Abstract
During pregnancy, the fetus that grows within the maternal uterus is not rejected by the maternal immune system. To enable both tolerance towards the fetus and defence against pathogens, modifications of the maternal immune system occur during gestation. These modifications are able to bring about a natural improvement in disease activity of some autoimmune diseases, such as rheumatoid arthritis (RA). Various mechanisms of the immune system contribute to the phenomenon of pregnancy-related improvement of RA, and the cessation of these immunomodulatory mechanisms after delivery correlates with postpartum disease flare. HLA disparity between mother and fetus, glycosylation of IgG, immunoregulatory pathways, and alterations in innate and adaptive immune cells and their cytokines have important roles in pregnancy and in pregnancy-related amelioration of RA.
Key points
-
Immune tolerance during pregnancy is most pronounced at the feto-maternal interface, but also has a systemic effect that supports amelioration of rheumatoid arthritis (RA) by rebalancing pro-inflammatory and anti-inflammatory influences.
-
A pro-inflammatory microenvironment is crucial for normal implantation and parturition, whereas a tolerogenic environment is induced during the course of pregnancy to enable normal placentation and fetal growth.
-
With regard to innate immune cells, monocyte gene activity in the peripheral blood is higher during pregnancy than postpartum, and a tolerogenic subset of innate cells is at work in the placenta during pregnancy.
-
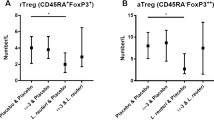
With regard to adaptive immune cells, there is no definite predominance of T helper 2 cells but a downregulation of effector T cell activity and an expansion of regulatory T cells, which support pregnancy-related RA improvement.
-
Galactosylation of IgG is a pregnancy-related phenomenon that renders disease-specific autoantibodies less pathogenic and could explain why a gestational increase in anti-cyclic citrullinated peptide antibody IgG galactosylation is associated with improved RA.
-
After delivery, the immunomodulatory effects mediated by fetal antigens and pregnancy hormones disappear, giving rise to T cell activity that, together with persisting monocyte activity, correlates with postpartum disease flares.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
17 February 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41584-020-0394-4
References
Ince-Askan, H. & Dolhain, R. J. Pregnancy and rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 29, 580–596 (2015).
Østensen, M., Villiger, P. M. & Förger, F. Interaction of pregnancy and autoimmune rheumatic disease. Autoimmun. Rev. 11, A437–A446 (2012).
Hench, P. S. The ameliorating effect of pregnancy on chronic atrophic (infectious, rheumatoid) arthritis, fibrositis, and intermittent hydrarthrosis. Mayo Clin. Proc. 13, 161–167 (1938).
Østensen, M. & Husby, G. A prospective clinical study of the effect of pregnancy on rheumatoid arthritis and ankylosing spondylitis. Arthritis Rheum. 26, 1155–1159 (1983).
Neely, N. T. & Persellin, R. H. Activity of rheumatoid arthritis during pregnancy. Tex. Med. 73, 59–63 (1977).
Østensen, M., Aune, B. & Husby, G. Effect of pregnancy and hormonal changes on the activity of rheumatoid arthritis. Scand. J. Rheumatol. 12, 69–72 (1983).
Zbinden, A., van den Brandt, S., Østensen, M., Villiger, P. M. & Förger, F. Risk for adverse pregnancy outcome in axial spondyloarthritis and rheumatoid arthritis: disease activity matters. Rheumatology 57, 1235–1242 (2018).
Förger, F. et al. Discontinuing TNF-inhibitors before gestational week 20 in well-controlled rheumatoid arthritis and juvenile arthritis is not associated with a disease worsening in late pregnancy. Arthritis Rheumatol. https://doi.org/10.1002/art.40821 (2019).
de Man, Y. A., Dolhain, R. J., van de Geijn, F. E., Willemsen, S. P. & Hazes, J. M. Disease activity of rheumatoid arthritis during pregnancy: results from a nationwide prospective study. Arthritis Rheum. 59, 1241–1248 (2008).
Barrett, J. H., Brennan, P., Fiddler, M. & Silman, A. J. Does rheumatoid arthritis remit during pregnancy and relapse postpartum? Results from a nationwide study in the United Kingdom performed prospectively from late pregnancy. Arthritis Rheum. 42, 1219–1227 (1999).
de Man, Y. A. et al. Women with rheumatoid arthritis negative for anti-cyclic citrullinated peptide and rheumatoid factor are more likely to improve during pregnancy, whereas in autoantibody-positive women autoantibody levels are not influenced by pregnancy. Ann. Rheum. Dis. 69, 420–423 (2010).
de Man, Y. A. et al. Association of higher rheumatoid arthritis disease activity during pregnancy with lower birth weight: results of a national prospective study. Arthritis Rheum. 60, 3196–3206 (2009).
Spector, T. D. Rheumatoid arthritis. Rheum. Dis. Clin. North Am. 16, 513–537 (1990).
Robertson, S. A. & Sharkey, D. J. Seminal fluid and fertility in women. Fertil. Steril. 106, 511–519 (2016).
Saito, S., Shima, T., Nakashima, A., Inada, K. & Yoshino, O. Role of paternal antigen-specific Treg cells in successful implantation. Am. J. Reprod. Immunol. 75, 310–316 (2016).
Chaouat, G., Dubanchet, S. & Ledee, N. Cytokines: important for implantation? J. Assist. Reprod. Genet. 24, 491–505 (2007).
Robertson, S. A., Care, A. S. & Moldenhauer, L. M. Regulatory T cells in embryo implantation and the immune response to pregnancy. J. Clin. Invest. 128, 4224–4235 (2018).
Arck, P. C. & Hecher, K. Fetomaternal immune cross-talk and its consequences for maternal and offspring's health. Nat. Med. 19, 548–556 (2013).
Medawar, P. B. Some immunological and endocrinological problems, raised by the evolution of viviparity in vertebrates. Nature 172, 603–606 (1953).
Lei, K. I., Chan, L. Y., Chan, W. Y., Johnson, P. J. & Lo, Y. M. Quantitative analysis of circulating cell-free Epstein-Barr virus (EBV) DNA levels in patients with EBV-associated lymphoid malignancies. Br. J. Haematol. 111, 239–246 (2000).
Triche, E. W. et al. Sharing and preeclampsia: variation in effects by seminal fluid exposure in a case-control study of nulliparous women in Iowa. J. Reprod. Immunol. 101–102, 111–119 (2014).
Ober, C. Current topic: HLA and reproduction: lessons from studies in the Hutterites. Placenta 16, 569–577 (1995).
Gonzalez, D. A., de Leon, A. C., Moncholi, C. V., Cordova Jde, C. & Hernandez, L. B. Arthritis in mice: allogeneic pregnancy protects more than syngeneic by attenuating cellular immune response. J. Rheumatol. 31, 30–34 (2004).
Nelson, J. L. et al. Remission of rheumatoid arthritis during pregnancy and maternal-fetal class II alloantigen disparity. Am. J. Reprod. Immunol. 28, 226–227 (1992).
Nelson, J. L. et al. Maternal-fetal disparity in HLA class II alloantigens and the pregnancy-induced amelioration of rheumatoid arthritis. N. Engl. J. Med. 329, 466–471 (1993).
van der Horst-Bruinsma, I. E. et al. Influence of HLA-class II incompatibility between mother and fetus on the development and course of rheumatoid arthritis of the mother. Ann. Rheum. Dis. 57, 286–290 (1998).
Zrour, S. H. et al. The impact of pregnancy on rheumatoid arthritis outcome: the role of maternofetal HLA class II disparity. Joint Bone Spine 77, 36–40 (2010).
Brennan, P. et al. Maternal-fetal HLA incompatibility and the course of inflammatory arthritis during pregnancy. J. Rheumatol. 27, 2843–2848 (2000).
McInnes, I. B. & Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 365, 2205–2219 (2011).
Reiding, K. R. et al. Serum protein N-glycosylation changes with rheumatoid arthritis disease activity during and after pregnancy. Front. Med. 4, 241 (2017).
Nesspor, T. C., Raju, T. S., Chin, C. N., Vafa, O. & Brezski, R. J. Avidity confers FcγR binding and immune effector function to aglycosylated immunoglobulin G1. J. Mol. Recognit. 25, 147–154 (2012).
Zenclussen, A. C., Gentile, T., Kortebani, G., Mazzolli, A. & Margni, R. Asymmetric antibodies and pregnancy. Am. J. Reprod. Immunol. 45, 289–294 (2001).
van Zeben, D. et al. Early agalactosylation of IgG is associated with a more progressive disease course in patients with rheumatoid arthritis: results of a follow-up study. Br. J. Rheumatol. 33, 36–43 (1994).
Rademacher, T. W., Williams, P. & Dwek, R. A. Agalactosyl glycoforms of IgG autoantibodies are pathogenic. Proc. Natl Acad. Sci. USA 91, 6123–6127 (1994).
van de Geijn, F. E. et al. Immunoglobulin G galactosylation and sialylation are associated with pregnancy-induced improvement of rheumatoid arthritis and the postpartum flare: results from a large prospective cohort study. Arthritis Res. Ther. 11, R193 (2009).
Bondt, A., Wuhrer, M., Kuijper, T. M., Hazes, J. M. & Dolhain, R. J. Fab glycosylation of immunoglobulin G does not associate with improvement of rheumatoid arthritis during pregnancy. Arthritis Res. Ther. 18, 274 (2016).
Bondt, A. et al. ACPA IgG galactosylation associates with disease activity in pregnant patients with rheumatoid arthritis. Ann. Rheum. Dis. 77, 1130–1136 (2018).
Van Dijk, W. & Mackiewicz, A. Interleukin-6-type cytokine-induced changes in acute phase protein glycosylation. Ann. NY Acad. Sci. 762, 319–330 (1995).
Ercan, A. et al. Estrogens regulate glycosylation of IgG in women and men. JCI Insight 2, e89703 (2017).
Fettke, F. et al. Maternal and fetal mechanisms of B cell regulation during pregnancy: human chorionic gonadotropin stimulates B cells to produce IL-10 while alpha-fetoprotein drives them into apoptosis. Front. Immunol. 7, 495 (2016).
Miranda, S., Canellada, A., Gentile, T. & Margni, R. Interleukin-6 and dexamethasone modulate in vitro asymmetric antibody synthesis and UDP-Glc glycoprotein glycosyltransferase activity. J. Reprod. Immunol. 66, 141–150 (2005).
Haupl, T. et al. Reactivation of rheumatoid arthritis after pregnancy: increased phagocyte and recurring lymphocyte gene activity. Arthritis Rheum. 58, 2981–2992 (2008).
Goin, D. E. et al. Pregnancy-induced gene expression changes in vivo among women with rheumatoid arthritis: a pilot study. Arthritis Res. Ther. 19, 104 (2017).
Weix, J. et al. Influence of pregnancy on the adipocytokine and peroxisome proliferator-activated receptor pathways in peripheral blood mononuclear cells from healthy donors and rheumatoid arthritis patients. Arthritis Rheum. 64, 2095–2103 (2012).
Lima, J. et al. Serum markers of B-cell activation in pregnancy during late gestation, delivery, and the postpartum period. Am. J. Reprod. Immunol. 81, e13090 (2019).
Weix, J., Haupl, T., Raio, L., Villiger, P. M. & Forger, F. The physiologic increase in expression of some type I IFN-inducible genes during pregnancy is not associated with improved disease activity in pregnant patients with rheumatoid arthritis. Transl. Res. 161, 505–512 (2013).
Schumacher, A. et al. Human chorionic gonadotropin attracts regulatory T cells into the fetal-maternal interface during early human pregnancy. J. Immunol. 182, 5488–5497 (2009).
Nagaeva, O., Jonsson, L. & Mincheva-Nilsson, L. Dominant IL-10 and TGF-β mRNA expression in γδT cells of human early pregnancy decidua suggests immunoregulatory potential. Am. J. Reprod. Immunol. 48, 9–17 (2002).
Bissenden, J. G., Ling, N. R. & Mackintosh, P. Suppression of mixed lymphocyte reactions by pregnancy serum. Clin. Exp. Immunol. 39, 195–202 (1980).
Nancy, P. et al. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science 336, 1317–1321 (2012).
Rieger, L. et al. Specific subsets of immune cells in human decidua differ between normal pregnancy and preeclampsia-a prospective observational study. Reprod. Biol. Endocrinol. 7, 132 (2009).
Ishihara, K. & Hirano, T. Molecular basis of the cell specificity of cytokine action. Biochim. Biophys. Acta 1592, 281–296 (2002).
Yockey, L. J. & Iwasaki, A. Interferons and proinflammatory cytokines in pregnancy and fetal development. Immunity 49, 397–412 (2018).
Racicot, K., Kwon, J. Y., Aldo, P., Silasi, M. & Mor, G. Understanding the complexity of the immune system during pregnancy. Am. J. Reprod. Immunol. 72, 107–116 (2014).
Vacca, P., Chiossone, L., Mingari, M. C. & Moretta, L. Heterogeneity of NK cells and other innate lymphoid cells in human and murine decidua. Front. Immunol. 10, 170 (2019).
Gomez-Lopez, N., StLouis, D., Lehr, M. A., Sanchez-Rodriguez, E. N. & Arenas-Hernandez, M. Immune cells in term and preterm labor. Cell Mol. Immunol. 11, 571–581 (2014).
Alijotas-Reig, J., Esteve-Valverde, E., Ferrer-Oliveras, R., Llurba, E. & Gris, J. M. Tumor necrosis factor-alpha and pregnancy: focus on biologics. An updated and comprehensive review. Clin. Rev. Allergy Immunol. 53, 40–53 (2017).
Logiodice, F. et al. Decidual interleukin-22-producing CD4+ T Cells (Th17/Th0/IL-22+ and Th17/Th2/IL-22+, Th2/IL-22+, Th0/IL-22+), which also produce IL-4, are involved in the success of pregnancy. Int. J. Mol. Sci. 20, 428 (2019).
Nicola, N. A. & Babon, J. J. Leukemia inhibitory factor (LIF). Cytokine Growth Factor Rev. 26, 533–544 (2015).
Winship, A., Correia, J., Zhang, J. G., Nicola, N. A. & Dimitriadis, E. Leukemia inhibitory factor (LIF) inhibition during mid-gestation impairs trophoblast invasion and spiral artery remodelling during pregnancy in mice. PLOS ONE 10, e0129110 (2015).
Ashkar, A. A., Di Santo, J. P. & Croy, B. A. Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J. Exp. Med. 192, 259–270 (2000).
Murphy, S. P. et al. Interferon gamma in successful pregnancies. Biol. Reprod. 80, 848–859 (2009).
Giaglis, S. et al. Neutrophil migration into the placenta: good, bad or deadly? Cell Adh. Migr. 10, 208–225 (2016).
Chen, C. P. et al. Expression of interferon gamma by decidual cells and natural killer cells at the human implantation site: implications for preeclampsia, spontaneous abortion, and intrauterine growth restriction. Reprod. Sci. 22, 1461–1467 (2015).
Jena, M. K., Nayak, N., Chen, K. & Nayak, N. R. Role of macrophages in pregnancy and related complications. Arch. Immunol. Ther. Exp. 67, 295–309 (2019).
Ziegler, S. M. et al. Innate immune responses to toll-like receptor stimulation are altered during the course of pregnancy. J. Reprod. Immunol. 128, 30–37 (2018).
Sur Chowdhury, C. et al. Enhanced neutrophil extracellular trap generation in rheumatoid arthritis: analysis of underlying signal transduction pathways and potential diagnostic utility. Arthritis Res. Ther. 16, R122 (2014).
Wegmann, T. G., Lin, H., Guilbert, L. & Mosmann, T. R. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol. Today 14, 353–356 (1993).
Fallon, P. G. et al. IL-4 induces characteristic Th2 responses even in the combined absence of IL-5, IL-9, and IL-13. Immunity 17, 7–17 (2002).
Ng, S. C. et al. Expression of intracellular Th1 and Th2 cytokines in women with recurrent spontaneous abortion, implantation failures after IVF/ET or normal pregnancy. Am. J. Reprod. Immunol. 48, 77–86 (2002).
Ostensen, M. et al. Pregnancy in patients with rheumatic disease: anti-inflammatory cytokines increase in pregnancy and decrease post partum. Ann. Rheum. Dis. 64, 839–844 (2005).
Santner-Nanan, B. et al. Fetal-maternal alignment of regulatory T cells correlates with IL-10 and Bcl-2 upregulation in pregnancy. J. Immunol. 191, 145–153 (2013).
Saito, S., Nakashima, A., Shima, T. & Ito, M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am. J. Reprod. Immunol. 63, 601–610 (2010).
Liu, Z. Z., Sun, G. Q., Hu, X. H., Kwak-Kim, J. & Liao, A. H. The transdifferentiation of regulatory T and Th17 cells in autoimmune/inflammatory diseases and its potential implications in pregnancy complications. Am. J. Reprod. Immunol. https://doi.org/10.1111/aji.12657 (2017).
Chaouat, G. et al. Th1/Th2 paradigm in pregnancy: paradigm lost? Cytokines in pregnancy/early abortion: reexamining the Th1/Th2 paradigm. Int. Arch. Allergy Immunol. 134, 93–119 (2004).
Roeleveld, D. M. & Koenders, M. I. The role of the Th17 cytokines IL-17 and IL-22 in rheumatoid arthritis pathogenesis and developments in cytokine immunotherapy. Cytokine 74, 101–107 (2015).
Furst, D. E. & Emery, P. Rheumatoid arthritis pathophysiology: update on emerging cytokine and cytokine-associated cell targets. Rheumatology 53, 1560–1569 (2014).
McInnes, I. B. & Schett, G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet 389, 2328–2337 (2017).
Huizinga, T. W., van der Linden, M. W., Deneys-Laporte, V. & Breedveld, F. C. Interleukin-10 as an explanation for pregnancy-induced flare in systemic lupus erythematosus and remission in rheumatoid arthritis. Rheumatology 38, 496–498 (1999).
de Steenwinkel, F. D. et al. Circulating maternal cytokines influence fetal growth in pregnant women with rheumatoid arthritis. Ann. Rheum. Dis. 72, 1995–2001 (2013).
Samstein, R. M., Josefowicz, S. Z., Arvey, A., Treuting, P. M. & Rudensky, A. Y. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell 150, 29–38 (2012).
Hierweger, A. M. et al. Progesterone modulates the T cell response via glucocorticoid receptor-dependent pathways. Am. J. Reprod. Immunol. 81, e13084 (2019).
Hori, S., Nomura, T. & Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061 (2003).
Fontenot, J. D., Gavin, M. A. & Rudensky, A. Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4, 330–336 (2003).
Habicht, A. et al. A link between PDL1 and T regulatory cells in fetomaternal tolerance. J. Immunol. 179, 5211–5219 (2007).
Sakaguchi, S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat. Immunol. 6, 345–352 (2005).
Aluvihare, V. R., Kallikourdis, M. & Betz, A. G. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 5, 266–271 (2004).
Tilburgs, T. et al. Evidence for a selective migration of fetus-specific CD4+CD25bright regulatory T cells from the peripheral blood to the decidua in human pregnancy. J. Immunol. 180, 5737–5745 (2008).
Somerset, D. A., Zheng, Y., Kilby, M. D., Sansom, D. M. & Drayson, M. T. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology 112, 38–43 (2004).
Sasaki, Y. et al. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol. Hum. Reprod. 10, 347–353 (2004).
Toldi, G. et al. The frequency of peripheral blood CD4+ CD25high FoxP3+ and CD4+ CD25− FoxP3+ regulatory T cells in normal pregnancy and pre-eclampsia. Am. J. Reprod. Immunol. 68, 175–180 (2012).
Marcoli, N. et al. Differential influence of maternal and fetal pregnancy factors on the in-vitro induction of human regulatory T cells: a preliminary study. Swiss Med. Wkly 145, w14172 (2015).
Metcalfe, S. M. LIF in the regulation of T-cell fate and as a potential therapeutic. Genes Immun. 12, 157–168 (2011).
Hosseini, A., Dolati, S., Hashemi, V., Abdollahpour-Alitappeh, M. & Yousefi, M. Regulatory T and T helper 17 cells: Their roles in preeclampsia. J. Cell Physiol. 233, 6561–6573 (2018).
Morgan, M. E. et al. Effective treatment of collagen-induced arthritis by adoptive transfer of CD25+ regulatory T cells. Arthritis Rheum. 52, 2212–2221 (2005).
Morgan, M. E. et al. CD25+ cell depletion hastens the onset of severe disease in collagen-induced arthritis. Arthritis Rheum. 48, 1452–1460 (2003).
Förger, F. et al. Pregnancy induces numerical and functional changes of CD4+CD25high regulatory T cells in patients with rheumatoid arthritis. Ann. Rheum. Dis. 67, 984–990 (2008).
Munoz-Suano, A., Kallikourdis, M., Sarris, M. & Betz, A. G. Regulatory T cells protect from autoimmune arthritis during pregnancy. J. Autoimmun. 38, J103–J108 (2012).
Koenen, H. J. et al. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood 112, 2340–2352 (2008).
Wang, T. et al. Regulatory T cells in rheumatoid arthritis showed increased plasticity toward Th17 but retained suppressive function in peripheral blood. Ann. Rheum. Dis. 74, 1293–1301 (2015).
Santner-Nanan, B. et al. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J. Immunol. 183, 7023–7030 (2009).
Sakaguchi, S., Vignali, D. A., Rudensky, A. Y., Niec, R. E. & Waldmann, H. The plasticity and stability of regulatory T cells. Nat. Rev. Immunol. 13, 461–467 (2013).
Ehrenstein, M. R. et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFα therapy. J. Exp. Med. 200, 277–285 (2004).
Komatsu, N. et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat. Med. 20, 62–68 (2014).
Jorgensen, K. T., Harpsoe, M. C., Jacobsen, S., Jess, T. & Frisch, M. Increased risk of rheumatoid arthritis in women with pregnancy complications and poor self-rated health: a study within the Danish National Birth Cohort. Rheumatology 53, 1513–1519 (2014).
Ma, K. K., Nelson, J. L., Guthrie, K. A., Dugowson, C. E. & Gammill, H. S. Adverse pregnancy outcomes and risk of subsequent rheumatoid arthritis. Arthritis Rheumatol. 66, 508–512 (2014).
Hayday, A. C. γδ T cells and the lymphoid stress-surveillance response. Immunity 31, 184–196 (2009).
Bonneville, M., O'Brien, R. L. & Born, W. K. γδ T cell effector functions: a blend of innate programming and acquired plasticity. Nat. Rev. Immunol. 10, 467–478 (2010).
Carding, S. R. & Egan, P. J. γδ T cells: functional plasticity and heterogeneity. Nat. Rev. Immunol. 2, 336–345 (2002).
Toussirot, E. et al. Increased production of soluble CTLA-4 in patients with spondylarthropathies correlates with disease activity. Arthritis Res. Ther. 11, R101 (2009).
Kimura, M., Hanawa, H., Watanabe, H., Ogawa, M. & Abo, T. Synchronous expansion of intermediate TCR cells in the liver and uterus during pregnancy. Cell Immunol. 162, 16–25 (1995).
Terzieva, A. et al. Early pregnancy human decidua is enriched with activated, fully differentiated and pro-inflammatory Gamma/Delta T Cells with diverse TCR repertoires. Int. J. Mol. Sci. 20, 687 (2019).
Barakonyi, A., Polgar, B. & Szekeres-Bartho, J. The role of gamma/delta T-cell receptor-positive cells in pregnancy: part II. Am. J. Reprod. Immunol. 42, 83–87 (1999).
Szereday, L., Barakonyi, A., Miko, E., Varga, P. & Szekeres-Bartho, J. γ/δT-cell subsets, NKG2A expression and apoptosis of Vδ2+ T cells in pregnant women with or without risk of premature pregnancy termination. Am. J. Reprod. Immunol. 50, 490–496 (2003).
Szekeres-Bartho, J. The role of progesterone in feto-maternal immunological cross talk. Med. Princ. Pract. 27, 301–307 (2018).
Szekeres-Bartho, J. & Wegmann, T. G. A progesterone-dependent immunomodulatory protein alters the Th1/Th2 balance. J. Reprod. Immunol. 31, 81–95 (1996).
Peterman, G. M., Spencer, C., Sperling, A. I. & Bluestone, J. A. Role of gamma delta T cells in murine collagen-induced arthritis. J. Immunol. 151, 6546–6558 (1993).
Smith, M. D. et al. Tγδ cells and their subsets in blood and synovial tissue from rheumatoid arthritis patients. Scand. J. Immunol. 32, 585–593 (1990).
Tham, M. et al. Reduced pro-inflammatory profile of γδT cells in pregnant patients with rheumatoid arthritis. Arthritis Res. Ther. 18, 26 (2016).
Ito, Y. et al. Gamma/delta T cells are the predominant source of interleukin-17 in affected joints in collagen-induced arthritis, but not in rheumatoid arthritis. Arthritis Rheum. 60, 2294–2303 (2009).
Shen, Y. et al. TCR γ/δ+ cell subsets in the synovial membranes of patients with rheumatoid arthritis and juvenile rheumatoid arthritis. Scand. J. Immunol. 36, 533–540 (1992).
Förger, F., Østensen, M., Schumacher, A. & Villiger, P. M. Impact of pregnancy on health related quality of life evaluated prospectively in pregnant women with rheumatic diseases by the SF-36 health survey. Ann. Rheum. Dis. 64, 1494–1499 (2005).
Østensen, M., Fuhrer, L., Mathieu, R., Seitz, M. & Villiger, P. M. A prospective study of pregnant patients with rheumatoid arthritis and ankylosing spondylitis using validated clinical instruments. Ann. Rheum. Dis. 63, 1212–1217 (2004).
Unger, A., Kay, A., Griffin, A. J. & Panayi, G. S. Disease activity and pregnancy associated alpha 2-glycoprotein in rheumatoid arthritis during pregnancy. Br. Med. J. 286, 750–752 (1983).
Jethwa, H., Lam, S., Smith, C. & Giles, I. Does rheumatoid arthritis really improve during pregnancy? A systematic review and metaanalysis. J. Rheumatol. 46, 245–250 (2019).
Østensen, M., Sicher, P., Forger, F. & Villiger, P. M. Activation markers of peripheral blood mononuclear cells in late pregnancy and after delivery: a pilot study. Ann. Rheum. Dis. 64, 318–320 (2005).
Recalde, G. et al. Contribution of sex steroids and prolactin to the modulation of T and B cells during autoimmunity. Autoimmun. Rev. 17, 504–512 (2018).
Vieira Borba, V. & Shoenfeld, Y. Prolactin, autoimmunity, and motherhood: when should women avoid breastfeeding? Clin. Rheumatol. 38, 1263–1270 (2019).
Pereira Suarez, A. L., Lopez-Rincon, G., Martinez Neri, P. A. & Estrada-Chavez, C. Prolactin in inflammatory response. Adv. Exp. Med. Biol. 846, 243–264 (2015).
Pellegrini, I., Lebrun, J. J., Ali, S. & Kelly, P. A. Expression of prolactin and its receptor in human lymphoid cells. Mol. Endocrinol. 6, 1023–1031 (1992).
Tang, M. W. et al. Rheumatoid arthritis and psoriatic arthritis synovial fluids stimulate prolactin production by macrophages. J. Leukoc. Biol. 102, 897–904 (2017).
Tang, M. W. et al. The prolactin receptor is expressed in rheumatoid arthritis and psoriatic arthritis synovial tissue and contributes to macrophage activation. Rheumatology 55, 2248–2259 (2016).
Ince-Askan, H., Hazes, J. M. W. & Dolhain, R. Breastfeeding among women with rheumatoid arthritis compared with the general population: results from a nationwide prospective cohort study. J. Rheumatol. 46, 1067–1074 (2019).
Zhang, F. et al. Are prolactin levels linked to suction pressure? Breastfeed. Med. 11, 461–468 (2016).
Chen, H., Wang, J., Zhou, W., Yin, H. & Wang, M. Breastfeeding and risk of rheumatoid arthritis: a systematic review and metaanalysis. J. Rheumatol. 42, 1563–1569 (2015).
Andreoli, L. et al. EULAR recommendations for women's health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann. Rheum. Dis. 76, 476–485 (2017).
Buyon, J. P. et al. Predictors of pregnancy outcomes in patients with lupus: a cohort study. Ann. Intern. Med. 163, 153–163 (2015).
Moroni, G. et al. Fetal outcome and recommendations of pregnancies in lupus nephritis in the 21st century. A prospective multicenter study. J. Autoimmun. 74, 6–12 (2016).
Gotestam Skorpen, C. et al. Disease activity during pregnancy and the first year postpartum in women with systemic lupus erythematosus. Arthritis Care Res. 69, 1201–1208 (2017).
Crow, M. K. & Ronnblom, L. Type I interferons in host defence and inflammatory diseases. Lupus Sci. Med. 6, e000336 (2019).
Kirou, K. A. et al. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 52, 1491–1503 (2005).
Andrade, D. et al. Interferon-alpha and angiogenic dysregulation in pregnant lupus patients who develop preeclampsia. Arthritis Rheumatol. 67, 977–987 (2015).
Ursin, K., Lydersen, S., Skomsvoll, J. F. & Wallenius, M. Disease activity during and after pregnancy in women with axial spondyloarthritis: a prospective multicentre study. Rheumatology 57, 1064–1071 (2018).
Lories, R. J. & Baeten, D. L. Differences in pathophysiology between rheumatoid arthritis and ankylosing spondylitis. Clin. Exp. Rheumatol. 27, S10–S14 (2009).
Förger, F., Villiger, P. M. & Østensen, M. Pregnancy in patients with ankylosing spondylitis: do regulatory T cells play a role? Arthritis Rheum. 61, 279–283 (2009).
Bhalla, A., Stone, P. R., Liddell, H. S., Zanderigo, A. & Chamley, L. W. Comparison of the expression of human leukocyte antigen (HLA)-G and HLA-E in women with normal pregnancy and those with recurrent miscarriage. Reproduction 131, 583–589 (2006).
Morandi, F. & Pistoia, V. Interactions between HLA-G and HLA-E in physiological and pathological conditions. Front. Immunol. 5, 394 (2014).
Li, S. et al. Estrogen induces indoleamine 2,3-dioxygenase expression via suppressors of cytokine signaling 3 in the chorionic villi and decidua of women in early pregnancy. Am. J. Reprod. Immunol. https://doi.org/10.1111/aji.13197 (2019).
Liu, W. et al. Relationship of SOCS3 and TGF-β with IDO expression in early pregnancy chorionic villi and decidua. Exp. Ther. Med. 14, 4817–4824 (2017).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Rheumatology thanks S. Saito and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Decidua
-
The maternal part of the placenta.
- Semiallogeneic graft
-
A transplant sharing half of its genes with the recipient
- Allograft
-
An organ, tissue or cells transplanted between genetically non-identical members of the same species.
- Microchimerism
-
The presence of a small number of genetically distinct cells in a host individual.
- Preeclampsia
-
A complication of pregnancy characterized by hypertension and proteinuria and signs of damage to other organs, usually occurring after 20 weeks of pregnancy.
- Mixed lymphocyte reaction
-
The proliferation response following co-culture of two populations of lymphocytes, used as a surrogate measure of T cell activation
- Trophoblast invasion
-
Invasion of the uterus by fetal cells during human placentation.
- Spiral artery remodelling
-
Transformation of spiral arteries into vessels of low resistance by trophoblast invasion.
- Parturition
-
The process of labour and delivery.
- Chorioamniotic membranes
-
Fetal membranes (amnion and chorion) making up the amniotic sac that surrounds and protects the fetus.
Rights and permissions
About this article
Cite this article
Förger, F., Villiger, P.M. Immunological adaptations in pregnancy that modulate rheumatoid arthritis disease activity. Nat Rev Rheumatol 16, 113–122 (2020). https://doi.org/10.1038/s41584-019-0351-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-019-0351-2
This article is cited by
-
Bidirectional association between autoimmune disease and perinatal depression: a nationwide study with sibling comparison
Molecular Psychiatry (2024)
-
Pregnancy and the Autoimmune Patient
Current Allergy and Asthma Reports (2024)
-
Maternal microbiota and gestational diabetes: impact on infant health
Journal of Translational Medicine (2023)
-
Local immune recognition of trophoblast in early human pregnancy: controversies and questions
Nature Reviews Immunology (2023)
-
Rheumatic Diseases in Reproductive Age—the Possibilities and the Risks
Reproductive Sciences (2023)