Abstract
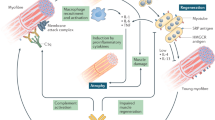
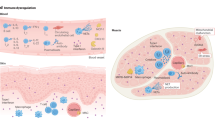
Inclusion body myositis (IBM) is often viewed as an enigmatic disease with uncertain pathogenic mechanisms and confusion around diagnosis, classification and prospects for treatment. Its clinical features (finger flexor and quadriceps weakness) and pathological features (invasion of myofibres by cytotoxic T cells) are unique among muscle diseases. Although IBM T cell autoimmunity has long been recognized, enormous attention has been focused for decades on several biomarkers of myofibre protein aggregates, which are present in <1% of myofibres in patients with IBM. This focus has given rise, together with the relative treatment refractoriness of IBM, to a competing view that IBM is not an autoimmune disease. Findings from the past decade that implicate autoimmunity in IBM include the identification of a circulating autoantibody (anti-cN1A); the absence of any statistically significant genetic risk factor other than the common autoimmune disease 8.1 MHC haplotype in whole-genome sequencing studies; the presence of a marked cytotoxic T cell signature in gene expression studies; and the identification in muscle and blood of large populations of clonal highly differentiated cytotoxic CD8+ T cells that are resistant to many immunotherapies. Mounting evidence that IBM is an autoimmune T cell-mediated disease provides hope that future therapies directed towards depleting these cells could be effective.
Key points
-
Inclusion body myositis (IBM) progresses slowly and is commonly misdiagnosed initially as arthritis or polymyositis; IBM is associated with cardiovascular complications and other autoimmune diseases and has a high economic cost.
-
IBM has unique physical examination features (such as finger flexor and knee extensor weakness) that distinguish it from most other muscle diseases.
-
IBM has a greater range of autoimmune T cell abnormalities than any other muscle disease; treatment refractoriness has paradoxically given rise to the view that IBM is not an autoimmune disease.
-
Degenerative abnormalities that can occur in IBM include numerous myofibre protein aggregates associated with endoplasmic reticulum stress.
-
Degenerative abnormalities might occur following autoimmunity in cell culture and mouse models and following immune cell dysfunction in patients infected with HIV or human T cell lymphotropic virus type 1.
-
Treatment refractoriness probably reflects the inability of current therapies to inhibit or deplete the highly differentiated population of effector memory and terminally differentiated effector T cells present in IBM.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Unverricht, H. Polymyositis acuta progressiva. Z. Klin. Med. 12, 533–549 (1887).
Bohan, A. History and classification of polymyositis and dermatomyositis. Clin. Dermatol. 6, 3–8 (1988).
Uverricht, H. Dermatomyositis acuta. Dtsch. Med. Wochenschr. 17, 41–44 (1891).
Levine, T. D. History of dermatomyositis. Arch. Neurol. 60, 780–782 (2003).
Carpenter, S., Karpati, G., Heller, I. & Eisen, A. Inclusion body myositis: a distinct variety of idiopathic inflammatory myopathy. Neurology 28, 8–17 (1978).
Emslie-Smith, A. M. & Engel, A. G. Necrotizing myopathy with pipestem capillaries, microvascular deposition of the complement membrane attack complex (MAC), and minimal cellular infiltration. Neurology 41, 936–939 (1991).
van der Meulen, M. F. et al. Polymyositis: an overdiagnosed entity. Neurology 61, 316–321 (2003).
Hoogendijk, J. E. et al. 119th ENMC international workshop: trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10-12 October 2003, Naarden, The Netherlands. Neuromuscul. Disord. 14, 337–345 (2004).
van de Vlekkert, J., Hoogendijk, J. E. & de Visser, M. Myositis with endomysial cell invasion indicates inclusion body myositis even if other criteria are not fulfilled. Neuromuscul. Disord. 25, 451–456 (2015).
Dalakas, M. C. Inflammatory muscle diseases. N. Engl. J. Med. 372, 1734–1747 (2015).
Wolstencroft, P. W. & Fiorentino, D. F. Dermatomyositis clinical and pathological phenotypes associated with myositis-specific autoantibodies. Curr. Rheumatol. Rep. 20, 28 (2018).
Schmidt, J. & Dalakas, M. C. Inclusion body myositis: from immunopathology and degenerative mechanisms to treatment perspectives. Expert Rev. Clin. Immunol. 9, 1125–1133 (2013).
Machado, P. M. et al. Ongoing developments in sporadic inclusion body myositis. Curr. Rheumatol. Rep. 16, 477 (2014).
Dimachkie, M. M. & Barohn, R. J. Inclusion body myositis. Neurol. Clin. 32, 629–646 (2014).
Mastaglia, F. L. & Needham, M. Inclusion body myositis: a review of clinical and genetic aspects, diagnostic criteria and therapeutic approaches. J. Clin. Neurosci. 22, 6–13 (2015).
Greenberg, S. A. Inclusion body myositis. Continuum 22, 1871–1888 (2016).
Gallay, L. & Petiot, P. Sporadic inclusion-body myositis: recent advances and the state of the art in 2016. Rev. Neurol. 172, 581–586 (2016).
Needham, M. & Mastaglia, F. L. Sporadic inclusion body myositis: a review of recent clinical advances and current approaches to diagnosis and treatment. Clin. Neurophysiol. 127, 1764–1773 (2016).
Schmidt, K. & Schmidt, J. Inclusion body myositis: advancements in diagnosis, pathomechanisms, and treatment. Curr. Opin. Rheumatol. 29, 632–638 (2017).
Benveniste, O. et al. Amyloid deposits and inflammatory infiltrates in sporadic inclusion body myositis: the inflammatory egg comes before the degenerative chicken. Acta Neuropathol. 129, 611–624 (2015).
Keller, C. W., Schmidt, J. & Lunemann, J. D. Immune and myodegenerative pathomechanisms in inclusion body myositis. Ann. Clin. Transl Neurol. 4, 422–445 (2017).
Chou, S. M. Myxovirus-like structures in a case of human chronic polymyositis. Science 158, 1453–1455 (1967).
Nishino, H., Engel, A. G. & Rima, B. K. Inclusion body myositis: the mumps virus hypothesis. Ann. Neurol. 25, 260–264 (1989).
Kallajoki, M. et al. Inclusion body myositis and paramyxoviruses. Hum. Pathol. 22, 29–32 (1991).
Fox, S. A., Ward, B. K., Robbins, P. D., Mastaglia, F. L. & Swanson, N. R. Inclusion body myositis: investigation of the mumps virus hypothesis by polymerase chain reaction. Muscle Nerve 19, 23–28 (1996).
Uruha, A. et al. Hepatitis C virus infection in inclusion body myositis: a case-control study. Neurology 86, 211–217 (2016).
Yunis, E. J. & Samaha, F. J. Inclusion body myositis. Lab. Invest. 25, 240–248 (1971).
Danon, M. J., Reyes, M. G., Perurena, O. H., Masdeu, J. C. & Manaligod, J. R. Inclusion body myositis. A corticosteroid-resistant idiopathic inflammatory myopathy. Arch. Neurol. 39, 760–764 (1982).
Eisen, A., Berry, K. & Gibson, G. Inclusion body myositis (IBM): myopathy or neuropathy? Neurology 33, 1109–1114 (1983).
Ringel, S. P., Kenny, C. E., Neville, H. E., Giorno, R. & Carry, M. R. Spectrum of inclusion body myositis. Arch. Neurol. 44, 1154–1157 (1987).
Calabrese, L. H., Mitsumoto, H. & Chou, S. M. Inclusion body myositis presenting as treatment-resistant polymyositis. Arthritis Rheum. 30, 397–403 (1987).
Lotz, B. P., Engel, A. G., Nishino, H., Stevens, J. C. & Litchy, W. J. Inclusion body myositis. Observations in 40 patients. Brain 112, 727–747 (1989).
Sayers, M. E., Chou, S. M. & Calabrese, L. H. Inclusion body myositis: analysis of 32 cases. J. Rheumatol. 19, 1385–1389 (1992).
Lindberg, C., Persson, L. I., Bjorkander, J. & Oldfors, A. Inclusion body myositis: clinical, morphological, physiological and laboratory findings in 18 cases. Acta Neurol. Scand. 89, 123–131 (1994).
Amato, A. A. et al. Inclusion body myositis: clinical and pathological boundaries. Ann. Neurol. 40, 581–586 (1996).
Felice, K. J., Relva, G. M. & Conway, S. R. Further observations on forearm flexor weakness in inclusion body myositis. Muscle Nerve 21, 659–661 (1998).
Phillips, B. A. et al. Patterns of muscle involvement in inclusion body myositis: clinical and magnetic resonance imaging study. Muscle Nerve 24, 1526–1534 (2001).
Badrising, U. A. et al. Inclusion body myositis. Clinical features and clinical course of the disease in 64 patients. J. Neurol. 252, 1448–1454 (2005).
Benveniste, O. et al. Long-term observational study of sporadic inclusion body myositis. Brain 134, 3176–3184 (2011).
Price, M. A. et al. Mortality and causes of death in patients with sporadic inclusion body myositis: survey study based on the clinical experience of specialists in Australia, Europe and the USA. J. Neuromuscul. Dis. 3, 67–75 (2016).
Felice, K. J. & North, W. A. Inclusion body myositis in Connecticut: observations in 35 patients during an 8-year period. Medicine 80, 320–327 (2001).
Needham, M. et al. Sporadic inclusion body myositis: phenotypic variability and influence of HLA-DR3 in a cohort of 57 Australian cases. J. Neurol. Neurosurg. Psychiatry 79, 1056–1060 (2008).
Cox, F. M. et al. A 12-year follow-up in sporadic inclusion body myositis: an end stage with major disabilities. Brain 134, 3167–3175 (2011).
Cortese, A. et al. Longitudinal observational study of sporadic inclusion body myositis: implications for clinical trials. Neuromuscul. Disord. 23, 404–412 (2013).
Hogrel, J. Y. et al. Four-year longitudinal study of clinical and functional endpoints in sporadic inclusion body myositis: implications for therapeutic trials. Neuromuscul. Disord. 24, 604–610 (2014).
Alfano, L. N. et al. Modeling functional decline over time in sporadic inclusion body myositis. Muscle Nerve 55, 526–531 (2016).
Rose, M. R. et al. A prospective natural history study of inclusion body myositis: implications for clinical trials. Neurology 57, 548–550 (2001).
Arahata, K. & Engel, A. G. Monoclonal antibody analysis of mononuclear cells in myopathies. I: Quantitation of subsets according to diagnosis and sites of accumulation and demonstration and counts of muscle fibers invaded by T cells. Ann. Neurol. 16, 193–208 (1984).
Engel, A. G. & Arahata, K. Monoclonal antibody analysis of mononuclear cells in myopathies. II: Phenotypes of autoinvasive cells in polymyositis and inclusion body myositis. Ann. Neurol. 16, 209–215 (1984).
Arahata, K. & Engel, A. G. Monoclonal antibody analysis of mononuclear cells in myopathies. III: Immunoelectron microscopy aspects of cell-mediated muscle fiber injury. Ann. Neurol. 19, 112–125 (1986).
Arahata, K. & Engel, A. G. Monoclonal antibody analysis of mononuclear cells in myopathies. IV: Cell-mediated cytotoxicity and muscle fiber necrosis. Ann. Neurol. 23, 168–173 (1988).
O’Hanlon, T. P., Dalakas, M. C., Plotz, P. H. & Miller, F. W. The αβT cell receptor repertoire in inclusion body myositis: diverse patterns of gene expression by muscle-infiltrating lymphocytes. J. Autoimmun. 7, 321–333 (1994).
Lindberg, C., Oldfors, A. & Tarkowski, A. Restricted use of T cell receptor V genes in endomysial infiltrates of patients with inflammatory myopathies. Eur. J. Immunol. 24, 2659–2663 (1994).
Lindberg, C., Oldfors, A. & Tarkowski, A. Local T cell proliferation and differentiation in inflammatory myopathies. Scand. J. Immunol. 41, 421–426 (1995).
Fyhr, I. M., Moslemi, A. R., Tarkowski, A., Lindberg, C. & Oldfors, A. Limited T cell receptor V gene usage in inclusion body myositis. Scand. J. Immunol. 43, 109–114 (1996).
Fyhr, I. M. et al. Oligoclonal expansion of muscle infiltrating T cells in inclusion body myositis. J. Neuroimmunol. 79, 185–189 (1997).
Bender, A., Behrens, L., Engel, A. G. & Hohlfeld, R. T cell heterogeneity in muscle lesions of inclusion body myositis. J. Neuroimmunol. 84, 86–91 (1998).
Amemiya, K., Granger, R. P. & Dalakas, M. C. Clonal restriction of T cell receptor expression by infiltrating lymphocytes in inclusion body myositis persists over time. Studies in repeated muscle biopsies. Brain 123, 2030–2039 (2000).
Muntzing, K., Lindberg, C., Moslemi, A. R. & Oldfors, A. Inclusion body myositis: clonal expansions of muscle-infiltrating T cells persist over time. Scand. J. Immunol. 58, 195–200 (2003).
Dimitri, D. et al. Shared blood and muscle CD8+T cell expansions in inclusion body myositis. Brain 129, 986–995 (2006).
Salajegheh, M. et al. T cell receptor profiling in muscle and blood lymphocytes in sporadic inclusion body myositis. Neurology 69, 1672–1679 (2007).
Pandya, J. M. et al. Expanded T cell receptor Vβ-restricted T cells from patients with sporadic inclusion body myositis are proinflammatory and cytotoxic CD28null T cells. Arthritis Rheum. 62, 3457–3466 (2010).
Allenbach, Y. et al. Th1 response and systemic treg deficiency in inclusion body myositis. PLOS ONE 9, e88788 (2014).
Greenberg, S. A., Pinkus, J. L., Amato, A. A., Kristensen, T. & Dorfman, D. M. Association of inclusion body myositis with T cell large granular lymphocytic leukaemia. Brain 139, 1348–1360 (2016).
Hohlfeld, R. & Schulze-Koops, H. Cytotoxic T cells go awry in inclusion body myositis. Brain 139, 1312–1314 (2016).
Lindberg, C., Trysberg, E., Tarkowski, A. & Oldfors, A. Anti-T-lymphocyte globulin treatment in inclusion body myositis: a randomized pilot study. Neurology 61, 260–262 (2003).
Dalakas, M. C. et al. Effect of Alemtuzumab (CAMPATH 1-H) in patients with inclusion-body myositis. Brain 132, 1536–1544 (2009).
Hogrel, J. Y. et al. Rapamycin vs. placebo for the treatment of inclusion body myositis: improvement of the 6 min walking distance, a functional scale, the FVC and muscle quantitative MRI. Arthritis Rheumatol. 69, 5L (2017).
Targoff, I. N. Autoantibodies and their significance in myositis. Curr. Rheumatol. Rep. 10, 333–340 (2008).
Nishikai, M. & Reichlin, M. Heterogeneity of precipitating antibodies in polymyositis and dermatomyositis. Characterization of the Jo-1 antibody system. Arthritis Rheum. 23, 881–888 (1980).
Reichlin, M. & Mattioli, M. Description of a serological reaction characteristic of polymyositis. Clin. Immunol. Immunopathol. 5, 12–20 (1976).
McHugh, N. J. & Tansley, S. L. Autoantibodies in myositis. Nat. Rev. Rheumatol. 14, 290–302 (2018).
Greenberg, S. A. et al. Molecular profiles of inflammatory myopathies. Neurology 59, 1170–1182 (2002).
Greenberg, S. A. et al. Plasma cells in muscle in inclusion body myositis and polymyositis. Neurology 65, 1782–1787 (2005).
Bradshaw, E. M. et al. A local antigen-driven humoral response is present in the inflammatory myopathies. J. Immunol. 178, 547–556 (2007).
Salajegheh, M. et al. Permissive environment for B cell maturation in myositis muscle in the absence of B cell follicles. Muscle Nerve 42, 576–583 (2010).
Ray, A. et al. Autoantibodies produced at the site of tissue damage provide evidence of humoral autoimmunity in inclusion body myositis. PLOS ONE 7, e46709 (2012).
Salajegheh, M., Lam, T. & Greenberg, S. A. Autoantibodies against a 43kDa muscle protein in inclusion body myositis. PLOS ONE 6, e20266 (2011).
Larman, H. B. et al. Cytosolic 5′-nucleotidase 1A autoimmunity in sporadic inclusion body myositis. Ann. Neurol. 73, 408–418 (2013).
Pluk, H. et al. Autoantibodies to cytosolic 5′-nucleotidase IA in inclusion body myositis. Ann. Neurol. 73, 397–407 (2013).
Mendell, J. R., Sahenk, Z., Gales, T. & Paul, L. Amyloid filaments in inclusion body myositis. Novel findings provide insight into nature of filaments. Arch. Neurol. 48, 1229–1234 (1991).
Oldfors, A., Larsson, N. G., Lindberg, C. & Holme, E. Mitochondrial DNA deletions in inclusion body myositis. Brain 116, 325–336 (1993).
Schmidt, J. et al. Interrelation of inflammation and APP in sIBM: IL-1β induces accumulation of β-amyloid in skeletal muscle. Brain 131, 1228–1240 (2008).
Freret, M. et al. Overexpression of MHC class I in muscle of lymphocyte-deficient mice causes a severe myopathy with induction of the unfolded protein response. Am. J. Pathol. 183, 893–904 (2013).
Rygiel, K. A. et al. Mitochondrial and inflammatory changes in sporadic inclusion body myositis. Neuropathol. Appl. Neurobiol. 41, 288–303 (2015).
Garlepp, M. J., Laing, B., Zilko, P. J., Ollier, W. & Mastaglia, F. L. HLA associations with inclusion body myositis. Clin. Exp. Immunol. 98, 40–45 (1994).
Koffman, B. M., Sivakumar, K., Simonis, T., Stroncek, D. & Dalakas, M. C. HLA allele distribution distinguishes sporadic inclusion body myositis from hereditary inclusion body myopathies. J. Neuroimmunol. 84, 139–142 (1998).
Lampe, J. B. et al. Analysis of HLA class I and II alleles in sporadic inclusion-body myositis. J. Neurol. 250, 1313–1317 (2003).
Price, P. et al. Two major histocompatibility complex haplotypes influence susceptibility to sporadic inclusion body myositis: critical evaluation of an association with HLA-DR3. Tissue Antigens 64, 575–580 (2004).
Scott, A. P. et al. Sporadic inclusion body myositis in Japanese is associated with the MHC ancestral haplotype 52.1. Neuromuscul. Disord. 16, 311–315 (2006).
Rojana-udomsart, A. et al. The association of sporadic inclusion body myositis and Sjögren’s syndrome in carriers of HLA-DR3 and the 8.1 MHC ancestral haplotype. Clin. Neurol. Neurosurg. 113, 559–563 (2011).
Rojana-udomsart, A. et al. High-resolution HLA-DRB1 genotyping in an Australian inclusion body myositis (s-IBM) cohort: an analysis of disease-associated alleles and diplotypes. J. Neuroimmunol. 250, 77–82 (2012).
Rojana-udomsart, A. et al. Analysis of HLA-DRB3 alleles and supertypical genotypes in the MHC class II region in sporadic inclusion body myositis. J. Neuroimmunol. 254, 174–177 (2013).
Rothwell, S. et al. Immune-array analysis in sporadic inclusion body myositis reveals HLA-DRB1 amino acid heterogeneity across the myositis spectrum. Arthritis Rheumatol. 69, 1090–1099 (2017).
Dalakas, M. C. et al. Treatment of inclusion-body myositis with IVIg: a double-blind, placebo-controlled study. Neurology 48, 712–716 (1997).
Greenberg, S. A., Pinkus, J. L. & Amato, A. A. Nuclear membrane proteins are present within rimmed vacuoles in inclusion-body myositis. Muscle Nerve 34, 406–416 (2006).
Chahin, N. & Engel, A. G. Correlation of muscle biopsy, clinical course, and outcome in PM and sporadic IBM. Neurology 70, 418–424 (2008).
Tawara, N. et al. Pathomechanisms of anti-cytosolic 5′-nucleotidase 1 A autoantibodies in sporadic inclusion body myositis. Ann. Neurol. 81, 512–525 (2017).
Johns Hopkins University. Myopathy, myofibrillar, 1; MFM1. OMIM https://www.omim.org/entry/601419 (2014).
Ahmed, M. et al. Targeting protein homeostasis in sporadic inclusion body myositis. Sci. Transl Med. 8, 331ra41 (2016).
Sivakumar, K., Semino-Mora, C. & Dalakas, M. C. An inflammatory, familial, inclusion body myositis with autoimmune features and a phenotype identical to sporadic inclusion body myositis. Studies in three families. Brain 120, 653–661 (1997).
Ranque-Francois, B. et al. Familial inflammatory inclusion body myositis. Ann. Rheum. Dis. 64, 634–637 (2005).
Tateyama, M. et al. Familial inclusion body myositis: a report on two Japanese sisters. Intern. Med. 42, 1035–1038 (2003).
Callan, A., Capkun, G., Vasanthaprasad, V., Freitas, R. & Needham, M. A. Systematic review and meta-analysis of prevalence studies of sporadic inclusion body myositis. J. Neuromuscul. Dis. 4, 127–137 (2017).
Tan, J. A. et al. Incidence and prevalence of idiopathic inflammatory myopathies in South Australia: a 30-year epidemiologic study of histology-proven cases. Int. J. Rheum. Dis. 16, 331–338 (2013).
Badrising, U. A. et al. Epidemiology of inclusion body myositis in the Netherlands: a nationwide study. Neurology 55, 1385–1387 (2000).
Lefter, S., Hardiman, O. & Ryan, A. M. A population-based epidemiologic study of adult neuromuscular disease in the Republic of Ireland. Neurology 88, 304–313 (2017).
Suzuki, N. et al. Increase in number of sporadic inclusion body myositis (sIBM) in Japan. J. Neurol. 259, 554–556 (2012).
Dobloug, G. C. et al. High prevalence of inclusion body myositis in Norway; a population-based clinical epidemiology study. Eur. J. Neurol. 22, 672 (2015).
Suzuki, N. et al. Multicenter questionnaire survey for sporadic inclusion body myositis in Japan. Orphanet J. Rare Dis. 11, 146 (2016).
Wilson, F. C., Ytterberg, S. R., St Sauver, J. L. & Reed, A. M. Epidemiology of sporadic inclusion body myositis and polymyositis in Olmsted County, Minnesota. J. Rheumatol. 35, 445–447 (2008).
Chilingaryan, A., Rison, R. A. & Beydoun, S. R. Misdiagnosis of inclusion body myositis: two case reports and a retrospective chart review. J. Med. Case Rep. 9, 169 (2015).
Paltiel, A. D. et al. Demographic and clinical features of inclusion body myositis in North America. Muscle Nerve 52, 527–533 (2015).
Keshishian, A., Greenberg, S. A., Agashivala, N., Baser, O. & Johnson, K. Health care costs and comorbidities for patients with inclusion body myositis. Curr. Med. Res. Opin. 34, 1679–1685 (2018).
Ko, E. H. & Rubin, A. D. Dysphagia due to inclusion body myositis: case presentation and review of the literature. Ann. Otol. Rhinol. Laryngol. 123, 605–608 (2014).
Cox, F. M. et al. Detecting dysphagia in inclusion body myositis. J. Neurol. 256, 2009–2013 (2009).
Houser, S. M., Calabrese, L. H. & Strome, M. Dysphagia in patients with inclusion body myositis. Laryngoscope 108, 1001–1005 (1998).
Oh, T. H., Brumfield, K. A., Hoskin, T. L., Kasperbauer, J. L. & Basford, J. R. Dysphagia in inclusion body myositis: clinical features, management, and clinical outcome. Am. J. Phys. Med. Rehabil. 87, 883–889 (2008).
Riminton, D. S., Chambers, S. T., Parkin, P. J., Pollock, M. & Donaldson, I. M. Inclusion body myositis presenting solely as dysphagia. Neurology 43, 1241–1243 (1993).
Verma, A., Bradley, W. G., Adesina, A. M., Sofferman, R. & Pendlebury, W. W. Inclusion body myositis with cricopharyngeus muscle involvement and severe dysphagia. Muscle Nerve 14, 470–473 (1991).
Rodriguez Cruz, P. M., Needham, M., Hollingsworth, P., Mastaglia, F. L. & Hillman, D. R. Sleep disordered breathing and subclinical impairment of respiratory function are common in sporadic inclusion body myositis. Neuromuscul. Disord. 24, 1036–1041 (2014).
Brady, S., Squier, W. & Hilton-Jones, D. Clinical assessment determines the diagnosis of inclusion body myositis independently of pathological features. J. Neurol. Neurosurg. Psychiatry 84, 1240–1246 (2013).
Dion, E. et al. Magnetic resonance imaging criteria for distinguishing between inclusion body myositis and polymyositis. J. Rheumatol. 29, 1897–1906 (2002).
Cox, F. M. et al. Magnetic resonance imaging of skeletal muscles in sporadic inclusion body myositis. Rheumatology 50, 1153–1161 (2011).
Inaishi, Y. et al. MRI for evaluation of flexor digitorum profundus muscle involvement in inclusion body myositis. Can. J. Neurol. Sci. 41, 780–781 (2014).
Tasca, G. et al. Magnetic resonance imaging pattern recognition in sporadic inclusion-body myositis. Muscle Nerve 52, 956–962 (2015).
Guimaraes, J. B. et al. Sporadic inclusion body myositis: MRI findings and correlation with clinical and functional parameters. AJR Am. J. Roentgenol. 209, 1340–1347 (2017).
Tsukita, K., Yagita, K., Sakamaki-Tsukita, H. & Suenaga, T. Sporadic inclusion body myositis: magnetic resonance imaging and ultrasound characteristics. QJM 111, 667–668 (2018).
Noto, Y. et al. Contrasting echogenicity in flexor digitorum profundus-flexor carpi ulnaris: a diagnostic ultrasound pattern in sporadic inclusion body myositis. Muscle Nerve 49, 745–748 (2014).
Nodera, H. et al. Intramuscular dissociation of echogenicity in the triceps surae characterizes sporadic inclusion body myositis. Eur. J. Neurol. 23, 588–596 (2016).
Albayda, J. et al. Pattern of muscle involvement in inclusion body myositis: a sonographic study. Clin. Exp. Rheumatol. 36, 996–1002 (2018).
Bachasson, D., Dubois, G. J. R., Allenbach, Y., Benveniste, O. & Hogrel, J. Y. Muscle shear wave elastography in inclusion body myositis: feasibility, reliability and relationships with muscle impairments. Ultrasound Med. Biol. 44, 1423–1432 (2018).
Olthoff, A. et al. Evaluation of dysphagia by novel real-time MRI. Neurology 87, 2132–2138 (2016).
Koffman, B. M., Rugiero, M. & Dalakas, M. C. Immune-mediated conditions and antibodies associated with sporadic inclusion body myositis. Muscle Nerve 21, 115–117 (1998).
Greenberg, S. A. Cytoplasmic 5′-nucleotidase autoantibodies in inclusion body myositis: Isotypes and diagnostic utility. Muscle Nerve 50, 488–492 (2014).
Goyal, N. A. et al. Seropositivity for NT5c1A antibody in sporadic inclusion body myositis predicts more severe motor, bulbar and respiratory involvement. J. Neurol. Neurosurg. Psychiatry 87, 373–378 (2016).
Lloyd, T. E. et al. Cytosolic 5′-nucleotidase 1A as a target of circulating autoantibodies in autoimmune diseases. Arthritis Care Res. 68, 66–71 (2016).
Kramp, S. L. et al. Development and evaluation of a standardized ELISA for the determination of autoantibodies against cN-1A (Mup44, NT5C1A) in sporadic inclusion body myositis. Auto Immun. Highlights 7, 16 (2016).
Felice, K. J. et al. Sensitivity and clinical utility of the anti-cytosolic 5’-nucleotidase 1 A (cN1A) antibody test in sporadic inclusion body myositis: Report of 40 patients from a single neuromuscular center. Neuromuscul. Disord. 28, 600–664 (2018).
Herbert, M. K. et al. Disease specificity of autoantibodies to cytosolic 5′-nucleotidase 1A in sporadic inclusion body myositis versus known autoimmune diseases. Ann. Rheum. Dis. 75, 696–701 (2016).
Muro, Y., Nakanishi, H., Katsuno, M., Kono, M. & Akiyama, M. Prevalence of anti-NT5C1A antibodies in Japanese patients with autoimmune rheumatic diseases in comparison with other patient cohorts. Clin. Chim. Acta 472, 1–4 (2017).
Rietveld, A. et al. Autoantibodies to cytosolic 5′-nucleotidase 1A in primary Sjögren’s syndrome and systemic lupus erythematosus. Front. Immunol. 9, 1200 (2018).
Mhiri, C. & Gherardi, R. Inclusion body myositis in French patients. A clinicopathological evaluation. Neuropathol. Appl. Neurobiol. 16, 333–344 (1990).
Askanas, V. & Engel, W. K. Molecular pathology and pathogenesis of inclusion-body myositis. Microsc. Res. Tech. 67, 114–120 (2005).
Rodriguez Cruz, P. M. et al. An analysis of the sensitivity and specificity of MHC-I and MHC-II immunohistochemical staining in muscle biopsies for the diagnosis of inflammatory myopathies. Neuromuscul. Disord. 24, 1025–1035 (2014).
Ikenaga, C. et al. Clinicopathologic features of myositis patients with CD8-MHC-1 complex pathology. Neurology 89, 1060–1068 (2017).
Temiz, P., Weihl, C. C. & Pestronk, A. Inflammatory myopathies with mitochondrial pathology and protein aggregates. J. Neurol. Sci. 278, 25–29 (2009).
Pestronk, A. Acquired immune and inflammatory myopathies: pathologic classification. Curr. Opin. Rheumatol. 23, 595–604 (2011).
van der Meulen, M. F. et al. Rimmed vacuoles and the added value of SMI-31 staining in diagnosing sporadic inclusion body myositis. Neuromuscul. Disord. 11, 447–451 (2001).
Dalakas, M. C. Polymyositis, dermatomyositis and inclusion-body myositis. N. Engl. J. Med. 325, 1487–1498 (1991).
Mastaglia, F. L. & Phillips, B. A. Idiopathic inflammatory myopathies: epidemiology, classification, and diagnostic criteria. Rheum. Dis. Clin. North Am. 28, 723–741 (2002).
Tawil, R. & Griggs, R. C. Inclusion body myositis. Curr. Opin. Rheumatol. 14, 653–657 (2002).
Verschuuren, J. J., van Engelen, B. G. M., van der Hoeven, H. & Hoogendijk, J. Inclusion body myositis diagnostic criteria. Inclusion Body Myositis. http://ibmmyositis.com/emery81.pdf (1997).
Griggs, R. C. et al. Inclusion body myositis and myopathies. Ann. Neurol. 38, 705–713 (1995).
Hilton-Jones, D. et al. Inclusion body myositis: MRC Centre for Neuromuscular Diseases, IBM workshop, London, 13 June 2008. Neuromuscul Disord. 20, 142–147 (2010).
Benveniste, O. & Hilton-Jones, D. International Workshop on Inclusion Body Myositis held at the Institute of Myology, Paris, on 29 May 2009. Neuromuscul. Disord. 20, 414–421 (2010).
Rose, M. R. 188th ENMC International Workshop: Inclusion Body Myositis, 2–4 December 2011, Naarden, The Netherlands. Neuromuscul. Disord. 23, 1044–1055 (2013).
Lloyd, T. E. et al. Evaluation and construction of diagnostic criteria for inclusion body myositis. Neurology 83, 426–433 (2014).
Kanellopoulos, P., Baltoyiannis, C. & Tzioufas, A. G. Primary Sjögren’s syndrome associated with inclusion body myositis. Rheumatology 41, 440–444 (2002).
Misterska-Skora, M., Sebastian, A., Dziegiel, P., Sebastian, M. & Wiland, P. Inclusion body myositis associated with Sjögren’s syndrome. Rheumatol. Int. 33, 3083–3086 (2013).
Colafrancesco, S. et al. Myositis in primary Sjögren’s syndrome: data from a multicentre cohort. Clin. Exp. Rheumatol. 33, 457–464 (2015).
Lloyd, T. E. et al. Overlapping features of polymyositis and inclusion body myositis in HIV-infected patients. Neurology 88, 1454–1460 (2017).
Hiniker, A., Daniels, B. H. & Margeta, M. T-cell-mediated inflammatory myopathies in HIV-positive individuals: a histologic study of 19 cases. J. Neuropathol. Exp. Neurol. 75, 239–245 (2016).
Cupler, E. J. et al. Inclusion body myositis in HIV-1 and HTLV-1 infected patients. Brain 119, 1887–1893 (1996).
Couture, P. et al. Inclusion body myositis and human immunodeficiency virus type 1: a new case report and literature review. Neuromuscul. Disord. 28, 334–338 (2018).
Matsuura, E. et al. Inclusion body myositis associated with human T-lymphotropic virus-type I infection: eleven patients from an endemic area in Japan. J. Neuropathol. Exp. Neurol. 67, 41–49 (2008).
Cox, F. M. et al. The heart in sporadic inclusion body myositis: a study in 51 patients. J. Neurol. 257, 447–451 (2010).
Limaye, V. S., Lester, S., Blumbergs, P. & Roberts-Thomson, P. J. Idiopathic inflammatory myositis is associated with a high incidence of hypertension and diabetes mellitus. Int. J. Rheum. Dis. 13, 132–137 (2010).
Lai, Y. T. et al. Dermatomyositis is associated with an increased risk of cardiovascular and cerebrovascular events: a Taiwanese population-based longitudinal follow-up study. Br. J. Dermatol. 168, 1054–1059 (2013).
Wang, H., Tang, J., Chen, X., Li, F. & Luo, J. Lipid profiles in untreated patients with dermatomyositis. J. Eur. Acad. Dermatol. Venereol. 27, 175–179 (2013).
Wang, H. et al. Altered lipid levels in untreated patients with early polymyositis. PLOS ONE 9, e89827 (2014).
Diederichsen, L. P. et al. Traditional cardiovascular risk factors and coronary artery calcification in adults with polymyositis and dermatomyositis: a Danish multicenter study. Arthritis Care Res. 67, 848–854 (2015).
Rai, S. K., Choi, H. K., Sayre, E. C. & Avina-Zubieta, J. A. Risk of myocardial infarction and ischaemic stroke in adults with polymyositis and dermatomyositis: a general population-based study. Rheumatology 55, 461–469 (2016).
Sherer, Y. & Shoenfeld, Y. Mechanisms of disease: atherosclerosis in autoimmune diseases. Nat. Clin. Pract. Rheumatol. 2, 99–106 (2006).
Ahearn, J., Shields, K. J., Liu, C. C. & Manzi, S. Cardiovascular disease biomarkers across autoimmune diseases. Clin. Immunol. 161, 59–63 (2015).
Alexanderson, H. Exercise in inflammatory myopathies, including inclusion body myositis. Curr. Rheumatol. Rep. 14, 244–251 (2012).
Arnardottir, S., Alexanderson, H., Lundberg, I. E. & Borg, K. Sporadic inclusion body myositis: pilot study on the effects of a home exercise program on muscle function, histopathology and inflammatory reaction. J. Rehabil. Med. 35, 31–35 (2003).
Johnson, L. G., Edwards, D. J., Walters, S. E., Thickbroom, G. W. & Mastaglia, F. L. The effectiveness of an individualized, home-based functional exercise program for patients with sporadic inclusion body myositis. J. Clin. Neuromuscul. Dis. 8, 187–194 (2007).
Parker, K. C. et al. Fast-twitch sarcomeric and glycolytic enzyme protein loss in inclusion body myositis. Muscle Nerve 39, 739–753 (2009).
Cherin, P. et al. Intravenous immunoglobulin for dysphagia of inclusion body myositis. Neurology 58, 326 (2002).
Pars, K. et al. Subcutaneous immunoglobulin treatment of inclusion-body myositis stabilizes dysphagia. Muscle Nerve 48, 838–839 (2013).
Cherin, P., Delain, J. C., de Jaeger, C. & Crave, J. C. Subcutaneous immunoglobulin use in inclusion body myositis: a review of 6 cases. Case Rep. Neurol. 7, 227–232 (2015).
Mendell, J. R. et al. Follistatin gene therapy for sporadic inclusion body myositis improves functional outcomes. Mol. Ther. 25, 870–879 (2017).
Greenberg, S. A. Unfounded claims of improved functional outcomes attributed to follistatin gene therapy in inclusion body myositis. Mol. Ther. 25, 2235–2237 (2017).
Walter, M. C. et al. High-dose immunoglobulin therapy in sporadic inclusion body myositis: a double-blind, placebo-controlled study. J. Neurol. 247, 22–28 (2000).
Dalakas, M. C. et al. A controlled study of intravenous immunoglobulin combined with prednisone in the treatment of IBM. Neurology 56, 323–327 (2001).
Badrising, U. A. et al. Comparison of weakness progression in inclusion body myositis during treatment with methotrexate or placebo. Ann. Neurol. 51, 369–372 (2002).
Muscle Study, G. Randomized pilot trial of βINF1a (Avonex) in patients with inclusion body myositis. Neurology 57, 1566–1570 (2001).
Muscle Study, G. Randomized pilot trial of high-dose βINF-1a in patients with inclusion body myositis. Neurology 63, 718–720 (2004).
Rutkove, S. B. et al. A pilot randomized trial of oxandrolone in inclusion body myositis. Neurology 58, 1081–1087 (2002).
Amato, A. A. et al. Treatment of sporadic inclusion body myositis with bimagrumab. Neurology 83, 2239–2246 (2014).
Amato, A. A. et al. A randomized, double-blind, placebo-controlled study of bimagrumab in patients with sporadic inclusion body myositis [abstract 8L]. Arthritis Rheumatol. 68, 4367–4369 (2016).
Chou, S. M. Myxovirus-like structures and accompanying nuclear changes in chronic polymyositis. Arch. Pathol. 86, 649–658 (1968).
Rifai, Z., Welle, S., Kamp, C. & Thornton, C. A. Ragged red fibers in normal aging and inflammatory myopathy. Ann. Neurol. 37, 24–29 (1995).
Oldfors, A. et al. Mitochondrial abnormalities in inclusion-body myositis. Neurology 66, S49–S55 (2006).
Askanas, V., Serdaroglu, P., Engel, W. K. & Alvarez, R. B. Immunolocalization of ubiquitin in muscle biopsies of patients with inclusion body myositis and oculopharyngeal muscular dystrophy. Neurosci. Lett. 130, 73–76 (1991).
Askanas, V., Engel, W. K. & Alvarez, R. B. Light and electron microscopic localization of β-amyloid protein in muscle biopsies of patients with inclusion-body myositis. Am. J. Pathol. 141, 31–36 (1992).
Askanas, V., Engel, W. K., Bilak, M., Alvarez, R. B. & Selkoe, D. J. Twisted tubulofilaments of inclusion body myositis muscle resemble paired helical filaments of Alzheimer brain and contain hyperphosphorylated tau. Am. J. Pathol. 144, 177–187 (1994).
Greenberg, S. A. Theories of the pathogenesis of inclusion body myositis. Curr. Rheumatol. Rep. 12, 221–228 (2010).
Askanas, V. & Engel, W. K. Proposed pathogenetic cascade of inclusion-body myositis: importance of amyloid-β, misfolded proteins, predisposing genes, and aging. Curr. Opin. Rheumatol. 15, 737–744 (2003).
Askanas, V. & Engel, W. K. Inclusion-body myositis: a myodegenerative conformational disorder associated with Aβ, protein misfolding, and proteasome inhibition. Neurology 66, S39–S48 (2006).
Askanas, V., Engel, W. K. & Nogalska, A. Sporadic inclusion-body myositis: a degenerative muscle disease associated with aging, impaired muscle protein homeostasis and abnormal mitophagy. Biochim. Biophys. Acta 1852, 633–643 (2015).
Greenberg, S. A. How citation distortions create unfounded authority: analysis of a citation network. BMJ 339, b2680 (2009).
Fergusson, D. Inappropriate referencing in research. BMJ 339, b2049 (2009).
Sarkozi, E., Askanas, V., Johnson, S. A., McFerrin, J. & Engel, W. K. Expression of β-amyloid precursor protein gene is developmentally regulated in human muscle fibers in vivo and in vitro. Exp. Neurol. 128, 27–33 (1994).
Askanas, V. & Engel, W. K. Sporadic inclusion-body myositis: conformational multifactorial ageing-related degenerative muscle disease associated with proteasomal and lysosomal inhibition, endoplasmic reticulum stress, and accumulation of amyloid-β42 oligomers and phosphorylated tau. Presse Med. 40, e219–e235 (2011).
Salajegheh, M. et al. Nature of “Tau” immunoreactivity in normal myonuclei and inclusion body myositis. Muscle Nerve 40, 520–528 (2009).
Pruitt, J. N. 2nd, Showalter, C. J. & Engel, A. G. Sporadic inclusion body myositis: counts of different types of abnormal fibers. Ann. Neurol. 39, 139–143 (1996).
Banwell, B. L. & Engel, A. G. αB-Crystallin immunolocalization yields new insights into inclusion body myositis. Neurology 54, 1033–1041 (2000).
Sherriff, F. E., Joachim, C. L., Squier, M. V. & Esiri, M. M. Ubiquitinated inclusions in inclusion-body myositis patients are immunoreactive for cathepsin D but not β-amyloid. Neurosci. Lett. 194, 37–40 (1995).
Dubourg, O. et al. Diagnostic value of markers of muscle degeneration in sporadic inclusion body myositis. Acta Myol 30, 103–108 (2011).
Hiniker, A., Daniels, B. H., Lee, H. S. & Margeta, M. Comparative utility of LC3, p62 and TDP-43 immunohistochemistry in differentiation of inclusion body myositis from polymyositis and related inflammatory myopathies. Acta Neuropathol. Commun. 1, 29 (2013).
Vattemi, G., Engel, W. K., McFerrin, J. & Askanas, V. Endoplasmic reticulum stress and unfolded protein response in inclusion body myositis muscle. Am. J. Pathol. 164, 1–7 (2004).
Lunemann, J. D. et al. β-amyloid is a substrate of autophagy in sporadic inclusion body myositis. Ann. Neurol. 61, 476–483 (2007).
Nakano, S., Oki, M. & Kusaka, H. The role of p62/SQSTM1 in sporadic inclusion body myositis. Neuromuscul. Disord. 27, 363–369 (2017).
Pinkus, J. L., Amato, A. A., Taylor, J. P. & Greenberg, S. A. Abnormal distribution of heterogeneous nuclear ribonucleoproteins in sporadic inclusion body myositis. Neuromuscul. Disord. 24, 611–616 (2014).
Weihl, C. C. et al. TDP-43 accumulation in inclusion body myopathy muscle suggests a common pathogenic mechanism with frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry 79, 1186–1189 (2008).
Salajegheh, M. et al. Sarcoplasmic redistribution of nuclear TDP-43 in inclusion body myositis. Muscle Nerve 40, 19–31 (2009).
Nogalska, A., Terracciano, C., D’Agostino, C., King Engel, W. & Askanas, V. p62/SQSTM1 is overexpressed and prominently accumulated in inclusions of sporadic inclusion-body myositis muscle fibers, and can help differentiating it from polymyositis and dermatomyositis. Acta Neuropathol. 118, 407–413 (2009).
Hengstman, G. J. & van Engelen, B. G. Polymyositis invasion of non-necrotic muscle fibres, and the art of repetition. BMJ 329, 1464–1467 (2004).
Callender, L. A. et al. Human CD8+ EMRA T cells display a senescence-associated secretory phenotype regulated by p38 MAPK. Aging Cell 17, e12675 (2018).
Chong, L. K. et al. Proliferation and interleukin 5 production by CD8hi CD57+ T cells. Eur. J. Immunol. 38, 995–1000 (2008).
Henson, S. M. & Akbar, A. N. KLRG1—more than a marker for T cell senescence. Age 31, 285–291 (2009).
Akbar, A. N. & Henson, S. M. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat. Rev. Immunol. 11, 289–295 (2011).
Melis, L., Van Praet, L., Pircher, H., Venken, K. & Elewaut, D. Senescence marker killer cell lectin-like receptor G1 (KLRG1) contributes to TNF-α production by interaction with its soluble E-cadherin ligand in chronically inflamed joints. Ann. Rheum. Dis. 73, 1223–1231 (2014).
Dumitriu, I. E. The life (and death) of CD4+CD28null T cells in inflammatory diseases. Immunology 146, 185–193 (2015).
Maly, K. & Schirmer, M. The story of CD4+CD28- T cells revisited: solved or still ongoing? J. Immunol. Res. 2015, 348746 (2015).
Lima, X. T. et al. Frequency and characteristics of circulating CD4+ CD28null T cells in patients with psoriasis. Br. J. Dermatol. 173, 998–1005 (2015).
Schirmer, M., Vallejo, A. N., Weyand, C. M. & Goronzy, J. J. Resistance to apoptosis and elevated expression of Bcl-2 in clonally expanded CD4+CD28- T cells from rheumatoid arthritis patients. J. Immunol. 161, 1018–1025 (1998).
Schirmer, M. et al. Circulating cytotoxic CD8+ CD28- T cells in ankylosing spondylitis. Arthritis Res. 4, 71–76 (2002).
Liaskou, E. et al. Loss of CD28 expression by liver-infiltrating T cells contributes to pathogenesis of primary sclerosing cholangitis. Gastroenterology 147, 221–232 (2014).
Dejaco, C. et al. NKG2D stimulated T cell autoreactivity in giant cell arteritis and polymyalgia rheumatica. Ann. Rheum. Dis. 72, 1852–1859 (2013).
Dejaco, C., Duftner, C., Klauser, A. & Schirmer, M. Altered T cell subtypes in spondyloarthritis, rheumatoid arthritis and polymyalgia rheumatica. Rheumatol. Int. 30, 297–303 (2010).
Duftner, C. et al. Prevalence, clinical relevance and characterization of circulating cytotoxic CD4+CD28- T cells in ankylosing spondylitis. Arthritis Res. Ther. 5, R292–R300 (2003).
Pinto-Medel, M. J. et al. The CD4+T cell subset lacking expression of the CD28 costimulatory molecule is expanded and shows a higher activation state in multiple sclerosis. J. Neuroimmunol. 243, 1–11 (2012).
Garcia de Tena, J. et al. Active Crohn’s disease patients show a distinctive expansion of circulating memory CD4+CD45RO+CD28null T cells. J. Clin. Immunol. 24, 185–196 (2004).
Leblanc, F., Zhang, D., Liu, X. & Loughran, T. P. Large granular lymphocyte leukemia: from dysregulated pathways to therapeutic targets. Future Oncol. 8, 787–801 (2012).
Mastaglia, F. L. et al. Polymorphism in the TOMM40 gene modifies the risk of developing sporadic inclusion body myositis and the age of onset of symptoms. Neuromuscul. Disord. 23, 969–974 (2013).
Gang, Q. et al. The effects of an intronic polymorphism in TOMM40 and APOE genotypes in sporadic inclusion body myositis. Neurobiol. Aging 36, 1766.e1–1766.e3 (2015).
De Paepe, B. & De Bleecker, J. L. The nonnecrotic invaded muscle fibers of polymyositis and sporadic inclusion body myositis: on the interplay of chemokines and stress proteins. Neurosci. Lett. 535, 18–23 (2013).
De Paepe, B., Creus, K. K. & De Bleecker, J. L. Chemokines in idiopathic inflammatory myopathies. Front. Biosci. 13, 2548–2577 (2008).
Ivanidze, J. et al. Inclusion body myositis: laser microdissection reveals differential up-regulation of IFN-γ signaling cascade in attacked versus nonattacked myofibers. Am. J. Pathol. 179, 1347–1359 (2011).
Mammen, A. L. Autoimmune myopathies. Continuum 22, 1852–1870 (2016).
Mammen, A. L. Autoimmune myopathies: autoantibodies, phenotypes and pathogenesis. Nat. Rev. Neurol. 7, 343–354 (2011).
Mammen, A. L. Which nonautoimmune myopathies are most frequently misdiagnosed as myositis? Curr. Opin. Rheumatol. 29, 618–622 (2017).
Britson, K. A., Yang, S. Y. & Lloyd, T. E. New developments in the genetics of inclusion body myositis. Curr. Rheumatol. Rep. 20, 26 (2018).
Olive, M. et al. Expression of mutant ubiquitin (UBB+1) and p62 in myotilinopathies and desminopathies. Neuropathol. Appl. Neurobiol. 34, 76–87 (2008).
Olive, M. et al. TAR DNA-binding protein 43 accumulation in protein aggregate myopathies. J. Neuropathol. Exp. Neurol. 68, 262–273 (2009).
Duleh, S., Wang, X., Komirenko, A. & Margeta, M. Activation of the Keap1/Nrf2 stress response pathway in autophagic vacuolar myopathies. Acta Neuropathol. Commun. 4, 115 (2016).
Arahata, K. et al. Inflammatory response in facioscapulohumeral muscular dystrophy (FSHD): immunocytochemical and genetic analyses. Muscle Nerve Suppl. 2, S56–S66 (1995).
Gallardo, E. et al. Inflammation in dysferlin myopathy: immunohistochemical characterization of 13 patients. Neurology 57, 2136–2138 (2001).
Castets, P., Frank, S., Sinnreich, M. & Ruegg, M. A. “Get the balance right”: pathological significance of autophagy perturbation in neuromuscular disorders. J. Neuromuscul. Dis. 3, 127–155 (2016).
Varadhachary, A. S., Weihl, C. C. & Pestronk, A. Mitochondrial pathology in immune and inflammatory myopathies. Curr. Opin. Rheumatol. 22, 651–657 (2010).
Meyer, A. et al. IFN-β-induced reactive oxygen species and mitochondrial damage contribute to muscle impairment and inflammation maintenance in dermatomyositis. Acta Neuropathol. 134, 655–666 (2017).
Nathan, J. A. et al. Immuno- and constitutive proteasomes do not differ in their abilities to degrade ubiquitinated proteins. Cell 152, 1184–1194 (2013).
Seifert, U. et al. Immunoproteasomes preserve protein homeostasis upon interferon-induced oxidative stress. Cell 142, 613–624 (2010).
Nagaraju, K. et al. Activation of the endoplasmic reticulum stress response in autoimmune myositis: potential role in muscle fiber damage and dysfunction. Arthritis Rheum. 52, 1824–1835 (2005).
Correia, A. S., Patel, P., Dutta, K. & Julien, J. P. Inflammation induces TDP-43 mislocalization and aggregation. PLOS ONE 10, e0140248 (2015).
Zhong, Z. et al. NF-κB restricts inflammasome activation via elimination of damaged mitochondria. Cell 164, 896–910 (2016).
Ozden, S. et al. Direct evidence for a chronic CD8+-T cell-mediated immune reaction to tax within the muscle of a human T cell leukemia/lymphoma virus type 1-infected patient with sporadic inclusion body myositis. J. Virol. 78, 10320–10327 (2004).
Green, D. R., Droin, N. & Pinkoski, M. Activation-induced cell death in T cells. Immunol. Rev. 193, 70–81 (2003).
Vallejo, A. N., Schirmer, M., Weyand, C. M. & Goronzy, J. J. Clonality and longevity of CD4+CD28null T cells are associated with defects in apoptotic pathways. J. Immunol. 165, 6301–6307 (2000).
Spaulding, C., Guo, W. & Effros, R. B. Resistance to apoptosis in human CD8+T cells that reach replicative senescence after multiple rounds of antigen-specific proliferation. Exp. Gerontol. 34, 633–644 (1999).
Posnett, D. N., Edinger, J. W., Manavalan, J. S., Irwin, C. & Marodon, G. Differentiation of human CD8 T cells: implications for in vivo persistence of CD8+CD28- cytotoxic effector clones. Int. Immunol. 11, 229–241 (1999).
Hodge, G. & Hodge, S. Steroid resistant CD8+CD28null NKT-like pro-inflammatory cytotoxic cells in chronic obstructive pulmonary disease. Front. Immunol. 7, 617 (2016).
Pandya, J. M. et al. Effects of conventional immunosuppressive treatment on CD244+(CD28null) and FOXP3+T cells in the inflamed muscle of patients with polymyositis and dermatomyositis. Arthritis Res. Ther. 18, 80 (2016).
Pearl, J. P. et al. Immunocompetent T cells with a memory-like phenotype are the dominant cell type following antibody-mediated T cell depletion. Am. J. Transplant. 5, 465–474 (2005).
Olnes, M. J. et al. Effects of systemically administered hydrocortisone on the human immunome. Sci. Rep. 6, 23002 (2016).
Bohan, A. & Peter, J. B. Polymyositis and dermatomyositis (first of two parts). N. Engl. J. Med. 292, 344–347 (1975).
Shah, M. V. et al. Molecular profiling of LGL leukemia reveals role of sphingolipid signaling in survival of cytotoxic lymphocytes. Blood 112, 770–781 (2008).
Bareau, B. et al. Analysis of a French cohort of patients with large granular lymphocyte leukemia: a report on 229 cases. Haematologica 95, 1534–1541 (2010).
Dumitriu, B. et al. Alemtuzumab in T cell large granular lymphocytic leukaemia: interim results from a single-arm, open-label, phase 2 study. Lancet Haematol. 3, e22–e29 (2016).
Mohan, S. R. et al. Therapeutic implications of variable expression of CD52 on clonal cytotoxic T cells in CD8+large granular lymphocyte leukemia. Haematologica 94, 1407–1414 (2009).
Gitelman, S. E. et al. Antithymocyte globulin therapy for patients with recent-onset type 1 diabetes: 2 year results of a randomised trial. Diabetologia 59, 1153–1161 (2016).
Scarsi, M. et al. The number of circulating recent thymic emigrants is severely reduced 1 year after a single dose of alemtuzumab in renal transplant recipients. Transpl. Int. 23, 786–795 (2010).
Neujahr, D. C. et al. Accelerated memory cell homeostasis during T cell depletion and approaches to overcome it. J. Immunol. 176, 4632–4639 (2006).
Crepin, T. et al. ATG-induced accelerated immune senescence: clinical implications in renal transplant recipients. Am. J. Transplant. 15, 1028–1038 (2015).
Macedo, C. et al. Long-term effects of alemtuzumab on regulatory and memory T cell subsets in kidney transplantation. Transplantation 93, 813–821 (2012).
Ramos-Casals, M. & Brito-Zeron, P. Emerging biological therapies in primary Sjögren’s syndrome. Rheumatology 46, 1389–1396 (2007).
Lombard, M. et al. Cyclosporin A treatment in primary biliary cirrhosis: results of a long-term placebo controlled trial. Gastroenterology 104, 519–526 (1993).
Mitchison, H. C. et al. A pilot, double-blind, controlled 1-year trial of prednisolone treatment in primary biliary cirrhosis: hepatic improvement but greater bone loss. Hepatology 10, 420–429 (1989).
Wiesner, R. H. et al. A controlled trial of cyclosporine in the treatment of primary biliary cirrhosis. N. Engl. J. Med. 322, 1419–1424 (1990).
Fujihara, T. et al. Preferential localization of CD8+αEβ7 +T cells around acinar epithelial cells with apoptosis in patients with Sjögren’s syndrome. J. Immunol. 163, 2226–2235 (1999).
Kita, H. Autoreactive CD8-specific T cell response in primary biliary cirrhosis. Hepatol. Res. 37 (Suppl. 3), 402–405 (2007).
Si, L., Whiteside, T. L., Schade, R. R., Starzl, T. E. & Van Thiel, D. H. T-Lymphocyte subsets in liver tissues of patients with primary biliary cirrhosis (PBC), patients with primary sclerosing cholangitis (PSC), and normal controls. J. Clin. Immunol. 4, 262–272 (1984).
Bjorkland, A. et al. Blood and liver-infiltrating lymphocytes in primary biliary cirrhosis: increase in activated T and natural killer cells and recruitment of primed memory T cells. Hepatology 13, 1106–1111 (1991).
Tasaki, S. et al. Multiomic disease signatures converge to cytotoxic CD8 T cells in primary Sjögren’s syndrome. Ann. Rheum. Dis. 76, 1458–1466 (2017).
Tsuda, M. et al. Fine phenotypic and functional characterization of effector cluster of differentiation 8 positive T cells in human patients with primary biliary cirrhosis. Hepatology 54, 1293–1302 (2011).
Yang, Z., Goronzy, J. J. & Weyand, C. M. Autophagy in autoimmune disease. J. Mol. Med. 93, 707–717 (2015).
Hosomi, S., Kaser, A. & Blumberg, R. S. Role of endoplasmic reticulum stress and autophagy as interlinking pathways in the pathogenesis of inflammatory bowel disease. Curr. Opin. Gastroenterol. 31, 81–88 (2015).
Grootjans, J., Kaser, A., Kaufman, R. J. & Blumberg, R. S. The unfolded protein response in immunity and inflammation. Nat. Rev. Immunol. 16, 469–484 (2016).
Sasaki, M., Miyakoshi, M., Sato, Y. & Nakanuma, Y. A possible involvement of p62/sequestosome-1 in the process of biliary epithelial autophagy and senescence in primary biliary cirrhosis. Liver Int. 32, 487–499 (2012).
Sasaki, M., Miyakoshi, M., Sato, Y. & Nakanuma, Y. Autophagy mediates the process of cellular senescence characterizing bile duct damages in primary biliary cirrhosis. Lab. Invest. 90, 835–843 (2010).
Sasaki, M., Yoshimura-Miyakoshi, M., Sato, Y. & Nakanuma, Y. A possible involvement of endoplasmic reticulum stress in biliary epithelial autophagy and senescence in primary biliary cirrhosis. J. Gastroenterol. 50, 984–995 (2015).
Katsiougiannis, S., Tenta, R. & Skopouli, F. N. Endoplasmic reticulum stress causes autophagy and apoptosis leading to cellular redistribution of the autoantigens Ro/Sjögren’s syndrome-related antigen A (SSA) and La/SSB in salivary gland epithelial cells. Clin. Exp. Immunol. 181, 244–252 (2015).
Leff, R. L., Miller, F. W., Hicks, J., Fraser, D. D. & Plotz, P. H. The treatment of inclusion body myositis: a retrospective review and a randomized, prospective trial of immunosuppressive therapy. Medicine 72, 225–235 (1993).
Soueidan, S. A. & Dalakas, M. C. Treatment of inclusion-body myositis with high-dose intravenous immunoglobulin. Neurology 43, 876–879 (1993).
Amato, A. A. et al. Inclusion body myositis: treatment with intravenous immunoglobulin. Neurology 44, 1516–1518 (1994).
Barohn, R. J., Amato, A. A., Sahenk, Z., Kissel, J. T. & Mendell, J. R. Inclusion body myositis: explanation for poor response to immunosuppressive therapy. Neurology 45, 1302–1304 (1995).
Barohn, R. J. et al. Pilot trial of etanercept in the treatment of inclusion-body myositis. Neurology 66, S123–S124 (2006).
Kosmidis, M. L., Alexopoulos, H., Tzioufas, A. G. & Dalakas, M. C. The effect of anakinra, an IL1 receptor antagonist, in patients with sporadic inclusion body myositis (sIBM): a small pilot study. J. Neurol. Sci. 334, 123–125 (2013).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT00079768 (2010).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT00917956 (2010).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01519349 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02483845 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02250443 (2018).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT00802815 (2014).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT00769860 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01423110 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01925209 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02481453 (2019).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02753530 (2018).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
S.A.G. is an inventor of intellectual property related to myositis diagnostics and therapeutics, owned and managed by Brigham and Women’s Hospital; he receives sponsored research from Pfizer, Inc. and is a founder of Abcuro, Inc.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Greenberg, S.A. Inclusion body myositis: clinical features and pathogenesis. Nat Rev Rheumatol 15, 257–272 (2019). https://doi.org/10.1038/s41584-019-0186-x
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-019-0186-x
This article is cited by
-
Face to Face: deciphering facial involvement in inclusion body myositis
Journal of Neurology (2024)
-
Complement and MHC patterns can provide the diagnostic framework for inflammatory neuromuscular diseases
Acta Neuropathologica (2024)
-
Effects of sporadic inclusion body myositis on skeletal muscle fibre type specific morphology and markers of regeneration and inflammation
Rheumatology International (2024)
-
Cytotoxic immune cells do not affect TDP-43 and p62 sarcoplasmic aggregation but influence TDP-43 localisation
Scientific Reports (2023)
-
Epidemiology of the idiopathic inflammatory myopathies
Nature Reviews Rheumatology (2023)