Abstract
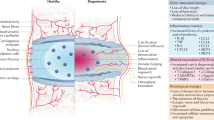
Intervertebral disc (IVD) degeneration is associated with low back pain. In IVDs, a high mechanical load, high osmotic pressure and hypoxic conditions create a hostile microenvironment for resident cells. How IVD homeostasis and function are maintained under stress remains to be understood; however, several research groups have reported isolating native endogenous progenitor-like or otherwise proliferative cells from the IVD. The isolation of such cells implies that the IVD might contain a quiescent progenitor-like population that could be activated for IVD repair and regeneration. Increased understanding of endogenous disc progenitor cells will improve our knowledge of IVD homeostasis and, when combined with tissue engineering techniques, might hold promise for future therapeutic applications. In this Review, the characteristics of progenitor cells in different IVD compartments are discussed, as well as the potency of different cell populations within the IVD. The stem cell characteristics of these cells are also compared with those of mesenchymal stromal cells. On the basis of existing evidence, whether and how IVD degeneration and the hostile microenvironment might affect endogenous progenitor cell function are considered, and ways to channel the potential of these cells for IVD repair are suggested.
Key points
-
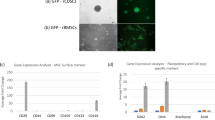
Intervertebral disc (IVD) progenitor cells express typical mesenchymal stromal cell (MSC) markers and pluripotency markers, as well as demonstrating tri-lineage differentiation potential similar to MSCs.
-
Cultured IVD progenitor cells are heterogeneous and cell subsets marked by expression of the tyrosine-protein kinase receptor TIE2 might possess increased multipotency.
-
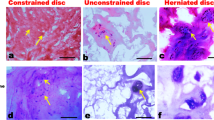
IVD degeneration and ageing affect the quantity and the properties of IVD progenitor cells.
-
The degenerated IVD microenvironment affects the fate of IVD progenitor cells, which should be considered and individually targeted when developing regenerative strategies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Vos, T. et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2163–2196 (2012).
Andersson, G. B. Epidemiological features of chronic low-back pain. Lancet 354, 581–585 (1999).
Walker, B. F. The prevalence of low back pain: a systematic review of the literature from 1966 to 1998. J. Spinal Disord. 13, 205–217 (2000).
Smith, L. J., Nerurkar, N. L., Choi, K. S., Harfe, B. D. & Elliott, D. M. Degeneration and regeneration of the intervertebral disc: lessons from development. Dis. Model. Mech. 4, 31–41 (2011).
Alini, M. et al. Are animal models useful for studying human disc disorders/degeneration? Eur. Spine J. 17, 2–19 (2008).
Cheung, K. M. et al. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine 34, 934–940 (2009).
Markolf, K. L. & Morris, J. M. The structural components of the intervertebral disc. A study of their contributions to the ability of the disc to withstand compressive forces. J. Bone Joint Surg. Am. 56, 675–687 (1974).
Debnath, S. et al. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature 562, 133–139 (2018).
Ito, Y. et al. Localization of chondrocyte precursors in periosteum. Osteoarthr. Cartil. 9, 215–223 (2001).
Lee, C. H. et al. Harnessing endogenous stem/progenitor cells for tendon regeneration. J. Clin. Invest. 125, 2690–2701 (2015).
Jiang, D. et al. Combined effect of ligament stem cells and umbilical-cord-blood-derived CD34+ cells on ligament healing. Cell Tissue Res. 362, 587–592 (2015).
Mifune, Y. et al. The effect of platelet-rich plasma on the regenerative therapy of muscle derived stem cells for articular cartilage repair. Osteoarthr. Cartil. 21, 175–185 (2013).
de Sousa, E. B., Casado, P. L., Moura Neto, V., Duarte, M. E. & Aguiar, D. P. Synovial fluid and synovial membrane mesenchymal stem cells: latest discoveries and therapeutic perspectives. Stem Cell Res. Ther. 5, 112 (2014).
Yang, F., Leung, V. Y., Luk, K. D., Chan, D. & Cheung, K. M. Mesenchymal stem cells arrest intervertebral disc degeneration through chondrocytic differentiation and stimulation of endogenous cells. Mol. Ther. 17, 1959–1966 (2009).
Sakai, D. et al. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat. Commun. 3, 1264 (2012).
Huang, S. et al. Coupling of small leucine-rich proteoglycans to hypoxic survival of a progenitor cell-like subpopulation in Rhesus Macaque intervertebral disc. Biomaterials 34, 6548–6558 (2013).
Henriksson, H. et al. Identification of cell proliferation zones, progenitor cells and a potential stem cell niche in the intervertebral disc region: a study in four species. Spine 34, 2278–2287 (2009).
Yasen, M. et al. Changes of number of cells expressing proliferation and progenitor cell markers with age in rabbit intervertebral discs. Acta Biochim. Biophys. Sin. 45, 368–376 (2013).
Blanco, J. F. et al. Isolation and characterization of mesenchymal stromal cells from human degenerated nucleus pulposus: comparison with bone marrow mesenchymal stromal cells from the same subjects. Spine 35, 2259–2265 (2010).
Feng, G. et al. Multipotential differentiation of human anulus fibrosus cells: an in vitro study. J. Bone Joint Surg. Am. 92, 675–685 (2010).
Huang, B. et al. Study to determine the presence of progenitor cells in the degenerated human cartilage endplates. Eur. Spine J. 21, 613–622 (2012).
Liu, L. T. et al. Characteristics of stem cells derived from the degenerated human intervertebral disc cartilage endplate. PLoS ONE 6, e26285 (2011).
Risbud, M. V. et al. Evidence for skeletal progenitor cells in the degenerate human intervertebral disc. Spine 32, 2537–2544 (2007).
Xiong, C. J. et al. Macrophage migration inhibitory factor inhibits the migration of cartilage end plate-derived stem cells by reacting with CD74. PLoS ONE 7, e43984 (2012).
Mizrahi, O. et al. Nucleus pulposus degeneration alters properties of resident progenitor cells. Spine J. 13, 803–814 (2013).
van den Akker, G. G. et al. Novel immortal human cell lines reveal subpopulations in the nucleus pulposus. Arthritis Res. Ther. 16, R135 (2014).
Doskocil, M., Valouch, P. & Pazderka, V. On vertebral body growth. Funct. Dev. Morphol. 3, 149–155 (1993).
Trout, J. J., Buckwalter, J. A., Moore, K. C. & Landas, S. K. Ultrastructure of the human intervertebral disc. I. Changes in notochordal cells with age. Tissue Cell 14, 359–369 (1982).
Kim, K. W. et al. The origin of chondrocytes in the nucleus pulposus and histologic findings associated with the transition of a notochordal nucleus pulposus to a fibrocartilaginous nucleus pulposus in intact rabbit intervertebral discs. Spine 28, 982–990 (2003).
Minogue, B. M., Richardson, S. M., Zeef, L. A., Freemont, A. J. & Hoyland, J. A. Transcriptional profiling of bovine intervertebral disc cells: implications for identification of normal and degenerate human intervertebral disc cell phenotypes. Arthritis Res. Ther. 12, R22 (2010).
Sive, J. I. et al. Expression of chondrocyte markers by cells of normal and degenerate intervertebral discs. Mol. Pathol. 55, 91–97 (2002).
Mwale, F., Roughley, P. & Antoniou, J. Distinction between the extracellular matrix of the nucleus pulposus and hyaline cartilage: a requisite for tissue engineering of intervertebral disc. Eur. Cell. Mater. 8, 58–63 (2004).
Henriksson, H. B., Svala, E., Skioldebrand, E., Lindahl, A. & Brisby, H. Support of concept that migrating progenitor cells from stem cell niches contribute to normal regeneration of the adult mammal intervertebral disc: a descriptive study in the New Zealand white rabbit. Spine 37, 722–732 (2012).
Erwin, W. M. et al. Intervertebral disc-derived stem cells: implications for regenerative medicine and neural repair. Spine 38, 211–216 (2013).
Dominici, M. et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315–317 (2006).
Pettine, K. A., Murphy, M. B., Suzuki, R. K. & Sand, T. T. Percutaneous injection of autologous bone marrow concentrate cells significantly reduces lumbar discogenic pain through 12 months. Stem Cells 33, 146–156 (2015).
Shu, C. C. et al. A histopathological scheme for the quantitative scoring of intervertebral disc degeneration and the therapeutic utility of adult mesenchymal stem cells for intervertebral disc regeneration. Int. J. Mol. Sci. 18, E1049 (2017).
Navone, S. E. et al. Expression of neural and neurotrophic markers in nucleus pulposus cells isolated from degenerated intervertebral disc. J. Orthop. Res. 30, 1470–1477 (2012).
Shen, Q., Zhang, L., Chai, B. & Ma, X. Isolation and characterization of mesenchymal stem-like cells from human nucleus pulposus tissue. Sci. China Life Sci. 58, 509–511 (2015).
Rui, Y. F. et al. Isolation, culture and identification of nucleus pulposus-derived mesenchymal stem cells from adult rats in vitro. Chinese J. Tissue Engineer. Res. 17, 8576–8582 (2013).
Tao, Y. et al. TGF-β3 and IGF-1 synergy ameliorates nucleus pulposus mesenchymal stem cell differentiation towards the nucleus pulposus cell type through MAPK/ERK signaling. Growth Factors 33, 326–336 (2015).
Zhang, H. et al. The ability to form cartilage of NPMSC and BMSC in SD rats. Int. J. Clin. Exp. Med. 8, 4989–4996 (2015).
Liu, C. et al. Identification of rabbit annulus fibrosus-derived stem cells. PLoS ONE 9, e108239 (2014).
Jin, L. et al. Annulus fibrosus cell characteristics are a potential source of intervertebral disc pathogenesis. PLoS ONE 9, e96519 (2014).
Nakai, T. et al. CD146 defines commitment of cultured annulus fibrosus cells to express a contractile phenotype. J. Orthop. Res. 34, 1361–1372 (2016).
Gruber, H. E. et al. Human annulus progenitor cells: analyses of this viable endogenous cell population. J. Orthop. Res. 34, 1351–1360 (2016).
Ishii, T. et al. Sciatic nerve regeneration by transplantation of in vitro differentiated nucleus pulposus progenitor cells. Regen. Med. 12, 365–376 (2017).
Li, Z. CD133: a stem cell biomarker and beyond. Exp. Hematol. Oncol. 2, 17 (2013).
Bonanno, G. et al. Human cord blood CD133+ cells immunoselected by a clinical-grade apparatus differentiate in vitro into endothelial- and cardiomyocyte-like cells. Transfusion 47, 280–289 (2007).
Takahashi, M. et al. CD133 is a positive marker for a distinct class of primitive human cord blood-derived CD34-negative hematopoietic stem cells. Leukemia 28, 1308–1315 (2014).
Sidney, L. E., Branch, M. J., Dunphy, S. E., Dua, H. S. & Hopkinson, A. Concise review: evidence for CD34 as a common marker for diverse progenitors. Stem Cells 32, 1380–1389 (2014).
Ning, Z. et al. Interleukin-1β affects the biological properties of rat nucleus pulposus-derived mesenchymal stem cells. Chinese J. Tissue Engineer. Res. 18, 4437–4443 (2014).
Henriksson, H. B. et al. Indications of that migration of stem cells is influenced by the extra cellular matrix architecture in the mammalian intervertebral disk region. Tissue Cell 47, 439–455 (2015).
Matta, A., Karim, M. Z., Isenman, D. E. & Erwin, W. M. Molecular therapy for degenerative disc disease: clues from secretome analysis of the notochordal cell-rich nucleus pulposus. Sci. Rep. 7, 45623 (2017).
da Silva Meirelles, L., Caplan, A. I. & Nardi, N. B. In search of the in vivo identity of mesenchymal stem cells. Stem Cells 26, 2287–2299 (2008).
Li, H. et al. Influence of hypoxia in the intervertebral disc on the biological behaviors of rat adipose- and nucleus pulposus-derived mesenchymal stem cells. Cells Tissues Organs 198, 266–277 (2013).
Li, X. C. et al. Characteristics and potentials of stem cells derived from human degenerated nucleus pulposus: potential for regeneration of the intervertebral disc. BMC Musculoskelet Disord. 18, 242 (2017).
Wu, H. et al. Comparison of nucleus pulposus stem/progenitor cells isolated from degenerated intervertebral discs with umbilical cord derived mesenchymal stem cells. Exp. Cell Res. 361, 324–332 (2017).
Wang, H. et al. Distinguishing characteristics of stem cells derived from different anatomical regions of human degenerated intervertebral discs. Eur. Spine J. 25, 2691–2704 (2016).
Shi, R. et al. The presence of stem cells in potential stem cell niches of the intervertebral disc region: an in vitro study on rats. Eur. Spine J. 24, 2411–2424 (2015).
Duff, S. E., Li, C., Garland, J. M. & Kumar, S. CD105 is important for angiogenesis: evidence and potential applications. FASEB J. 17, 984–992 (2003).
Kong, D. H., Kim, Y. K., Kim, M. R., Jang, J. H. & Lee, S. Emerging roles of vascular cell adhesion molecule-1 (VCAM-1) in immunological disorders and cancer. Int. J. Mol. Sci. 19, E1057 (2018).
Wang, H. et al. Utilization of stem cells in alginate for nucleus pulposus tissue engineering. Tissue Eng. Part A. 20, 908–920 (2014).
Brown, S. et al. Cell clusters are indicative of stem cell activity in the degenerate intervertebral disc: can their properties be manipulated to improve intrinsic repair of the disc? Stem Cells Dev. 27, 147–165 (2018).
Turner, S., Balain, B., Caterson, B., Morgan, C. & Roberts, S. Viability, growth kinetics and stem cell markers of single and clustered cells in human intervertebral discs: implications for regenerative therapies. Eur. Spine J. 23, 2462–2472 (2014).
Liu, M. H., Cui, Y. H. & Zhou, Y. Cellular mechanical properties reflect the differentiation potential of nucleus pulposus-derived progenitor cells. Am. J. Transl Res. 8, 4446–4454 (2016).
Tekari, A., Chan, S. C., Sakai, D., Grad, S. & Gantenbein, B. Angiopoietin-1 receptor Tie2 distinguishes multipotent differentiation capability in bovine coccygeal nucleus pulposus cells. Stem Cell Res. Ther. 7, 75 (2016).
Benz, K., Stippich, C., Freudigmann, C., Mollenhauer, J. A. & Aicher, W. K. Maintenance of “stem cell” features of cartilage cell sub-populations during in vitro propagation. J. Transl Med. 11, 27 (2013).
Jia, Z. et al. Comparison of biological characteristics of nucleus pulposus mesenchymal stem cells derived from non-degenerative and degenerative human nucleus pulposus. Exp. Ther. Med. 13, 3574–3580 (2017).
Zhao, Y. et al. Age-related changes in nucleus pulposus mesenchymal stem cells: an in vitro study in rats. Stem Cells Int. 2017, 6761572 (2017).
Molinos, M. et al. Age-correlated phenotypic alterations in cells isolated from human degenerated intervertebral discs with contained hernias. Spine 43, E274–E284 (2018).
Barker, T. H. & Hagood, J. S. Getting a grip on Thy-1 signaling. Biochim. Biophys. Acta 1793, 921–923 (2009).
Zhi, X. et al. RNA interference of ecto-5′-nucleotidase (CD73) inhibits human breast cancer cell growth and invasion. Clin. Exp. Metastasis 24, 439–448 (2007).
Miettinen, M. & Lasota, J. KIT (CD117): a review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl. Immunohistochem. Mol. Morphol. 13, 205–220 (2005).
Yeh, C. H., Jin, L., Shen, F., Balian, G. & Li, X. J. miR-221 attenuates the osteogenic differentiation of human annulus fibrosus cells. Spine J. 16, 896–904 (2016).
Melrose, J. Strategies in regenerative medicine for intervertebral disc repair using mesenchymal stem cells and bioscaffolds. Regen. Med. 11, 705–724 (2016).
Diamant, B., Karlsson, J. & Nachemson, A. Correlation between lactate levels and pH in discs of patients with lumbar rhizopathies. Experientia 24, 1195–1196 (1968).
Bartels, E. M., Fairbank, J. C., Winlove, C. P. & Urban, J. P. Oxygen and lactate concentrations measured in vivo in the intervertebral discs of patients with scoliosis and back pain. Spine 23, 1–7 (1998).
Lv, F. J. et al. Matrix metalloproteinase 12 is an indicator of intervertebral disc degeneration co-expressed with fibrotic markers. Osteoarthritis Cartilage 24, 1826–1836 (2016).
Phillips, K. L. et al. Potential roles of cytokines and chemokines in human intervertebral disc degeneration: interleukin-1 is a master regulator of catabolic processes. Osteoarthritis Cartilage 23, 1165–1177 (2015).
Peng, Y. & Lv, F.-J. Symptomatic versus asymptomatic intervertebral disc degeneration: is inflammation the key? Crit. Rev. Eukaryot. Gene Expr. 25, 13–21 (2015).
Li, Y. Y. et al. Delivering mesenchymal stem cells in collagen microsphere carriers to rabbit degenerative disc: reduced risk of osteophyte formation. Tissue Eng. Part A. 20, 1379–1391 (2014).
Setton, L. A. & Chen, J. Mechanobiology of the intervertebral disc and relevance to disc degeneration. J. Bone Joint Surg. Am. 88 (Suppl. 2), 52–57 (2006).
Huang, Y. C., Urban, J. P. & Luk, K. D. Intervertebral disc regeneration: do nutrients lead the way? Nat. Rev. Rheumatol. 10, 561–566 (2014).
Han, B. et al. Nucleus pulposus mesenchymal stem cells in acidic conditions mimicking degenerative intervertebral discs give better performance than adipose tissue-derived mesenchymal stem cells. Cells Tissues Organs 199, 342–352 (2014).
Liu, J. et al. Biological behavior of human nucleus pulposus mesenchymal stem cells in response to changes in the acidic environment during intervertebral disc degeneration. Stem Cells Dev. 26, 901–911 (2017).
Yao, Y. et al. A genome-wide analysis of the gene expression profiles and alternative splicing events during the hypoxia-regulated osteogenic differentiation of human cartilage endplate-derived stem cells. Mol. Med. Rep. 16, 1991–2001 (2017).
Yao, Y. et al. MIF plays a key role in regulating tissue-specific chondro-osteogenic differentiation fate of human cartilage endplate stem cells under hypoxia. Stem Cell Rep. 7, 249–262 (2016).
Navaro, Y. et al. Matrix stiffness determines the fate of nucleus pulposus-derived stem cells. Biomaterials 49, 68–76 (2015).
Liu, C. et al. The effect of the fibre orientation of electrospun scaffolds on the matrix production of rabbit annulus fibrosus-derived stem cells. Bone Res. 3, 15012 (2015).
Yuan, C. et al. [Stress regulating osteogenic differentiation of human intervertebral disc cartilage endplate-derived stem cells]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 29, 351–355 (2015).
Yuan, C., Pu, L., He, Z. & Wang, J. BNIP3/Bcl-2-mediated apoptosis induced by cyclic tensile stretch in human cartilage endplate-derived stem cells. Exp. Ther. Med. 15, 235–241 (2018).
He, Z., Pu, L., Yuan, C., Jia, M. & Wang, J. Nutrition deficiency promotes apoptosis of cartilage endplate stem cells in a caspase-independent manner partially through upregulating BNIP3. Acta Biochim. Biophys. Sin. (Shanghai) 49, 25–32 (2017).
Vadala, G. et al. Mesenchymal stem cells injection in degenerated intervertebral disc: cell leakage may induce osteophyte formation. J. Tissue Eng. Regen. Med. 6, 348–355 (2012).
Huang, Y. Z. et al. Species variation in the spontaneous calcification of bone marrow-derived mesenchymal stem cells. Cytotherapy 15, 323–329 (2013).
Lv, F., Lu, M., Cheung, K. M., Leung, V. Y. & Zhou, G. Intrinsic properties of mesemchymal stem cells from human bone marrow, umbilical cord and umbilical cord blood comparing the different sources of MSC. Curr. Stem Cell Res. Ther. 7, 389–399 (2012).
Sasaki, N. et al. Physical exercise affects cell proliferation in lumbar intervertebral disc regions in rats. Spine 37, 1440–1447 (2012).
Saraiya, M. et al. Reversine enhances generation of progenitor-like cells by dedifferentiation of annulus fibrosus cells. Tissue Eng. Part A. 16, 1443–1455 (2010).
Zhu, C. et al. Modulation of the gene expression of annulus fibrosus-derived stem cells using poly(ether carbonate urethane)urea scaffolds of tunable elasticity. Acta Biomater. 29, 228–238 (2016).
Pratsinis, H. & Kletsas, D. PDGF, bFGF and IGF-I stimulate the proliferation of intervertebral disc cells in vitro via the activation of the ERK and Akt signaling pathways. Eur. Spine J. 16, 1858–1866 (2007).
Leung, V. Y. L. et al. Bone morphogenetic protein-2 and -7 mediate the anabolic function of nucleus pulposus cells with discrete mechanisms. Connect. Tissue Res. 58, 573–585 (2017).
Kumar, H. et al. Safety and tolerability of intradiscal implantation of combined autologous adipose-derived mesenchymal stem cells and hyaluronic acid in patients with chronic discogenic low back pain: 1-year follow-up of a phase I study. Stem Cell Res. Ther. 8, 262 (2017).
Elabd, C. et al. Intra-discal injection of autologous, hypoxic cultured bone marrow-derived mesenchymal stem cells in five patients with chronic lower back pain: a long-term safety and feasibility study. J. Transl Med. 14, 253 (2016).
US National Library of Medicine. ClinialTrials.gov https://clinicaltrials.gov/ct2/show/NCT03347708 (2018).
Brisby, H. et al. The presence of local mesenchymal progenitor cells in human degenerated intervertebral discs and possibilities to influence these in vitro: a descriptive study in humans. Stem Cells Dev. 22, 804–814 (2013).
Shang, J., Fan, X., Shangguan, L., Liu, H. & Zhou, Y. Global gene expression profiling and alternative splicing events during the chondrogenic differentiation of human cartilage endplate-derived stem cells. Biomed Res. Int. 2015, 604972 (2015).
Sang, C., Cao, X., Chen, F., Yang, X. & Zhang, Y. Differential characterization of two kinds of stem cells isolated from rabbit nucleus pulposus and annulus fibrosus. Stem Cells Int. 2016, 8283257 (2016).
Lin, L. et al. Use of limiting dilution method for isolation of nucleus pulposus mesenchymal stem/progenitor cells and effects of plating density on biological characteristics and plasticity. Biomed Res. Int. 2017, 9765843 (2017).
Acknowledgements
The work of the authors was supported by the National Natural Science Foundation of China (grant 81702191 to F.-J.L.), the Fundamental Research Funds for the Central Universities, South China University of Technology (grant 2018MS70 to F.-J.L.) and the general research fund (grant 17126615 to V.Y.L.) and theme-based research scheme (grant T12-708/12N to K.M.C.) of the Research Grant Council of Hong Kong.
Review criteria
A search for original articles published between inception and 2018 without language restriction was performed in MEDLINE, PubMed, Ovid and SCOPUS. The search terms used were ‘intervertebral disc’, ‘nucleus pulposus’, ‘endplate’ or ‘annulus fibrosus’ in combination with ‘stem cells’ or ‘progenitor’. All articles identified were reviewed manually for eligibility. Confusion regarding the description of cells in these articles was noted for in vitro studies. For example, in some articles, the studied cells were defined as intervertebral disc stem or progenitor cells without full examination of the stem cell-like phenotype and function of the cells. Such articles were excluded from further analysis.
Reviewer information
Nature Reviews Rheumatology thanks J. Melrose, M. Alini and H. Brisby for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
F.-J.L. researched data for the article. All authors made substantial contributions to discussion of the content of the article, wrote the article and reviewed and/or edited the article before submission.
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Lyu, FJ., Cheung, K.M., Zheng, Z. et al. IVD progenitor cells: a new horizon for understanding disc homeostasis and repair. Nat Rev Rheumatol 15, 102–112 (2019). https://doi.org/10.1038/s41584-018-0154-x
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-018-0154-x
This article is cited by
-
Future of low back pain: unravelling IVD components and MSCs’ potential
Cell Regeneration (2024)
-
The most influential articles on stem cells in intervertebral disc degeneration
BMC Musculoskeletal Disorders (2024)
-
Senescent-like macrophages mediate angiogenesis for endplate sclerosis via IL-10 secretion in male mice
Nature Communications (2024)
-
The Role of Immunocyte Infiltration Regulatory Network Based on hdWGCNA and Single-Cell Bioinformatics Analysis in Intervertebral Disc Degeneration
Inflammation (2024)
-
Transplantation of active nucleus pulposus cells with a keep-charging hydrogel microsphere system to rescue intervertebral disc degeneration
Journal of Nanobiotechnology (2023)