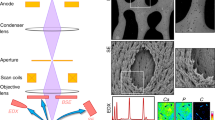
Abstract
Bone is organized in a hierarchical 3D architecture. Traditionally, analysis of the skeletal system was based on bone mass assessment by radiographic methods or on the examination of bone structure by 2D histological sections. Advanced imaging technologies and big data analysis now enable the unprecedented examination of bone and provide new insights into its 3D macrostructure and microstructure. These technologies comprise ex vivo and in vivo methods including high-resolution computed tomography (CT), synchrotron-based imaging, X-ray microscopy, ultra-high-field magnetic resonance imaging (MRI), light-sheet fluorescence microscopy, confocal and intravital two-photon imaging. In concert, these techniques have been used to detect and quantify a novel vascular system of trans-cortical vessels in bone. Furthermore, structures such as the lacunar network, which harbours and connects osteocytes, become accessible for 3D imaging and quantification using these methods. Next-generation imaging of the skeletal system and its blood supply are anticipated to contribute to an entirely new understanding of bone tissue composition and function, from macroscale to nanoscale, in health and disease. These insights could provide the basis for early detection and precision-type intervention of bone disorders in the future.
Key points
-
Bone is a complex tissue that has functional elements at size scales spanning five orders of magnitude.
-
Studying all the different structures of bone is necessary to comprehensively understand bone function in health and disease in experimental and clinical settings.
-
Comprehensive analysis of bone requires an array of different imaging approaches, including X-ray, magnetic resonance, and optical and electron microscopy imaging modalities.
-
Fundamental improvements of methodology in all these imaging approaches now enable a completely new view of bone, resulting in novel insights into its function and blood supply.
-
Massive amounts of imaging data emerging from such analyses require innovative image reconstruction algorithms, such as machine vision and deep learning, to extract meaningful information.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sommerfeldt, D. W. & Rubin, C. T. Biology of bone and how it orchestrates the form and function of the skeleton. Eur. Spine J. 10 (Suppl. 2), S86–S95 (2001).
Clarke, B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 3 (Suppl. 3), S131–S139 (2008).
Agas, D., Marchetti, L., Douni, E. & Sabbieti, M. G. The unbearable lightness of bone marrow homeostasis. Cytokine Growth Factor Rev. 26, 347–359 (2015).
McKee, M. D. & Cole, W. G. (ed.) Bone Matrix and Mineralization. 2 edn (Elsevier, 2012).
Sims, N. A. & Martin, T. J. Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. Bonekey Rep. 3, 481 (2014).
Bozec, A. & Zaiss, M. M. T. Regulatory cells in bone remodelling. Curr. Osteoporos. Rep. 15, 121–125 (2017).
Parra-Torres, A. Y., Valdés-Flores, M., Orozco, L. & Velázquez-Cruz, R. In Topics in Osteoporosis. https://doi.org/10.5772/54905 (InTech Open, 2013).
Shetty, S., Kapoor, N., Bondu, J. D., Thomas, N. & Paul, T. V. Bone turnover markers: emerging tool in the management of osteoporosis. Indian J. Endocrinol. Metab. 20, 846–852 (2016).
Hernlund, E. et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. Arch. Osteoporos. 8, 136 (2013).
Suen, P. K. & Qin, L. Sclerostin, an emerging therapeutic target for treating osteoporosis and osteoporotic fracture: a general review. J. Orthop. Translat. 4, 1–13 (2016).
Grüneboom, A. et al. A network of trans-cortical capillaries as mainstay for blood circulation in long bones. Nat. Metab. 1, 236–250 (2019).
Andrews, J. C. et al. A high resolution, hard x-ray bio-imaging facility at SSRL. Synchrotron Radiat. News 21, 17–26 (2008).
Arboleya, L. & Castañedab, S. Osteoimmunology: the study of the relationship between the immune system and bone tissue. Reumatol. Clin. 9, 303–315 (2014).
Bach-Gansmo, F. L. et al. Osteocyte lacunar properties and cortical microstructure in human iliac crest as a function of age and sex. Bone 91, 11–19 (2016).
Florencio-Silva, R., Sasso, G. R., Sasso-Cerri, E., Simoes, M. J. & Cerri, P. S. Biology of bone tissue: structure, function, and factors that influence bone cells. Biomed. Res. Int. 2015, 421746 (2015).
Dallas, S. L. & Bonewald, L. F. Dynamics of the transition from osteoblast to osteocyte. Ann. N. Y. Acad. Sci. 1192, 437–443 (2010).
Cardoso, L., Fritton, S. P., Gailani, G., Benalla, M. & Cowin, S. C. Advances in assessment of bone porosity, permeability and interstitial fluid flow. J. Biomech. 46, 253–265 (2013).
Bonewald, L. F. & Johnson, M. L. Osteocytes, mechanosensing and Wnt signaling. Bone 42, 606–615 (2008).
Röntgen, W. C. Ueber eine neue Art von Strahlen. Vorläufige Mitteilung. Sitzungsberichte der Würzburger Physik.-medic. Gesellschaft, 137–147 (1895).
Zhu, Y., Zhang, J., Li, A., Zhang, Y. & Fan, C. Synchrotron-based X-ray microscopy for sub-100nm resolution cell imaging. Curr. Opin. Chem. Biol. 39, 11–16 (2017).
Varga, P. et al. Synchrotron X-ray phase nano-tomography-based analysis of the lacunar-canalicular network morphology and its relation to the strains experienced by osteocytes in situ as predicted by case-specific finite element analysis. Biomech. Model Mechanobiol. 14, 267–282 (2015).
Sanderson, M. J., Smith, I., Parker, I. & Bootman, M. D. Fluorescence microscopy. Cold Spring Harb. Protoc. 2014, https://doi.org/10.1101/pdb.top071795 (2014).
Wolf, D. E. Fundamentals of fluorescence and fluorescence microscopy. Methods Cell Biol. 81, 63–91 (2007).
Engelke, K. et al. Clinical use of quantitative computed tomography and peripheral quantitative computed tomography in the management of osteoporosis in adults: The 2007 ISCD official positions. J. Clin. Densitom. 11, 123–162 (2008).
Museyko, O., Bousson, V., Adams, J., Laredo, J. D. & Engelke, K. QCT of the proximal femur – which parameters should be measured to discriminate hip fracture? Osteoporos. Int. 27, 1137–1147 (2016).
Wang, X. et al. Prediction of new clinical vertebral fractures in elderly men using finite element analysis of CT scans. J. Bone Miner. Res. 27, 808–816 (2012).
Imai, K., Ohnishi, I., Matsumoto, T., Yamamoto, S. & Nakamura, K. Assessment of vertebral fracture risk and therapeutic effects of alendronate in postmenopausal women using a quantitative computed tomography-based nonlinear finite element method. Osteoporos. Int. 20, 801–810 (2009).
Figueiredo, C. P. et al. Methods for segmentation of rheumatoid arthritis bone erosions in high-resolution peripheral quantitative computed tomography (HR-pQCT). Semin. Arthritis Rheum. 47, 611–618 (2017).
Engelke, K. et al. Clinical use of quantitative computed tomography-based advanced techniques in the management of osteoporosis in adults: the 2015 ISCD official positions-part III. J. Clin. Densitom. 18, 393–407 (2015).
Gausden, E. B., Nwachukwu, B. U., Schreiber, J. J., Lorich, D. G. & Lane, J. M. Opportunistic use of CT imaging for osteoporosis screening and bone density assessment: A qualitative systematic review. J. Bone Joint Surg. Am. 99, 1580–1590 (2017).
Muhlberg, A. et al. Three-dimensional distribution of muscle and adipose tissue of the thigh at CT: association with acute hip fracture. Radiology 290, 426–434 (2018).
Engelke, K. et al. Reanalysis precision of 3D quantitative computed tomography (QCT) of the spine. Bone 44, 566–572 (2009).
Lang, T. F. et al. Volumetric quantitative computed tomography of the proximal femur: precision and relation to bone strength. Bone 21, 101–108 (1997).
Gluer, C. C. 30 years of DXA technology innovations. Bone 104, 7–12 (2017).
Engelke, K. et al. Quantitative computed tomography (QCT) of the forearm using general purpose spiral whole-body CT scanners: accuracy, precision and comparison with dual-energy X-ray absorptiometry (DXA). Bone 45, 110–118 (2009).
Engelke, K. Quantitative computed tomography – current status and new developments. J. Clin. Densitom. 20, 309–321 (2017).
Lin, E. & Alessio, A. What are the basic concepts of temporal, contrast, and spatial resolution in cardiac CT? J. Cardiovasc. Comput. Tomogr. 3, 403–408 (2009).
Li, Y. et al. CT slice thickness and convolution kernel affect performance of a radiomic model for predicting EGFR status in non-small cell lung cancer: a preliminary study. Sci. Rep. 8, 17913 (2018).
Dougherty, G. & Newman, D. Measurement of thickness and density of thin structures by computed tomography: a simulation study. Med. Phys. 26, 1341–1348 (1999).
Newman, D. L., Dougherty, G., al Obaid, A. & al Hajrasy, H. Limitations of clinical CT in assessing cortical thickness and density. Phys. Med. Biol. 43, 619–626 (1998).
Zebaze, R. M. et al. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet 375, 1729–1736 (2010).
Museyko, O., Gerner, B. & Engelke, K. A new method to determine cortical bone thickness in CT images using a hybrid approach of parametric profile representation and local adaptive thresholds: accuracy results. PLOS ONE 12, e0187097 (2017).
Treece, G. M. & Gee, A. H. Independent measurement of femoral cortical thickness and cortical bone density using clinical CT. Med. Image Anal. 20, 249–264 (2015).
Treece, G. M., Poole, K. E. & Gee, A. H. Imaging the femoral cortex: thickness, density and mass from clinical CT. Med. Image Anal. 16, 952–965 (2012).
Damm, T. et al. Improved accuracy in the assessment of vertebral cortical thickness by quantitative computed tomography using the Iterative Convolution OptimizatioN (ICON) method. Bone 120, 194–203 (2018).
Zysset, P. et al. Clinical use of quantitative computed tomography-based finite element analysis of the hip and spine in the management of osteoporosis in adults: the 2015 ISCD official positions – part II. J. Clin. Densitom. 18, 359–392 (2015).
Bousson, V. D. et al. In vivo discrimination of hip fracture with quantitative computed tomography: results from the prospective European Femur Fracture Study (EFFECT). JBMR 26, 881–893 (2011).
Chappard, C. et al. Prediction of femoral fracture load: cross-sectional study of texture analysis and geometric measurements on plain radiographs versus bone mineral density. Radiology 255, 536–543 (2010).
Cheng, X., Li, J., Lu, Y., Keyak, J. & Lang, T. Proximal femoral density and geometry measurements by quantitative computed tomography: association with hip fracture. Bone 40, 169–174 (2007).
Yang, L. et al. Association of incident hip fracture with the estimated femoral strength by finite element analysis of DXA scans in the Osteoporotic Fractures in Men (MrOS) study. Osteoporos. Int. 29, 643–651 (2017).
Yang, L., Udall, W. J., McCloskey, E. V. & Eastell, R. Distribution of bone density and cortical thickness in the proximal femur and their association with hip fracture in postmenopausal women: a quantitative computed tomography study. Osteoporos. Int. 25, 251–263 (2014).
Borggrefe, J. et al. Association of 3D geometric measures derived from quantitative computed tomography with hip fracture risk in older men. J. Bone Miner. Res. 31, 1550–1558 (2016).
Bouxsein, M. L. et al. Age- and sex-specific differences in the factor of risk for vertebral fracture: a population-based study using QCT. J. Bone Miner. Res. 21, 1475–1482 (2006).
Bruno, A. G. et al. Vertebral size, bone density, and strength in men and women matched for age and areal spine BMD. J. Bone Miner. Res. 29, 562–569 (2014).
Hahn, M. H. & Won, Y. Y. Bone mineral density and fatty degeneration of thigh muscles measured by computed tomography in hip fracture patients. J. Bone. Metab. 23, 215–221 (2016).
Lang, T. et al. Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: the health, aging, and body composition study. J. Bone Miner. Res. 25, 513–519 (2010).
Lang, T. et al. Pelvic body composition measurements by quantitative computed tomography: association with recent hip fracture. Bone 42, 798–805 (2008).
Wong, A. K. et al. Bone-muscle indices as risk factors for fractures in men: the Osteoporotic Fractures in Men (MrOS) Study. J. Musculoskelet. Neuronal Interact. 14, 246–254 (2014).
Lee, D. C., Hoffmann, P. F., Kopperdahl, D. L. & Keaveny, T. M. Phantomless calibration of CT scans for measurement of BMD and bone strength-Inter-operator reanalysis precision. Bone 103, 325–333 (2017).
Boutroy, S., Bouxsein, M. L., Munoz, F. & Delmas, P. D. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J. Clin. Endocrinol. Metab. 90, 6508–6515 (2005).
Burghardt, A. J., Link, T. M. & Majumdar, S. High-resolution computed tomography for clinical imaging of bone microarchitecture. Clin. Orthop. Relat. Res. 469, 2179–2193 (2011).
Nishiyama, K. K. & Shane, E. Clinical imaging of bone microarchitecture with HR-pQCT. Curr. Osteoporos. Rep. 11, 147–155 (2013).
Burghardt, A. J. et al. Multicenter precision of cortical and trabecular bone quality measures assessed by high-resolution peripheral quantitative computed tomography. J. Bone Miner. Res. 28, 524–536 (2013).
Manske, S. L., Davison, E. M., Burt, L. A., Raymond, D. A. & Boyd, S. K. The estimation of second-generation HR-pQCT from first-generation HR-pQCT using in vivo cross-calibration. J. Bone Miner. Res. 32, 1514–1524 (2017).
Laib, A. & Ruegsegger, P. Calibration of trabecular bone structure measurements of in vivo three-dimensional peripheral quantitative computed tomography with 28-microm-resolution microcomputed tomography. Bone 24, 35–39 (1999).
Manske, S. L., Zhu, Y., Sandino, C. & Boyd, S. K. Human trabecular bone microarchitecture can be assessed independently of density with second generation HR-pQCT. Bone 79, 213–221 (2015).
Tjong, W., Nirody, J., Burghardt, A. J., Carballido-Gamio, J. & Kazakia, G. J. Structural analysis of cortical porosity applied to HR-pQCT data. Med. Phys. 41, 013701 (2014).
Bousson, V. et al. Distribution of intracortical porosity in human midfemoral cortex by age and gender. J. Bone Miner. Res. 16, 1308–1317 (2001).
Paccou, J. et al. Bone microarchitecture in men and women with diabetes: the importance of cortical porosity. Calcif. Tissue Int. 98, 465–473 (2016).
Patsch, J. M. et al. Increased cortical porosity in type 2 diabetic postmenopausal women with fragility fractures. J. Bone Miner. Res. 28, 313–324 (2013).
Cheung, A. M. et al. High-resolution peripheral quantitative computed tomography for the assessment of bone strength and structure: a review by the Canadian Bone Strength Working Group. Curr. Osteoporos. Rep. 11, 136–146 (2013).
Nishiyama, K., Dal’Ara, E. & Engelke, K. In Advanced Techniques of Bone Mass Measurements in Adults (Bilezikian, J. P., ed.) (Wiley, 2018).
Schett, G. & Gravallese, E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat. Rev. Rheumatol. 8, 656–664 (2012).
Regensburger, A. et al. A comparative analysis of magnetic resonance imaging and high-resolution peripheral quantitative computed tomography of the hand for the detection of erosion repair in rheumatoid arthritis. Rheumatology 54, 1573–1581 (2015).
Bhatnagar, S., Krishnamurthy, V. & Pagare, S. S. Diagnostic efficacy of panoramic radiography in detection of osteoporosis in post-menopausal women with low bone mineral density. J. Clin. Imaging Sci. 3, 23 (2013).
de Charry, C. et al. Clinical cone beam computed tomography compared to high-resolution peripheral computed tomography in the assessment of distal radius bone. Osteoporos. Int. 27, 3073–3082 (2016).
Posadzy, M., Desimpel, J. & Vanhoenacker, F. Cone beam CT of the musculoskeletal system: clinical applications. Insights Imaging 9, 35–45 (2018).
Nardi, C. et al. The role of cone beam CT in the study of symptomatic total knee arthroplasty (TKA): a 20 cases report. Br. J. Radiol. 90, 20160925 (2017).
Mys, K., Stockmans, F., Vereecke, E. & van Lenthe, G. H. Quantification of bone microstructure in the wrist using cone-beam computed tomography. Bone 114, 206–214 (2018).
Paulus, M. J., Gleason, S. S., Kennel, S. J., Hunsicker, P. R. & Johnson, D. K. High resolution X-ray computed tomography: an emerging tool for small animal cancer research. Neoplasia 2, 62–70 (2000).
Schneider, P., Meier, M., Wepf, R. & Müller, R. Serial FIB/SEM imaging for quantitative 3D assessment of the osteocyte lacuno-canalicular network. Bone 49, 304–311 (2011).
Langer, M. et al. X-Ray phase nanotomography resolves the 3D human bone ultrastructure. PLOS ONE 7, e35691 (2012).
Hasegawa, T. et al. Three-dimensional ultrastructure of osteocytes assessed by focused ion beam-scanning electron microscopy (FIB-SEM). Histochem. Cell Biol. 149, 423–432 (2018).
Carl Zeiss Microscopy GmbH. Multi-length Scale Imaging Bridging the 3D Resolution Gap. Zeiss.de https://www.zeiss.de/content/dam/Microscopy/us/download/pdf/technical-notes/x-ray-microscopy/multi-length-scale-imaging.pdf (2013).
Bonse, U. (ed.) Developments in X-ray Tomography III. Vol. 4503, 1–386 (SPIE Press, 2002).
Langer, M. & Peyrin, F. 3D X-ray ultra-microscopy of bone tissue. Osteoporos. Int. 27, 441–455 (2016).
Muller, R. Hierarchical microimaging of bone structure and function. Nat. Rev. Rheumatol. 5, 373–381 (2009).
Schneider, P. et al. Simultaneous 3D visualization and quantification of murine bone and bone vasculature using micro-computed tomography and vascular replica. Microsc. Res. Tech. 72, 690–701 (2009).
Schropp, A. et al. Hard x-ray scanning microscopy with coherent radiation: beyond the resolution of conventional x-ray microscopes. Appl. Phys. Lett. 100, 253112–253112 (2012).
Ma, S. et al. Synchrotron imaging assessment of bone quality. Clin. Rev. Bone Miner. Metab. 14, 150–160 (2016).
Paul, G. R., Malhotra, A. & Muller, R. Mechanical stimuli in the local in vivo environment in bone: computational approaches linking organ-scale loads to cellular signals. Curr. Osteoporos. Rep. 16, 395–403 (2018).
Zizak, I. et al. Characteristics of mineral particles in the human bone/cartilage interface. J. Struct. Biol. 141, 208–217 (2003).
Gupta, H. S. et al. Nanoscale deformation mechanisms in bone. Nano Lett. 5, 2108–2111 (2005).
Hammond, M. A., Gallant, M. A., Burr, D. B. & Wallace, J. M. Nanoscale changes in collagen are reflected in physical and mechanical properties of bone at the microscale in diabetic rats. Bone 60, 26–32 (2013).
Gelb, J. Functionality to failure: materials engineering in the 4th dimension. Adv. Mater. Process. 170, 14–18 (2012).
Tkachuk, A. et al. X-ray computed tomography in Zernike phase contrast mode at 8 keV with 50-nm resolution using Cu rotating anode X-ray source. Z. Kristallogr. 222, 650–655 (2007).
Gelb, J. et al. Non-destructive local X-ray tomography for multi-length scale analysis of reservoir rocks: validations and observations. in Int. Symposium of the Society of Core Analysts. 1–6 (2012).
Wang, J. et al. Automated markerless full field hard x-ray microscopic tomography at sub-50 nm 3-dimension spatial resolution. Appl. Phys. Lett. 100, 143107 (2012).
Tian, Y. et al. High resolution hard x-ray microscope on a second generation synchrotron source. Rev. Sci. Instrum. 79, 103708/103701 (2008).
Chu, Y. S. et al. Hard-x-ray microscopy with Fresnel zone plates reaches 40 nm Rayleigh resolution. Appl. Phys. Lett. 92, 103119 (2008).
Ladd, M. E. et al. Pros and cons of ultra-high-field MRI/MRS for human application. Progr. Nucl. Magn. Reson. Spectrosc. 109, 1–50 (2018).
van Wijk, D. F. et al. Increasing spatial resolution of 3T MRI scanning improves reproducibility of carotid arterial wall dimension measurements. MAGMA 27, 219–226 (2014).
Nowogrodzki, A. The world’s strongest MRI machines are pushing human imaging to new limits. Nature 563, 24–26 (2018).
Kraff, O. & Quick, H. H. 7T: Physics, safety, and potential clinical applications. J. Magn. Reson. Imaging 46, 1573–1589 (2017).
Krug, R., Stehling, C., Kelley, D. A. C., Majumdar, S. & Link, T. M. Imaging of the musculoskeletal system in vivo using ultra-high field magnetic resonance at 7 T. Invest. Radiol. 44, 613–618 (2009).
Chang, G. et al. MRI of the hip at 7T: feasibility of bone microarchitecture, high-resolution cartilage, and clinical imaging. J. Magn. Reson. Imaging 39, 1384–1393 (2014).
Chang, G. et al. 7T MRI detects deterioration in subchondral bone microarchitecture in subjects with mild knee osteoarthritis as compared with healthy controls. J. Magn. Reson. Imaging 41, 1311–1317 (2015).
Lazik, A. et al. 7 Tesla quantitative hip MRI: T1, T2 and T2* mapping of hip cartilage in healthy volunteers. Eur. Radiol. 26, 1245–1253 (2015).
Lazik-Palm, A. et al. Morphological and quantitative 7 T MRI of hip cartilage transplants in comparison to 3 T – initial experiences. Invest. Radiol. 51, 552–559 (2016).
Juras, V. et al. The compositional difference between ankle and knee cartilage demonstrated by T2 mapping at 7 Tesla MR. Eur. J. Radiol. 85, 771–777 (2016).
Krug, R. et al. In vivo ultra-high-field magnetic resonance imaging of trabecular bone microarchitecture at 7 T. J. Magn. Reson. Imaging 27, 854–859 (2008).
Wright, A. C. et al. Helmholtz-pair transmit coil with integrated receive array for high-resolution MRI of trabecular bone in the distal tibia at 7T. J. Magn. Reson. 210, 113–122 (2011).
Griffin, L. M. et al. 7T MRI of distal radius trabecular bone microarchitecture: how trabecular bone quality varies depending on distance from end-of-bone. J. Magn. Reson. Imaging 45, 872–878 (2016).
Bhagat, Y. A. et al. Performance of μMRI-based virtual bone biopsy for structural and mechanical analysis at the distal tibia at 7T field strength. J. Magn. Reson. Imaging 33, 372–381 (2011).
Chang, G. et al. In vivo estimation of bone stiffness at the distal femur and proximal tibia using ultra-high-field 7-Tesla magnetic resonance imaging and micro-finite element analysis. J. Bone Miner. Metab. 30, 243–251 (2011).
Weiger, M., Stampanoni, M. & Pruessmann, K. P. Direct depiction of bone microstructure using MRI with zero echo time. Bone 54, 44–47 (2013).
Wehrli, F. W. & Fernandez-Seara, M. A. Nuclear magnetic resonance studies of bone water. Ann. Biomed. Eng. 33, 79–86 (2005).
Jara, H., Wehrli, F. W., Chung, H. & Ford, J. C. High-resolution variable flip angle 3D MR imaging of trabecular microstructure in vivo. Magn. Reson. Med. 29, 528–539 (1993).
Liu, X. S. et al. Accuracy of high-resolution in vivo micro magnetic resonance imaging for measurements of microstructural and mechanical properties of human distal tibial bone. J. Bone Miner. Res. 25, 2039–2050 (2010).
Anumula, S., Wehrli, S. L., Magland, J., Wright, A. C. & Wehrli, F. W. Ultra-short echo-time MRI detects changes in bone mineralization and water content in OVX rat bone in response to alendronate treatment. Bone 46, 1391–1399 (2010).
Krug, R. et al. Ultrashort echo time MRI of cortical bone at 7 Tesla field strength: a feasibility study. J. Magn. Reson. Imaging 34, 691–695 (2011).
Lazik-Palm, A. et al. Morphological imaging and T2 and T2* mapping of hip cartilage at 7 Tesla MRI under the influence of intravenous gadolinium. Eur. Radiol. 26, 3923–3931 (2016).
Rietsch, S. H. G. et al. An 8-channel transceiver 7-channel receive RF coil setup for high SNR ultrahigh-field MRI of the shoulder at 7T. Med. Phys. 44, 6195–6208 (2017).
Johst, S., Wrede, K. H., Ladd, M. E. & Maderwald, S. Time-of-flight magnetic resonance angiography at 7 T using venous saturation pulses with reduced flip angles. Invest. Radiol. 47, 445–450 (2012).
Mattern, H. et al. Prospective motion correction enables highest resolution time-of-flight angiography at 7T. Magn. Reson. Med. 80, 248–258 (2017).
Fu, R. et al. Ultra-wide bore 900MHz high-resolution NMR at the national high magnetic field laboratory. J. Magn. Reson. 177, 1–8 (2005).
Schepkin, V. D., Brey, W. W., Gor’kov, P. L. & Grant, S. C. Initial in vivo rodent sodium and proton MR imaging at 21.1 T. Magn. Reson. Imaging 28, 400–407 (2010).
Budinger, T. F. & Bird, M. D. MRI and MRS of the human brain at magnetic fields of 14 T to 20 T: technical feasibility, safety, and neuroscience horizons. NeuroImage 168, 509–531 (2018).
Budinger, T. F. et al. Toward 20 T magnetic resonance for human brain studies: opportunities for discovery and neuroscience rationale. Magn. Reson. Mater. Phys. Biol. Med. 29, 617–639 (2016).
Schulz, R. B. & Semmler, W. Fundamentals of optical imaging Handb. Exp. Pharmacol. 185, 3–22 (2008).
Hell, S. W. Far-field optical nanoscopy. Science 316, 1153–1158 (2007).
Sigal, Y. M., Zhou, R. & Zhuang, X. Visualizing and discovering cellular structures with super-resolution microscopy. Science 361, 880–887 (2018).
Adams, M. W., Loftus, A. F., Dunn, S. E., Joens, M. S. & Fitzpatrick, J. A. Light sheet fluorescence microscopy (LSFM). Curr. Protoc. Cytom. 71, 12 37 11–12 37 15 (2015).
Santi, P. A. Light sheet fluorescence microscopy: a review. J. Histochem. Cytochem. 59, 129–138 (2011).
Keller, P. J. & Stelzer, E. H. Quantitative in vivo imaging of entire embryos with digital scanned laser light sheet fluorescence microscopy. Curr. Opin. Neurobiol. 18, 624–632 (2008).
Richardson, D. S. & Lichtman, J. W. Clarifying tissue clearing. Cell 162, 246–257 (2015).
Klingberg, A. et al. Fully automated evaluation of total glomerular number and capillary tuft size in nephritic kidneys using lightsheet microscopy. J. Am. Soc. Nephrol. 28, 452–459 (2017).
Chatterjee, K., Pratiwi, F. W., Wu, F. C. M., Chen, P. & Chen, B. C. Recent progress in light sheet microscopy for biological applications. Appl. Spectrosc. 72, 1137–1169 (2018).
Keller, P. J., Schmidt, A. D., Wittbrodt, J. & Stelzer, E. H. Digital scanned laser light-sheet fluorescence microscopy (DSLM) of zebrafish and Drosophila embryonic development. Cold Spring Harb. Protoc. 2011, 1235–1243 (2011).
Kromm, D., Thumberger, T. & Wittbrodt, J. An eye on light-sheet microscopy. Methods Cell Biol. 133, 105–123 (2016).
Dean, K. M. & Fiolka, R. Uniform and scalable light-sheets generated by extended focusing. Opt. Express 22, 26141–26152 (2014).
Dean, K. M., Roudot, P., Welf, E. S., Danuser, G. & Fiolka, R. Deconvolution-free subcellular imaging with axially swept light sheet microscopy. Biophys. J. 108, 2807–2815 (2015).
Genina, E. A., Bashkatov, A. N. & Tuchin, V. V. Optical clearing of cranial bone. Adv. Opt. Technol. 2008, 1–8 (2008).
Vigouroux, R. J., Belle, M. & Chedotal, A. Neuroscience in the third dimension: shedding new light on the brain with tissue clearing. Mol. Brain 10, 33 (2017).
Zundler, S. et al. Three-dimensional cross-sectional light-sheet microscopy imaging of the inflamed mouse gut. Gastroenterology 153, 898–900 (2017).
Männ, L. et al. CD11c.DTR mice develop a fatal fulminant myocarditis after local or systemic treatment with diphtheria toxin. Eur. J. Immunol. 46, 2028–2042 (2016).
Tuchin, V. V. Optical clearing of tissues and blood using the immersion method. J. Phys. D: Appl. Phys. 38, 2497–2518 (2005).
Ariel, P. A beginner’s guide to tissue clearing. Int. J. Biochem. Cell Biol. 84, 35–39 (2017).
Renier, N. et al. iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell 159, 896–910 (2014).
Becker, K., Jahrling, N., Saghafi, S., Weiler, R. & Dodt, H. U. Chemical clearing and dehydration of GFP expressing mouse brains. PLOS ONE 7, e33916 (2012).
Becker, K., Jahrling, N., Kramer, E. R., Schnorrer, F. & Dodt, H. U. Ultramicroscopy: 3D reconstruction of large microscopical specimens. J. Biophotonics 1, 36–42 (2008).
Schwarz, M. K. et al. Fluorescent-protein stabilization and high-resolution imaging of cleared, intact mouse brains. PLOS ONE 10, e0124650 (2015).
Zukor, K. A., Kent, D. T. & Odelberg, S. J. Fluorescent whole-mount method for visualizing three-dimensional relationships in intact and regenerating adult newt spinal cords. Dev. Dyn. 239, 3048–3057 (2010).
Acar, M. et al. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature 526, 126–130 (2015).
Chen, J. Y. et al. Hoxb5 marks long-term haematopoietic stem cells and reveals a homogenous perivascular niche. Nature 530, 223–227 (2016).
de Saint-Georges, L. & Miller, S. C. The microcirculation of bone and marrow in the diaphysis of the rat hemopoietic long bones. Anat. Rec. 233, 169–177 (1992).
Herisson, F. et al. Direct vascular channels connect skull bone marrow and the brain surface enabling myeloid cell migration. Nat. Neurosci. 21, 1209–1217 (2018).
Augustin, H. G. & Koh, G. Y. Organotypic vasculature: from descriptive heterogeneity to functional pathophysiology. Science 357, eaal2379 (2017).
Ramasamy, S. K. et al. Blood flow controls bone vascular function and osteogenesis. Nat. Commun. 7, 13601 (2016).
Ramasamy, S. K. Structure and functions of blood vessels and vascular niches in bone. Stem Cells Int. 2017, 5046953 (2017).
Jonkman, J. & Brown, C. M. Any way you slice it – a comparison of confocal microscopy techniques. J. Biomol. Tech. 26, 54–65 (2015).
Takaku, T. et al. Hematopoiesis in 3 dimensions: human and murine bone marrow architecture visualized by confocal microscopy. Blood 116, e41–e55 (2010).
Morrison, S. J. & Scadden, D. T. The bone marrow niche for haematopoietic stem cells. Nature 505, 327–334 (2014).
Kusumbe, A. P., Ramasamy, S. K. & Adams, R. H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 507, 323–328 (2014).
Ramasamy, S. K., Kusumbe, A. P., Wang, L. & Adams, R. H. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature 507, 376–380 (2014).
Kunisaki, Y. et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 502, 637–643 (2013).
Mizuhashi, K. et al. Resting zone of the growth plate houses a unique class of skeletal stem cells. Nature 563, 254–258 (2018).
Cahalan, M. D., Parker, I., Wei, S. H. & Miller, M. J. Two-photon tissue imaging: seeing the immune system in a fresh light. Nat. Rev. Immunol. 2, 872–880 (2002).
Erturk, A. et al. Three-dimensional imaging of solvent-cleared organs using 3DISCO. Nat. Protoc. 7, 1983–1995 (2012).
Tanaka, K. et al. Intravital imaging of gastrointestinal diseases in preclinical models using two-photon laser scanning microscopy. Surg. Today 43, 123–129 (2013).
Phan, T. G. & Bullen, A. Practical intravital two-photon microscopy for immunological research: faster, brighter, deeper. Immunol. Cell Biol. 88, 438–444 (2010).
Perry, S. W., Burke, R. M. & Brown, E. B. Two-photon and second harmonic microscopy in clinical and translational cancer research. Ann. Biomed. Eng. 40, 277–291 (2012).
Bousso, P. & Moreau, H. D. Functional immunoimaging: the revolution continues. Nat. Rev. Immunol. 12, 858–864 (2012).
Germain, R. N., Robey, E. A. & Cahalan, M. D. A decade of imaging cellular motility and interaction dynamics in the immune system. Science 336, 1676–1681 (2012).
Niesner, R. A., Andresen, V. & Gunzer, M. Intravital 2-photon microscopy – focus on speed and time resolved imaging modalities. Immunol. Rev. 221, 7–25 (2008).
Wang, T. et al. Three-photon imaging of mouse brain structure and function through the intact skull. Nat. Methods 15, 789–792 (2018).
Carriles, R. et al. Invited review article: imaging techniques for harmonic and multiphoton absorption fluorescence microscopy. Rev. Sci. Instrum. 80, 081101 (2009).
Vielreicher, M. et al. Taking a deep look: modern microscopy technologies to optimize the design and functionality of biocompatible scaffolds for tissue engineering in regenerative medicine. J. R. Soc. Interface 10, 20130263 (2013).
Georgiadis, M., Muller, R. & Schneider, P. Techniques to assess bone ultrastructure organization: orientation and arrangement of mineralized collagen fibrils. J. R. Soc. Interface 13, 20160088 (2016).
Genthial, R. et al. Label-free imaging of bone multiscale porosity and interfaces using third-harmonic generation microscopy. Sci. Rep. 7, 3419 (2017).
Saitou, T., Kiyomatsu, H. & Imamura, T. Quantitative morphometry for osteochondral tissues using second harmonic generation microscopy and image texture information. Sci. Rep. 8, 2826 (2018).
Okada, T., Takahashi, S., Ishida, A. & Ishigame, H. In vivo multiphoton imaging of immune cell dynamics. Pflugers Arch. 468, 1793–1801 (2016).
Mazo, I. B. et al. Hematopoietic progenitor cell rolling in bone marrow microvessels: parallel contributions by endothelial selectins and vascular cell adhesion molecule 1. J. Exp. Med. 188, 465–474 (1998).
Vandoorne, K. et al. Imaging the vascular bone marrow niche during inflammatory stress. Circ. Res. 123, 415–427 (2018).
Bixel, M. G. et al. Flow dynamics and HSPC homing in bone marrow microvessels. Cell. Rep. 18, 1804–1816 (2017).
Hasenberg, A. et al. Catchup: a mouse model for imaging-based tracking and modulation of neutrophil granulocytes. Nat. Methods 12, 445–452 (2015).
Spencer, J. A. et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 508, 269–273 (2014).
Köhler, A. et al. G-CSF mediated thrombopoietin release triggers neutrophil motility and mobilization from bone marrow via induction of Cxcr2 ligands. Blood 117, 4349–4357 (2011).
Junt, T. et al. Dynamic visualization of thrombopoiesis within bone marrow. Science 317, 1767–1770 (2007).
Massberg, S. et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell 131, 994–1008 (2007).
Devi, S. et al. Neutrophil mobilization via plerixafor-mediated CXCR4 inhibition arises from lung demargination and blockade of neutrophil homing to the bone marrow. J. Exp. Med. 210, 2321–2336 (2013).
Lassailly, F., Foster, K., Lopez-Onieva, L., Currie, E. & Bonnet, D. Multimodal imaging reveals structural and functional heterogeneity in different bone marrow compartments: functional implications on hematopoietic stem cells. Blood 122, 1730–1740 (2013).
Köhler, A. et al. Altered cellular dynamics and endosteal location of aged early hematopoietic progenitor cells revealed by time-lapse intravital imaging in long bones. Blood 114, 290–298 (2009).
Kiel, M. J., Iwashita, T., Yilmaz, O. H. & Morrison, S. J. Spatial differences in hematopoiesis but not in stem cells indicate a lack of regional patterning in definitive hematopoietic stem cells. Dev. Biol. 283, 29–39 (2005).
Chan, C. K. et al. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature 457, 490–494 (2009).
Nombela-Arrieta, C. & Manz, M. G. Quantification and three-dimensional microanatomical organization of the bone marrow. Blood Adv. 1, 407–416 (2017).
Otto, L., Zelinskyy, G., Schuster, M., Dittmer, U. & Gunzer, M. Imaging of cytotoxic antiviral immunity while considering the 3R principle of animal research. J. Mol. Med. 96, 349–360 (2018).
Mill, L. et al. In Bildverarbeitung für die Medizin 2018. Springer, 115–120 (2018).
Aichert, A. et al. Epipolar consistency in transmission imaging. IEEE Trans Med Imaging 34, 2205–2219 (2015).
Bier, B. et al. X-ray-transform invariant anatomical landmark detection for pelvic trauma surgery. arXiv e-prints https://arxiv.org/abs/1803.08608 (2018).
Choi, J. H. et al. Fiducial marker-based correction for involuntary motion in weight-bearing C-arm CT scanning of knees. II. Experiment. Med. Phys. 41, 061902 (2014).
Pan, X., Sidky, E. Y. & Vannier, M. Why do commercial CT scanners still employ traditional, filtered back-projection for image reconstruction? Inverse Probl. 25, 1230009 (2009).
Würfl, T., Ghesu, F. C., Christlein, V. & Maier, A. in Medical Image Computing and Computer-Assisted Intervention – MICCAI 2016. 432–440 (2016).
Zhu, B., Liu, J. Z., Cauley, S. F., Rosen, B. R. & Rosen, M. S. Image reconstruction by domain-transform manifold learning. Nature 555, 487–492 (2018).
Ye, J. C., Han, Y. & Cha, E. Deep convolutional framelets: a general deep learning framework for inverse problems. arXiv e-prints https://arxiv.org/abs/1707.00372 (2017).
Kobler, E., Klatzer, T., Hammernik, K. & Pock, T. Variational networks: connecting variational methods and deep learning, in German Conference on Pattern Recognition. Springer, 281–293 (2017).
Halabi, S. S. et al. The RSNA pediatric bone age machine learning challenge. Radiology 290, 498–503 (2018).
Ellmann, S. et al. Prediction of early metastatic disease in experimental breast cancer bone metastasis by combining PET/CT and MRI parameters to a model-averaged neural network. Bone 120, 254–261 (2018).
Yune, S. et al. Beyond human perception: sexual dimorphism in hand and wrist radiographs is discernible by a deep learning model. J. Digit. Imaging https://doi.org/10.1007/s10278-018-0148-x (2018).
Maier, A., Syben, C., Lasser, T. & Riess, C. A gentle introduction to deep learning in medical image processing. Zeitschrift für Medizinische Physik 29, 86–101 (2018).
Maier, A. et al. Precision learning: towards use of known operators in neural networks. arXiv e-prints https://arxiv.org/abs/1712.00374 (2017).
Andrews, J. C. et al. Nanoscale X-ray microscopic imaging of mammalian mineralized tissue. Microsc. Microanal. 16, 327–336 (2010).
Stelzer, E. H. Light-sheet fluorescence microscopy for quantitative biology. Nat. Methods 12, 23–26 (2014).
Pan, C. et al. Shrinkage-mediated imaging of entire organs and organisms using uDISCO. Nat. Methods 13, 859–867 (2016).
Moser, E., Stadlbauer, A., Windischberger, C., Quick, H. H. & Ladd, M. E. Magnetic resonance imaging methodology. Eur. J. Nucl. Med. Mol. Imaging 36 (Suppl. 1), S30–S41 (2009).
Acknowledgements
The authors thank the IMaging Centre ESsen, the Optical Imaging Centre Erlangen and the Erwin L. Hahn Institute for Magnetic Resonance Imaging at University Duisburg-Essen for support with imaging. The authors’ work is supported by funding from the German Research Foundation (SPP1480 Immunobone), to M.G. and G.S.; the Collaborative Research Centre (CRC) 1181, to G.S.; and the European Union (EU HEALTH-2013-INNOVATION-1, MATHIAS), to M.G.. The work of S.K., A.M. and G.S. is also supported by the European Research Council (ERC) Synergy grant NanoScope (grant no. 810316) and the work of G.S. is also supported by the Innovative Medicine Initiative (IMI)-funded project RTCure.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all aspects of the manuscript, including researching data for the article, providing substantial contributions to discussions of its content, writing the article, and reviewing and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Glossary
- Haversian system
-
Structural unit of bone consisting of a central Haversian canal, which surrounds blood vessels and nerves, surrounded by concentric rings called lamellae.
- Lacuno-canalicular network
-
(LCN). A system of sub-micrometre-sized channels (canaliculi) inside cortical bone that form a network with osteocyte-containing lacunae, through which osteocyte membrane processes make contact with other osteocytes or endothelial cells of trans-cortical blood vessels.
- Pixels
-
Elements (or ‘picture elements’) of a 2D digital image, typically numbering in the hundreds of thousands or millions and arranged in rows and columns. Each pixel has a level of brightness (grey level) and, for coloured images, a combination of several (typically three) colour components.
- Voxels
-
Similar to a pixel, a voxel (or ‘volume pixel’) is an image element in a 3D image such as a tomographic scan.
- Synchrotron-based imaging
-
Imaging modality comprising X-ray-based CT coupled to a powerful source of X-rays (synchrotron).
- Absorption coefficient
-
Describes how easily a tissue can be penetrated by X-ray radiation, expressed as the fraction of an X-ray beam that is absorbed or scattered per unit of thickness.
- Hounsfield units
-
(HU). Values representing linearly transformed absorption coefficients, used to standardize CT scanners; by definition, the radiodensity of water is zero HU and air negative 1,000 HU. Bone is between +500 and +1,500 HU and fat is negative ~100 HU.
- Calibration phantom
-
An object containing artificial materials with known physical density and X-ray absorption behaviour that is imaged by CT together with a patient to enable precise assessment of these properties in the patient´s tissue.
- Areal BMD
-
A measurement of bone mineral content, determined by dividing the amount of bone mineral (in grams) by the area of the 2D bone site scanned (in square centimetres).
- Kernels
-
In the context of CT image reconstruction, a special function that can be varied within a certain range in order to tune the pixel noise and the geometrical resolution.
- Partial volume artefacts
-
Occur when a CT voxel encompasses tissues with different absorptions, so that the beam attenuation represents the average value of these tissues.
- Fresnel zone plate
-
A device to focus X-rays, consisting of a group of radially symmetrical, alternately opaque and transparent rings (zones); an X-ray wave hitting the zone plate diffracts around the opaque zones, and the zones can be engineered in such a way that the waves are focused.
- Tesla
-
(T). An SI unit that describes the strength of a magnetic field. 1 Tesla is ~20,000 times larger than Earth’s natural magnetic field.
- Signal-to-noise ratio
-
(SNR). Describes the ratio of the power of an anticipated signal to the power of background noise.
- Ultra-short echo time
-
Technique used in MRI sequences that enables visualization of tissues with very short transverse relaxation times by starting spatial encoding and data acquisition as soon as possible after the radiofrequency pulse.
- Zero echo time
-
Technique used in MRI sequences that enables visualization of tissues with very short transverse relaxation times by starting spatial encoding before the radiofrequency pulse and starting data acquisition as soon as possible after the radiofrequency pulse.
- Time-of-flight magnetic resonance angiography
-
(TOF MRA). An MRI technique used for high-resolution imaging of blood vessels, based on the principle that, when using short echo times, unsaturated blood entering the imaging slice gives a much higher (brighter) signal than the surrounding static tissue, which is saturated and thus remains dark.
- Refractive index
-
(RI). A descriptor of how fast light propagates through a material, expressed as the ratio of the velocity of light in a vacuum to its velocity in that material.
- Excitation maximum
-
A fluorophore fluoresces when its electrons absorb incoming photons (excitation) and then return to their original energy level, releasing excess energy in the form of a red-shifted photon; the excitation maximum is the optimal energy (i.e. wavelength or colour) that incoming photons must have to make this process most effective.
Rights and permissions
About this article
Cite this article
Grüneboom, A., Kling, L., Christiansen, S. et al. Next-generation imaging of the skeletal system and its blood supply. Nat Rev Rheumatol 15, 533–549 (2019). https://doi.org/10.1038/s41584-019-0274-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-019-0274-y
This article is cited by
-
Osteocyte mitochondria regulate angiogenesis of transcortical vessels
Nature Communications (2024)
-
Bone disease imaging through the near-infrared-II window
Nature Communications (2023)
-
Ultrafast (600 ps) α-ray scintillators
PhotoniX (2022)
-
Fast volumetric scanning of living tissue
Nature Biomedical Engineering (2022)
-
Intravital microscopy imaging of kidney injury and regeneration
Renal Replacement Therapy (2021)