Abstract
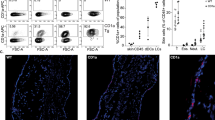
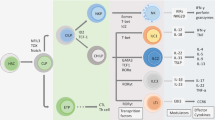
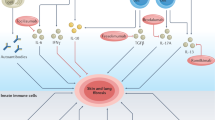
Cutaneous lupus erythematosus (CLE) is an autoimmune disease that can present as an isolated skin disease or as a manifestation within the spectrum of systemic lupus erythematosus. The clinical spectrum of CLE is broad, ranging from isolated discoid plaques to widespread skin lesions. Histologically, skin lesions present as interface dermatitis (inflammation of the skin mediated by anti-epidermal responses), which is orchestrated by type I and type III interferon-regulated cytokines and chemokines. Both innate and adaptive immune pathways are strongly activated in the formation of skin lesions owing to continuous re-activation of innate pathways via pattern recognition receptors (PRRs). These insights into the molecular pathogenesis of skin lesions in CLE have improved our understanding of the mechanisms underlying established therapies and have triggered the development of targeted treatment strategies that focus on immune cells (for example, B cells, T cells or plasmacytoid dendritic cells), as well as immune response pathways (for example, PRR signalling, Janus kinase (JAK)–signal transducer and activator of transcription (STAT) signalling and nuclear factor-κB signalling) and their cytokines and chemokines (for example, type I interferons, CXC-chemokine ligand 10 (CXCL10), IL-6 and IL-12).
Key points
-
Cutaneous lupus erythematosus (CLE) occurs as isolated skin disease or in the context of systemic lupus erythematosus.
-
Skin lesions in CLE are characterized by an interferon-orchestrated cytotoxic anti-epidermal immune response (known as interface dermatitis).
-
Genetic variations in immune-regulation genes (such as genes involved in the type I interferon pathway, cell death, clearance of cell debris, antigen presentation, antibody production and immune cell regulation) predispose individuals to CLE.
-
The chronic pathological cycle of CLE is fuelled by a continuous re-activation of innate immune pathways through adaptive effector mechanisms.
-
Pharmacological inhibition of both adaptive and innate immune responses can be effective in the treatment of patients with CLE.
-
New treatment strategies are being developed that mainly target type I interferon-producing cells (such as plasmacytoid dendritic cells) and their pathways (such as IFNAR or Janus kinase signalling).
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kuhn, A., Wenzel, J. & Bijl, M. Lupus erythematosus revisited. Semin. Immunopathol. 38, 97–112 (2016).
Stannard, J. N. & Kahlenberg, J. M. Cutaneous lupus erythematosus: updates on pathogenesis and associations with systemic lupus. Curr. Opin. Rheumatol. 28, 453–459 (2016).
Hejazi, E. Z. & Werth, V. P. Cutaneous lupus erythematosus: an update on pathogenesis, diagnosis and treatment. Am. J. Clin. Dermatol. 17, 135–146 (2016).
Kuhn, A., Rondinone, R., Doria, A. & Shoenfeld, Y. 1st international conference on cutaneous lupus erythematosus Düsseldorf, Germany, September 1–5, 2004. Autoimmun. Rev. 4, 66–78 (2005).
Gilliam, J. N. & Sontheimer, R. D. Distinctive cutaneous subsets in the spectrum of lupus erythematosus. J. Am. Acad. Dermatol. 4, 471–475 (1981).
Grönhagen, C. M., Fored, C. M., Granath, F. & Nyberg, F. Cutaneous lupus erythematosus and the association with systemic lupus erythematosus: a population-based cohort of 1088 patients in Sweden. Br. J. Dermatol. 164, 1335–1341 (2011).
Biazar, C. et al. Cutaneous lupus erythematosus: first multicenter database analysis of 1002 patients from the European Society of Cutaneous Lupus Erythematosus (EUSCLE). Autoimmun. Rev. 12, 444–454 (2013).
Jarukitsopa, S. et al. Epidemiology of systemic lupus erythematosus and cutaneous lupus erythematosus in a predominantly white population in the United States. Arthritis Care Res. 67, 817–828 (2015).
Durosaro, O., Davis, M. D. P., Reed, K. B. & Rohlinger, A. L. Incidence of cutaneous lupus erythematosus, 1965–2005: a population-based study. Arch. Dermatol. 145, 249–253 (2009).
Patel, P. & Werth, V. Cutaneous lupus erythematosus: a review. Dermatol. Clin. 20, 373–385 (2002).
Lipsker, D. The need to revisit the nosology of cutaneous lupus erythematosus: the current terminology and morphologic classification of cutaneous LE: difficult, incomplete and not always applicable. Lupus 19, 1047–1049 (2010).
Lin, J. H., Dutz, J. P., Sontheimer, R. D. & Werth, V. P. Pathophysiology of cutaneous lupus erythematosus. Clin. Rev. Allergy Immunol. 33, 85–106 (2007).
Wieczorek, I. T., Propert, K. J., Okawa, J. & Werth, V. P. Systemic symptoms in the progression of cutaneous to systemic lupus erythematosus. JAMA Dermatol. 150, 291–296 (2014).
Sticherling, M. Kutaner lupus erythematodes und Hautveränderungen beim systemischen lupus erythematodes [German]. Z. Rheumatol. 72, 429–435 (2013).
Kuhn, A. & Landmann, A. The classification and diagnosis of cutaneous lupus erythematosus. J. Autoimmun. 48-49, 14–19 (2014).
Uva, L. et al. Cutaneous manifestations of systemic lupus erythematosus. Autoimmune Dis. 2012, 834291 (2012).
Wenzel, J. et al. The expression pattern of interferon-inducible proteins reflects the characteristic histological distribution of infiltrating immune cells in different cutaneous lupus erythematosus subsets. Br. J. Dermatol. 157, 752–757 (2007).
Wenzel, J. & Tuting, T. An IFN-associated cytotoxic cellular immune response against viral, self-, or tumor antigens is a common pathogenetic feature in “interface dermatitis”. J. Invest. Dermatol. 128, 2392–2402 (2008).
Lauffer, F. et al. Type I immune response induces keratinocyte necroptosis and is associated with interface dermatitis. J. Invest. Dermatol. 38, 1785–1794 (2018).
Obermoser, G., Sontheimer, R. D. & Zelger, B. Overview of common, rare and atypical manifestations of cutaneous lupus erythematosus and histopathological correlates. Lupus 19, 1050–1070 (2010).
Scholtissek, B. et al. Immunostimulatory endogenous nucleic acids drive the lesional inflammation in cutaneous lupus erythematosus. J. Invest. Dermatol. 137, 1484–1492 (2017).
Sarkar, M. K. et al. Photosensitivity and type I IFN responses in cutaneous lupus are driven by epidermal-derived interferon kappa. Ann. Rheum. Dis. 77, 1653–1664 (2018).
Zahn, S. et al. Evidence for a pathophysiological role of keratinocyte-derived type III interferon (IFNλ) in cutaneous lupus erythematosus. J. Invest. Dermatol. 131, 133–140 (2011).
Wenzel, J., Zahn, S., Bieber, T. & Tuting, T. Type I interferon-associated cytotoxic inflammation in cutaneous lupus erythematosus. Arch. Dermatol. Res. 301, 83–86 (2009).
Kuhn, A. et al. S2k guideline for treatment of cutaneous lupus erythematosus-guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV). J. Eur. Acad. Dermatol. Venereol. 31, 389–404 (2017).
Filotico, R. & Mastrandrea, V. Cutaneous lupus erythematosus: clinico-pathologic correlation. G. Ital. Dermatol. Venereol. 153, 216–229 (2018).
Sunderkötter, C. H. et al. Nomenclature of cutaneous vasculitis: dermatologic addendum to the 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Arthritis Rheumatol. 70, 171–184 (2018).
Udompanich, S., Chanprapaph, K. & Suchonwanit, P. Hair and scalp changes in cutaneous and systemic lupus erythematosus. Am. J. Clin. Dermatol. 19, 679–694 (2018).
Reich, A., Meurer, M., Viehweg, A. & Muller, D. J. Narrow-band UVB-induced externalization of selected nuclear antigens in keratinocytes: implications for lupus erythematosus pathogenesis. Photochem. Photobiol. 85, 1–7 (2009).
Casciola-Rosen, L. & Rosen, A. Ultraviolet light-induced keratinocyte apoptosis: a potential mechanism for the induction of skin lesions and autoantibody production in LE. Lupus 6, 175–180 (1997).
Zhang, Y.-P., Wu, J., Han, Y.-F., Shi, Z.-R. & Wang, L. Pathogenesis of cutaneous lupus erythema associated with and without systemic lupus erythema. Autoimmun. Rev. 16, 735–742 (2017).
Wenzel, J. et al. Scarring skin lesions of discoid lupus erythematosus are characterized by high numbers of skin-homing cytotoxic lymphocytes associated with strong expression of the type I interferon-induced protein MxA. Br. J. Dermatol. 153, 1011–1015 (2005).
Mak, A. & Kow, N. Y. The pathology of T cells in systemic lupus erythematosus. J. Immunol. Res. 2014, 419029 (2014).
Grassi, M., Capello, F., Bertolino, L., Seia, Z. & Pippione, M. Identification of granzyme B-expressing CD-8-positive T cells in lymphocytic inflammatory infiltrate in cutaneous lupus erythematosus and in dermatomyositis. Clin. Exp. Dermatol. 34, 910–914 (2009).
Herrada, A. A. et al. Innate immune cells’ contribution to systemic lupus erythematosus. Front. Immunol. 10, 772 (2019).
Sestak, A. L., Fürnrohr, B. G., Harley, J. B., Merrill, J. T. & Namjou, B. The genetics of systemic lupus erythematosus and implications for targeted therapy. Ann. Rheum. Dis. 70, i37–i43 (2011).
Peschke, K. et al. Deregulated type I IFN response in TREX1-associated familial chilblain lupus. J. Invest. Dermatol. 134, 1456–1459 (2014).
Hersh, A. O., Arkin, L. M. & Prahalad, S. Immunogenetics of cutaneous lupus erythematosus. Curr. Opin. Pediatr. 28, 470–475 (2016).
Foering, K. et al. Characterization of clinical photosensitivity in cutaneous lupus erythematosus. J. Am. Acad. Dermatol. 69, 205–213 (2013).
Gehrke, N. et al. Oxidative damage of DNA confers resistance to cytosolic nuclease TREX1 degradation and potentiates STING-dependent immune sensing. Immunity 39, 482–495 (2013).
Kaczmarczyk-Sekuła, K. et al. Mast cells in systemic and cutaneous lupus erythematosus. Pol. J. Pathol. 4, 397–402 (2015).
Gerl, V. et al. The intracellular 52-kd Ro/SSA autoantigen in keratinocytes is up-regulated by tumor necrosis factor α via tumor necrosis factor receptor I. Arthritis Rheum. 52, 531–538 (2005).
Patsinakidis, N. et al. Suppression of UV-induced damage by a liposomal sunscreen: a prospective, open-label study in patients with cutaneous lupus erythematosus and healthy controls. Exp. Dermatol. 21, 958–961 (2012).
Katayama, S. et al. Delineating the healthy human skin UV response and early induction of interferon pathway in cutaneous lupus erythematosus. J. Invest. Dermatol. https://doi.org/10.1016/j.jid.2019.02.035 (2019).
Chang, J. & Werth, V. P. Therapeutic options for cutaneous lupus erythematosus: recent advances and future prospects. Expert Rev. Clin. Immunol. 12, 1109–1121 (2016).
Piette, E. W. et al. Impact of smoking in cutaneous lupus erythematosus. Arch. Dermatol. 148, 317–322 (2012).
White, P. C. et al. Cigarette smoke modifies neutrophil chemotaxis, neutrophil extracellular trap formation and inflammatory response-related gene expression. J. Periodont. Res. 53, 525–535 (2018).
Vaglio, A. et al. Drug-induced lupus: traditional and new concepts. Autoimmun. Rev. 17, 912–918 (2018).
Sandholdt, L. H., Laurinaviciene, R. & Bygum, A. Proton pump inhibitor-induced subacute cutaneous lupus erythematosus. Br. J. Dermatol. 170, 342–351 (2014).
Biermann, M. H. C. et al. The role of dead cell clearance in the etiology and pathogenesis of systemic lupus erythematosus: dendritic cells as potential targets. Expert Rev. Clin. Immunol. 10, 1151–1164 (2014).
Shovman, O., Tamar, S., Amital, H., Watad, A. & Shoenfeld, Y. Diverse patterns of anti-TNF-α-induced lupus: case series and review of the literature. Clin. Rheumatol. 37, 563–568 (2018).
Levine, D., Switlyk, S. A. & Gottlieb, A. Cutaneous lupus erythematosus and anti-TNF-α therapy: a case report with review of the literature. J. Drugs Dermatol. 9, 1283–1287 (2010).
Fiorentino, D. F. The Yin and Yang of TNF-α inhibition. Arch. Dermatol. 143, 233–236 (2007).
Arrue, I., Saiz, A., Ortiz-Romero, P. L. & Rodríguez-Peralto, J. L. Lupus-like reaction to interferon at the injection site: report of five cases. J. Cutan. Pathol. 34, 18–21 (2007).
Curran, C. S., Gupta, S., Sanz, I. & Sharon, E. PD-1 immunobiology in systemic lupus erythematosus. J. Autoimmun. 97, 1–9 (2018).
Sinha, A. A. & Dey-Rao, R. Genomic investigation of lupus in the skin. J. Invest. Dermatol. Symp. Proc. 18, S75–S80 (2017).
Dey-Rao, R. & Sinha, A. A. Genome-wide transcriptional profiling data from skin of chronic cutaneous lupus erythematosus (CCLE) patients. Data Brief 4, 47–49 (2015).
Zahn, S. et al. Interferon-α stimulates TRAIL expression in human keratinocytes and peripheral blood mononuclear cells: implications for the pathogenesis of cutaneous lupus erythematosus. Br. J. Dermatol. 165, 1118–1123 (2011).
Muskardin, T. L. W. & Niewold, T. B. Type I interferon in rheumatic diseases. Nat. Rev. Rheumatol. 14, 214–228 (2018).
Yin, Q. et al. Ultraviolet B irradiation induces skin accumulation of plasmacytoid dendritic cells: a possible role for chemerin. Autoimmunity 47, 185–192 (2014).
Liu, L., Xu, G., Dou, H. & Deng, G.-M. The features of skin inflammation induced by lupus serum. Clin. Immunol. 165, 4–11 (2016).
Dudhgaonkar, S. et al. Selective IRAK4 inhibition attenuates disease in murine lupus models and demonstrates steroid sparing activity. J. Immunol. 198, 1308–1319 (2017).
Mande, P. et al. Fas ligand promotes an inducible TLR-dependent model of cutaneous lupus-like inflammation. J. Clin. Invest. 128, 2966–2978 (2018).
König, N. et al. Familial chilblain lupus due to a gain-of-function mutation in STING. Ann. Rheum. Dis. 76, 468–472 (2017).
Sabrautzki, S. et al. An ENU mutagenesis-derived mouse model with a dominant Jak1 mutation resembling phenotypes of systemic autoimmune disease. Am. J. Pathol. 183, 352–368 (2013).
Means, T. K. et al. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J. Clin. Invest. 115, 407–417 (2005).
Eloranta, M.-L. et al. Regulation of the interferon-α production induced by RNA-containing immune complexes in plasmacytoid dendritic cells. Arthritis Rheum. 60, 2418–2427 (2009).
Saadeh, D., Kurban, M. & Abbas, O. Update on the role of plasmacytoid dendritic cells in inflammatory/autoimmune skin diseases. Exp. Dermatol. 25, 415–421 (2016).
Liu, Z. & Davidson, A. Taming lupus — a new understanding of pathogenesis is leading to clinical advances. Nat. Med. 18, 871–882 (2012).
Rönnblom, L. The type I interferon system in the etiopathogenesis of autoimmune diseases. Ups. J. Med. Sci. 116, 227–237 (2011).
Kalali, B. N. et al. Double-stranded RNA induces an antiviral defense status in epidermal keratinocytes through TLR3-, PKR-, and MDA5/RIG-I-mediated differential signaling. J. Immunol. 181, 2694–2704 (2008).
Skouboe, M. K. et al. STING agonists enable antiviral cross-talk between human cells and confer protection against genital herpes in mice. PLOS Pathog. 14, e1006976 (2018).
Stannard, J. N. et al. Lupus skin is primed for IL-6 inflammatory responses through a keratinocyte-mediated autocrine type I interferon loop. J. Invest. Dermatol. 137, 115–122 (2017).
Liu, Y. et al. TWEAK/Fn14 activation participates in Ro52-mediated photosensitization in cutaneous lupus erythematosus. Front. Immunol. 8, 651 (2017).
Zahn, S. et al. Ultraviolet light protection by a sunscreen prevents interferon-driven skin inflammation in cutaneous lupus erythematosus. Exp. Dermatol. 23, 516–518 (2014).
Wenzel, J. et al. Enhanced type I interferon signalling promotes Th1-biased inflammation in cutaneous lupus erythematosus. J. Pathol. 205, 435–442 (2005).
Kuhn, A. et al. Accumulation of apoptotic cells in the epidermis of patients with cutaneous lupus erythematosus after ultraviolet irradiation. Arthritis Rheum. 54, 939–950 (2006).
Kuechle, M. K. & Elkon, K. B. Shining light on lupus and UV. Arthritis Res. Ther. 9, 101 (2007).
Mistry, P. & Kaplan, M. J. Cell death in the pathogenesis of systemic lupus erythematosus and lupus nephritis. Clin. Immunol. 185, 59–73 (2017).
Wang, D., Drenker, M., Eiz-Vesper, B., Werfel, T. & Wittmann, M. Evidence for a pathogenetic role of interleukin-18 in cutaneous lupus erythematosus. Arthritis Rheum. 58, 3205–3215 (2008).
Caneparo, V., Landolfo, S., Gariglio, M. & De Andrea, M. The absent in melanoma 2-like receptor IFN-inducible protein 16 as an inflammasome regulator in systemic lupus erythematosus: the dark side of sensing microbes. Front. Immunol. 9, 1180 (2018).
Kreuter, A. et al. Expression of antimicrobial peptides in different subtypes of cutaneous lupus erythematosus. J. Am. Acad. Dermatol. 65, 125–133 (2011).
Chamilos, G. et al. Cytosolic sensing of extracellular self-DNA transported into monocytes by the antimicrobial peptide LL37. Blood 120, 3699–3707 (2012).
Drake, L. A. et al. Guidelines of care for cutaneous lupus erythematosus. American Academy of Dermatology. J. Am. Acad. Dermatol. 34, 830–836 (1996).
Kuhn, A., Landmann, A. & Wenzel, J. Advances in the treatment of cutaneous lupus erythematosus. Lupus 25, 830–837 (2016).
Kuhn, A. et al. Photoprotective effects of a broad-spectrum sunscreen in ultraviolet-induced cutaneous lupus erythematosus: a randomized, vehicle-controlled, double-blind study. J. Am. Acad. Dermatol. 64, 37–48 (2011).
Kuhn, A. et al. Efficacy of tacrolimus 0.1% ointment in cutaneous lupus erythematosus: a multicenter, randomized, double-blind, vehicle-controlled trial. J. Am. Acad. Dermatol. 65, 54–64.e2 (2011).
Alves, P. et al. Quinacrine suppresses tumor necrosis factor-α and IFN-α in dermatomyositis and cutaneous lupus erythematosus. J. Investig. Dermatol. Symp. Proc. 18, S57–S63 (2017).
Kuznik, A. et al. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J. Immunol. 186, 4794–4804 (2011).
Yokogawa, N. et al. Effects of hydroxychloroquine in patients with cutaneous lupus erythematosus: a multicenter, double-blind, randomized, parallel-group trial. Arthritis Rheumatol. 69, 791–799 (2017).
Zeidi, M., Kim, H. J. & Werth, V. P. Increased myeloid dendritic cells and TNF-α expression predicts poor response to hydroxychloroquine in cutaneous lupus erythematosus. J. Invest. Dermatol. 139, 324–332 (2019).
Wenzel, J. Methotrexate in systemic lupus erythematosus. Lupus 14, 569 (2005).
Klebes, M., Wutte, N. & Aberer, E. Dapsone as second-line treatment for cutaneous lupus erythematosus? A retrospective analysis of 34 patients and a review of the literature. Dermatology 232, 91–96 (2016).
Shornick, J. K., Formica, N. & Parke, A. L. Isotretinoin for refractory lupus erythematosus. J. Am. Acad. Dermatol. 24, 49–52 (1991).
Chan, E. S. L. & Cronstein, B. N. Methotrexate—how does it really work? Nat. Rev. Rheumatol. 6, 175–178 (2010).
Thomas, S. et al. Methotrexate is a JAK/STAT pathway inhibitor. PLOS ONE 10, e0130078 (2015).
Klaeschen, A. S. & Wenzel, J. Upcoming therapeutic targets in cutaneous lupus erythematous. Expert Rev. Clin. Pharmacol. 9, 567–578 (2016).
Felten, R. et al. The 2018 pipeline of targeted therapies under clinical development for systemic lupus erythematosus: a systematic review of trials. Autoimmun. Rev. 17, 781–790 (2018).
Presto, J. K., Hejazi, E. Z. & Werth, V. P. Biological therapies in the treatment of cutaneous lupus erythematosus. Lupus 26, 115–118 (2017).
Hofmann, S. C., Leandro, M. J., Morris, S. D. & Isenberg, D. A. Effects of rituximab-based B-cell depletion therapy on skin manifestations of lupus erythematosus—report of 17 cases and review of the literature. Lupus 22, 932–939 (2013).
Berghen, N., Vulsteke, J.-B., Westhovens, R., Lenaerts, J. & De Langhe, E. Rituximab in systemic autoimmune rheumatic diseases: indications and practical use. Acta Clin. Belg. 74, 272–279 (2018).
Vital, E. M. et al. Brief report: responses to rituximab suggest B cell-independent inflammation in cutaneous systemic lupus erythematosus. Arthritis Rheumatol. 67, 1586–1591 (2015).
Vashisht, P., Borghoff, K. & O’Dell, J. R. Hearth-Holmes, M. Belimumab for the treatment of recalcitrant cutaneous lupus. Lupus 26, 857–864 (2017).
Iaccarino, L. et al. Effects of belimumab on flare rate and expected damage progression in patients with active systemic lupus erythematosus. Arthritis Care Res. (Hoboken) 69, 115–123 (2017).
European Medicines Agency. EU Clinical Trials Register https://www.clinicaltrialsregister.eu/ctr-search/trial/2017-003051-35/DE (2018).
Wenzel, J., Landmann, A., Vorwerk, G. & Kuhn, A. High expression of B lymphocyte stimulator in lesional keratinocytes of patients with cutaneous lupus erythematosus. Exp. Dermatol. 27, 95–97 (2018).
Merrill, J. T. et al. Efficacy and safety of atacicept in patients with systemic lupus erythematosus: results of a twenty-four-week, multicenter, randomized, double-blind, placebo-controlled, parallel-arm, phase IIb study. Arthritis Rheumatol. 70, 266–276 (2018).
Kohler, S. et al. Bortezomib in antibody-mediated autoimmune diseases (TAVAB): study protocol for a unicentric, non-randomised, non-placebo controlled trial. BMJ Open 9, e024523 (2019).
Shirley, M. Ixazomib: first global approval. Drugs 76, 405–411 (2016).
Alexander, T. et al. Proteasome inhibition with bortezomib induces a therapeutically relevant depletion of plasma cells in SLE but does not target their precursors. Eur. J. Immunol. 48, 1573–1579 (2018).
Aguayo-Leiva, I., Vano-Galvan, S., Carrillo-Gijon, R. & Jaén-Olasolo, P. Lupus tumidus induced by bortezomib not requiring discontinuation of the drug. J. Eur. Acad. Dermatol. Venereol. 24, 1363–1364 (2010).
Gensous, N. et al. T follicular helper cells in autoimmune disorders. Front. Immunol. 9, 1637 (2018).
Katsuyama, T., Tsokos, G. C. & Moulton, V. R. Aberrant T cell signaling and subsets in systemic lupus erythematosus. Front. Immunol. 9, 1088 (2018).
Dehesa, L., Abuchar, A., Nuno-Gonzalez, A., Vitiello, M. & Kerdel, F. A. The use of cyclosporine in dermatology. J. Drugs Dermatol. 11, 979–987 (2012).
Wu, Q. & Kuca, K. Metabolic pathway of cyclosporine A and its correlation with nephrotoxicity. Curr. Drug Metab. 20, 84–90 (2018).
Sin, F. E. & Isenberg, D. An evaluation of voclosporin for the treatment of lupus nephritis. Expert Opin. Pharmacother. 19, 1613–1621 (2018).
Spee-Mayer, C. Von et al. Low-dose interleukin-2 selectively corrects regulatory T cell defects in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 75, 1407–1415 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03312335 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02770170 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02804763 (2019).
Danion, F. et al. Efficacy of abatacept in systemic lupus erythematosus: a retrospective analysis of 11 patients with refractory disease. Lupus 25, 1440–1447 (2016).
Tarazi, M., Aiempanakit, K. & Werth, V. P. Subacute cutaneous lupus erythematosus and systemic lupus erythematosus associated with abatacept. JAAD Case Rep. 4, 698–700 (2018).
Biliouris, K. et al. A pre-clinical quantitative model predicts the pharmacokinetics/pharmacodynamics of an anti-BDCA2 monoclonal antibody in humans. J. Pharmacokinet. Pharmacodyn. 45, 817–827 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02847598 (2019).
Furie, R. et al. BIIB059, a monoclonal antibody targeting BDCA2, shows evidence of biological activity and early clinical proof of concept in subjects with active cutaneous LE. Ann. Rheum. Dis. 76, (Suppl. 2), 857 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03817424 (2019).
Kalunian, K. C. et al. A phase II study of the efficacy and safety of rontalizumab (rhuMAb interferon-α) in patients with systemic lupus erythematosus (ROSE). Ann. Rheum. Dis. 75, 196–202 (2016).
Merrill, J. T. et al. Safety profile and clinical activity of sifalimumab, a fully human anti-interferon α monoclonal antibody, in systemic lupus erythematosus: a phase I, multicentre, double-blind randomised study. Ann. Rheum. Dis. 70, 1905–1913 (2011).
Werth, V. P. et al. Brief report: pharmacodynamics, safety, and clinical efficacy of AMG 811, a human anti-interferon-γ antibody, in patients with discoid lupus erythematosus. Arthritis Rheumatol. 69, 1028–1034 (2017).
Furie, R. et al. Anifrolumab, an anti-interferon-α receptor monoclonal antibody, in moderate-to-severe systemic lupus erythematosus. Arthritis Rheumatol. 69, 376–386 (2017).
Santos, F. P. S. & Verstovsek, S. Efficacy of ruxolitinib for myelofibrosis. Expert Opin. Pharmacother. 15, 1465–1473 (2014).
Spoerl, S. et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood 123, 3832–3842 (2014).
Hornung, T. et al. Remission of recalcitrant dermatomyositis treated with ruxolitinib. N. Engl. J. Med. 371, 2537–2538 (2014).
Hornung, T., Wolf, D. & Wenzel, J. More on remission of recalcitrant dermatomyositis treated with ruxolitinib. N. Engl. J. Med. 372, 1273–1274 (2015).
Klaeschen, A. S., Wolf, D., Brossart, P., Bieber, T. & Wenzel, J. JAK inhibitor ruxolitinib inhibits the expression of cytokines characteristic of cutaneous lupus erythematosus. Exp. Dermatol. 26, 728–730 (2017).
Wenzel, J. et al. JAK1/2 inhibitor ruxolitinib controls a case of chilblain lupus erythematosus. J. Invest. Dermatol. 136, 1281–1283 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03159936 (2019).
Wallace, D. J. et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 392, 222–231 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03134222 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03920267 (2019).
Deng, G.-M., Liu, L., Bahjat, F. R., Pine, P. R. & Tsokos, G. C. Suppression of skin and kidney disease by inhibition of spleen tyrosine kinase in lupus-prone mice. Arthritis Rheum. 62, 2086–2092 (2010).
Deng, G.-M. & Tsokos, G. C. The role of Syk in cutaneous lupus erythematosus. Exp. Dermatol. 25, 674–675 (2016).
Braegelmann, C. et al. Spleen tyrosine kinase (SYK) is a potential target for the treatment of cutaneous lupus erythematosus patients. Exp. Dermatol. 25, 375–379 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02927457 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02936375 (2018).
Iwata, Y. et al. p38 mitogen-activated protein kinase contributes to autoimmune renal injury in MRL-Fas lpr mice. J. Am. Soc. Nephrol. 14, 57–67 (2003).
Jin, N. et al. The selective p38 mitogen-activated protein kinase inhibitor, SB203580, improves renal disease in MRL/lpr mouse model of systemic lupus. Int. Immunopharmacol. 11, 1319–1326 (2011).
Kuhn, A. et al. Fumaric acid ester treatment in cutaneous lupus erythematosus (CLE): a prospective, open-label, phase II pilot study. Lupus 25, 1357–1364 (2016).
Dey-Rao, R., Smith, J. R., Chow, S. & Sinha, A. A. Differential gene expression analysis in CCLE lesions provides new insights regarding the genetics basis of skin vs. systemic disease. Genomics 104, 144–155 (2014).
Norman, R., Greenberg, R. G. & Jackson, J. M. Case reports of etanercept in inflammatory dermatoses. J. Am. Acad. Dermatol. 54, S139–S142 (2006).
Drosou, A., Kirsner, R. S., Welsh, E., Sullivan, T. P. & Kerdel, F. A. Use of infliximab, an anti-tumor necrosis α antibody, for inflammatory dermatoses. J. Cutan. Med. Surg. 7, 382–386 (2003).
Aringer, M. & Smolen, J. S. Efficacy and safety of TNF-blocker therapy in systemic lupus erythematosus. Expert Opin. Drug Saf. 7, 411–419 (2008).
European Medicines Agency. EU Clinical Trials Register https://www.clinicaltrialsregister.eu/ctr-search/trial/2015-001602-33/GB (2015).
Van Vollenhoven, R. F. et al. Efficacy and safety of ustekinumab, an IL-12 and IL-23 inhibitor, in patients with active systemic lupus erythematosus: results of a multicentre, double-blind, phase 2, randomised, controlled study. Lancet 392, 1330–1339 (2018).
Souza, A., De Ali-Shaw, T., Strober, B. E. & Franks, A. G. Successful treatment of subacute lupus erythematosus with ustekinumab. Arch. Dermatol. 147, 896–898 (2011).
Tierney, E., Kirthi, S., Ramsay, B. & Ahmad, K. Ustekinumab-induced subacute cutaneous lupus. JAAD Case Rep. 5, 271–273 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01405196 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01702740 (2012).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02437890 (2019).
Kunz, M. et al. Genome-wide association study identifies new susceptibility loci for cutaneous lupus erythematosus. Exp. Dermatol. 24, 510–515 (2015).
Millard, T. P. et al. A candidate gene analysis of three related photosensitivity disorders: cutaneous lupus erythematosus, polymorphic light eruption and actinic prurigo. Br. J. Dermatol. 145, 229–236 (2001).
Ruiz-Larrañaga, O. et al. Genetic association study of systemic lupus erythematosus and disease subphenotypes in European populations. Clin. Rheumatol. 35, 1161–1168 (2016).
Järvinen, T. M. et al. Tyrosine kinase 2 and interferon regulatory factor 5 polymorphisms are associated with discoid and subacute cutaneous lupus erythematosus. Exp. Dermatol. 19, 123–131 (2010).
Skonieczna, K. et al. Genetic similarities and differences between discoid and systemic lupus erythematosus patients within the Polish population. Postepy Dermatol. Alergol. 34, 228–232 (2017).
Levy, S. B., Pinnell, S. R., Meadows, L., Snyderman, R. & Ward, F. E. Hereditary C2 deficiency associated with cutaneous lupus erythematosus: clinical, laboratory, and genetic studies. Arch. Dermatol. 115, 57–61 (1979).
Agnello, V., Gell, J. & Tye, M. J. Partial genetic deficiency of the C4 component of complement in discoid lupus erythematosus and urticaria/angioedema. J. Am. Acad. Dermatol. 9, 894–898 (1983).
Racila, D. M. et al. Homozygous single nucleotide polymorphism of the complement C1QA gene is associated with decreased levels of C1q in patients with subacute cutaneous lupus erythematosus. Lupus 12, 124–132 (2003).
Lipsker, D. & Hauptmann, G. Cutaneous manifestations of complement deficiencies. Lupus 19, 1096–1106 (2010).
Sanchez, E. et al. Phenotypic associations of genetic susceptibility loci in systemic lupus erythematosus. Ann. Rheum. Dis. 70, 1752–1757 (2011).
Järvinen, T. M. et al. Polymorphisms of the ITGAM gene confer higher risk of discoid cutaneous than of systemic lupus erythematosus. PLOS ONE 5, e14212 (2010).
Da Silva Fonseca, A. M. et al. Polymorphisms in STK17A gene are associated with systemic lupus erythematosus and its clinical manifestations. Gene 527, 435–439 (2013).
Azevêdo Silva, J. De et al. Vitamin D receptor (VDR) gene polymorphisms and susceptibility to systemic lupus erythematosus clinical manifestations. Lupus 22, 1110–1117 (2013).
Zhong, H. et al. Replicated associations of TNFAIP3, TNIP1 and ETS1 with systemic lupus erythematosus in a southwestern Chinese population. Arthritis Res. Ther. 13, R186 (2011).
Vigato-Ferreira, I. C. C. et al. FcγRIIa and FcγRIIIb polymorphisms and associations with clinical manifestations in systemic lupus erythematosus patients. Autoimmunity 47, 451–458 (2014).
Harley, I. T. W. et al. The role of genetic variation near interferon-kappa in systemic lupus erythematosus. J. Biomed. Biotechnol. 2010, 706825 (2010).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03122431 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02176148 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03260166 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02428309 (2019).
European Medicines Agency. EU Clinical Trials Register https://www.clinicaltrialsregister.eu/ctr-search/trial/2017-001203-79/HU (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03288324 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03517722 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03866317 (2019).
European Medicines Agency. EU Clinical Trials Register https://www.clinicaltrialsregister.eu/ctr-search/trial/2016-003246-93/PL (2016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
J.W. declares that he has received financial support from GSK (for clinical studies, investigator-initiated trials and advisory board fees), Incyte (for investigator-initiated trials), Spirig (for an investigator-initiated trial), Medac (for advisory board fees), Actelion (for advisory board fees), Celgene (for advisory board fees), Biogen (for advisory board fees), Roche (for advisory board fees and clinical studies), Leo (for advisory board fees and clinical studies), Merck Serono (for clinical studies) and ArrayBio (for clinical studies).
Additional information
Peer review information
Nature Reviews Rheumatology thanks M. Caproni, F. Furukawa and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Interface dermatitis
-
Cytotoxic, anti-epithelial inflammation at the dermo-epidermal junction, characterized by hydropic degeneration, keratinocytic cell death and colloid bodies
- Papulosquamous
-
A medium-sized (3–10 mm) elevated skin lesion with scaling
- Psoriasiform
-
A ‘psoriasis-like’, well-circumscribed, elevated skin lesion with scaling
- Annular
-
A ring-shaped skin lesion
- Polycyclic
-
Skin lesions formed of several erythematous rings
- Erythrosquamous
-
A red and scaling skin lesion
- Bullae
-
Large blisters (>1 cm)
- Target lesions
-
Annular skin lesions with similarity to an archer’s bullseye with a central papule or vesicle, surrounded by pale oedema, and a peripheral ring-shaped erythema
- Colloid bodies
-
Pale, hyaline residue material derived from dead keratinocytes seen in the lower epidermis and the upper dermis (also known as Civatte bodies)
Rights and permissions
About this article
Cite this article
Wenzel, J. Cutaneous lupus erythematosus: new insights into pathogenesis and therapeutic strategies. Nat Rev Rheumatol 15, 519–532 (2019). https://doi.org/10.1038/s41584-019-0272-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-019-0272-0
This article is cited by
-
Screening of Genes Associated with Immune Infiltration of Discoid Lupus Erythematosus Based on Weighted Gene Co-expression Network Analysis
Biochemical Genetics (2024)
-
The differential panorama of clinical features of lupus erythematosus patients with different onset ages: a cross-sectional multicenter study from China
Clinical Rheumatology (2023)
-
Cutaneous Lupus Erythematosus: An Update on Pathogenesis and Future Therapeutic Directions
American Journal of Clinical Dermatology (2023)
-
Novel role of long non-coding RNAs in autoimmune cutaneous disease
Journal of Cell Communication and Signaling (2022)
-
Long-term risk of adverse cardiovascular outcomes associated with cutaneous lupus erythematosus: a nationwide cohort study
Clinical Rheumatology (2022)