematosus
Advances in Rheumatology (2023)