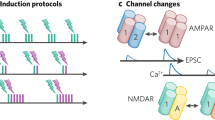
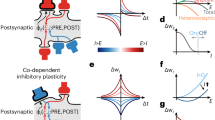
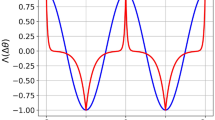
Abstract
Hebb postulated that, to store information in the brain, assemblies of excitatory neurons coding for a percept are bound together via associative long-term synaptic plasticity. In this view, it is unclear what role, if any, is carried out by inhibitory interneurons. Indeed, some have argued that inhibitory interneurons are not plastic. Yet numerous recent studies have demonstrated that, similar to excitatory neurons, inhibitory interneurons also undergo long-term plasticity. Here, we discuss the many diverse forms of long-term plasticity that are found at inputs to and outputs from several types of cortical inhibitory interneuron, including their plasticity of intrinsic excitability and their homeostatic plasticity. We explain key plasticity terminology, highlight key interneuron plasticity mechanisms, extract overarching principles and point out implications for healthy brain functionality as well as for neuropathology. We introduce the concept of the plasticitome — the synaptic plasticity counterpart to the genome or the connectome — as well as nomenclature and definitions for dealing with this rich diversity of plasticity. We argue that the great diversity of interneuron plasticity rules is best understood at the circuit level, for example as a way of elucidating how the credit-assignment problem is solved in deep biological neural networks.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bliss, T. V. & Collingridge, G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361, 31–39 (1993).
Malenka, R. C. & Bear, M. F. LTP and LTD: an embarrassment of riches. Neuron 44, 5–21 (2004).
Markram, H., Gerstner, W. & Sjöström, P. J. Spike-timing-dependent plasticity: a comprehensive overview. Front. Synaptic Neurosci. 4, 2 (2012).
Katz, L. C. & Shatz, C. J. Synaptic activity and the construction of cortical circuits. Science 274, 1133–1138 (1996).
Hensch, T. K. Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 6, 877–888 (2005).
Cline, H. T. Topographic maps: developing roles of synaptic plasticity. Curr. Biol. 8, R836–R839 (1998).
Markram, H., Gerstner, W. & Sjöström, P. J. A history of spike-timing-dependent plasticity. Front. Synaptic Neurosci. 3, 4 (2011).
Hebb, D. O. The Organization of Behaviour (Wiley, 1949).
Shatz, C. J. The developing brain. Sci. Am. 267, 60–67 (1992).
Löwel, S. & Singer, W. Selection of intrinsic horizontal connections in the visual cortex by correlated neuronal activity. Science 255, 209–212 (1992).
Hebb, D. O. A Textbook of Psychology (W. B. Saunders, 1972).
Hopfield, J. J. & Tank, D. W. Computing with neural circuits: a model. Science 233, 625–633 (1986).
McBain, C. J., Freund, T. F. & Mody, I. Glutamatergic synapses onto hippocampal interneurons: precision timing without lasting plasticity. Trends Neurosci. 22, 228–235 (1999).
Sjöström, P. J., Rancz, E. A., Roth, A. & Häusser, M. Dendritic excitability and synaptic plasticity. Physiol. Rev. 88, 769–840 (2008).
Maheux, J., Froemke, R. C. & Sjöström, P. J. in Dendrites Ch. 18 (eds Stuart, G., Spruston, N. & Häusser, M.) 465–498 (Oxford Univ. Press, 2016).
Yazaki-Sugiyama, Y., Kang, S., Cateau, H., Fukai, T. & Hensch, T. K. Bidirectional plasticity in fast-spiking GABA circuits by visual experience. Nature 462, 218–221 (2009). In this work, in vivo visual cortex recordings following monocular deprivation reveal that BCs have an unexpected initial preference for the occluded eye before a late preference for the open eye, in keeping with temporally symmetric STDP at excitatory inputs to BCs.
Udakis, M., Pedrosa, V., Chamberlain, S. E. L., Clopath, C. & Mellor, J. R. Interneuron-specific plasticity at parvalbumin and somatostatin inhibitory synapses onto CA1 pyramidal neurons shapes hippocampal output. Nat. Commun. 11, 4395 (2020). Using computer modelling, this study demonstrates that timing-dependent LTP at SST+ IN inputs onto CA1 PCs stabilizes hippocampal place cells and prevents interference in new environments, whereas timing-dependent LTD at PV+ IN inputs maintains place cell spike output.
Vogels, T. P., Sprekeler, H., Zenke, F., Clopath, C. & Gerstner, W. Inhibitory plasticity balances excitation and inhibition in sensory pathways and memory networks. Science 334, 1569–1573 (2011).
Kullmann, D. M. & Lamsa, K. P. LTP and LTD in cortical GABAergic interneurons: emerging rules and roles. Neuropharmacology 60, 712–719 (2011).
Lamsa, K. P., Heeroma, J. H., Somogyi, P., Rusakov, D. A. & Kullmann, D. M. Anti-Hebbian long-term potentiation in the hippocampal feedback inhibitory circuit. Science 315, 1262–1266 (2007).
Markram, H. et al. Interneurons of the neocortical inhibitory system. Nat. Rev. Neurosci. 5, 793–807 (2004).
Ascoli, G. A. et al. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat. Rev. Neurosci. 9, 557–568 (2008).
Gouwens, N. W. et al. Integrated morphoelectric and transcriptomic classification of cortical GABAergic cells. Cell 183, 935–953.e19 (2020).
Larsen, R. S. & Sjöström, P. J. Synapse-type-specific plasticity in local circuits. CONB 35, 127–135 (2015). This review defines the research field of synapse type-specific plasticity in local circuits.
Sjöström, P. J. Grand challenge at the frontiers of synaptic neuroscience. Front. Synaptic Neurosci. 13, 748937 (2021).
Abbott, L. F. et al. The mind of a mouse. Cell 182, 1372–1376 (2020).
Gregory, S. G. et al. A physical map of the mouse genome. Nature 418, 743–750 (2002).
Kepecs, A. & Fishell, G. Interneuron cell types are fit to function. Nature 505, 318–326 (2014).
Rudy, B., Fishell, G., Lee, S. & Hjerling-Leffler, J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev. Neurobiol. 71, 45–61 (2011).
Tremblay, R., Lee, S. & Rudy, B. GABAergic interneurons in the neocortex: from cellular properties to circuits. Neuron 91, 260–292 (2016).
Pfeffer, C. K., Xue, M., He, M., Huang, Z. J. & Scanziani, M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat. Neurosci. 16, 1068–1076 (2013).
Kawaguchi, Y. & Kubota, Y. Correlation of physiological subgroupings of nonpyramidal cells with parvalbumin- and calbindinD28k-immunoreactive neurons in layer V of rat frontal cortex. J. Neurophysiol. 70, 387–396 (1993).
Mele, M., Leal, G. & Duarte, C. B. Role of GABAA R trafficking in the plasticity of inhibitory synapses. J. Neurochem. 139, 997–1018 (2016).
Kullmann, D. M., Moreau, A. W., Bakiri, Y. & Nicholson, E. Plasticity of inhibition. Neuron 75, 951–962 (2012).
Chiu, C. Q., Barberis, A. & Higley, M. J. Preserving the balance: diverse forms of long-term GABAergic synaptic plasticity. Nat. Rev. Neurosci. 20, 272–281 (2019).
Capogna, M., Castillo, P. E. & Maffei, A. The ins and outs of inhibitory synaptic plasticity: neuron types, molecular mechanisms and functional roles. Eur. J. Neurosci. 54, 6882–6901 (2021).
Wu, Y. K., Miehl, C. & Gjorgjieva, J. Regulation of circuit organization and function through inhibitory synaptic plasticity. Trends Neurosci. https://doi.org/10.1016/j.tins.2022.10.006 (2022).
Sprekeler, H. Functional consequences of inhibitory plasticity: homeostasis, the excitation–inhibition balance and beyond. Curr. Opin. Neurobiol. 43, 198–203 (2017).
Topolnik, L. & Tamboli, S. The role of inhibitory circuits in hippocampal memory processing. Nat. Rev. Neurosci. https://doi.org/10.1038/s41583-022-00599-0 (2022).
Hensch, T. K. et al. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science 282, 1504–1508 (1998).
Maffei, A., Nataraj, K., Nelson, S. B. & Turrigiano, G. G. Potentiation of cortical inhibition by visual deprivation. Nature 443, 81–84 (2006). This work shows that visual deprivation leaves excitatory connections in L4 unaffected but potentiates BC inhibition of PCs, which shifts the E/I balance in PCs to favour inhibition and may, thus, underlie deprivation-induced degradation of visual function.
Sjöström, P. J. & Gerstner, W. Spike-timing dependent plasticity. Scholarpedia 5, 1362 (2010).
Song, S., Miller, K. D. & Abbott, L. F. Competitive Hebbian learning through spike-timing-dependent synaptic plasticity. Nat. Neurosci. 3, 919–926 (2000).
Vickers, E. D. et al. Parvalbumin-interneuron output synapses show spike-timing-dependent plasticity that contributes to auditory map remodeling. Neuron 99, 720–735.e6 (2018). Using paired recordings in L4 of auditory cortex, this work shows how critical period sound exposure transforms the sign of plasticity from LTD to LTP at PV+ IN to PC synapses, which may provide disinhibition during critical period plasticity.
Field, R. E. et al. Heterosynaptic plasticity determines the set point for cortical excitatory–inhibitory balance. Neuron https://doi.org/10.1016/j.neuron.2020.03.002 (2020). Using electrode stimulation arrays, this work finds that, in developing auditory cortex, homosynaptic and heterosynaptic excitatory and inhibitory inputs to L5 PCs all exhibit STDP; however, compared with homosynaptic inputs, heterosynaptic inputs have a stronger influence on the set point for overall E/I balance.
Wang, L. & Maffei, A. Inhibitory plasticity dictates the sign of plasticity at excitatory synapses. J. Neurosci. 34, 1083–1093 (2014).
D’Amour, J. A. & Froemke, R. C. Inhibitory and excitatory spike-timing-dependent plasticity in the auditory cortex. Neuron https://doi.org/10.1016/j.neuron.2015.03.014 (2015). This work shows how both inhibitory and excitatory neocortical synapses are modified by STDP and how inhibitory plasticity depends on the initial E/I ratio, which helps maintain E/I balance.
Lourenço, J. et al. Non-associative potentiation of perisomatic inhibition alters the temporal coding of neocortical layer 5 pyramidal neurons. PLoS Biol. 12, e1001903 (2014). This work shows that, in L5 PCs, the selective potentiation of perisomatic inhibition via nitric oxide retrograde signalling alters the ability to integrate excitatory inputs and improves spiking precision.
Wiesel, T. N. & Hubel, D. H. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J. Neurophysiol. 28, 1029–1040 (1965).
Kuhlman, S. J. et al. A disinhibitory microcircuit initiates critical-period plasticity in the visual cortex. Nature 501, 543–546 (2013).
Lu, J. T., Li, C. Y., Zhao, J. P., Poo, M. M. & Zhang, X. H. Spike-timing-dependent plasticity of neocortical excitatory synapses on inhibitory interneurons depends on target cell type. J. Neurosci. 27, 9711–9720 (2007). This work shows that PC–MC synapses exhibit the classical temporally asymmetric STDP also found at PC–PC connections, although plasticity at these two synapse types relies on different mechanisms, whereas PC–BC synapses connections depress irrespective of relative timing.
Xue, M., Atallah, B. V. & Scanziani, M. Equalizing excitation–inhibition ratios across visual cortical neurons. Nature 511, 596–600 (2014).
Hennequin, G., Agnes, E. J. & Vogels, T. P. Inhibitory plasticity: balance, control, and codependence. Annu. Rev. Neurosci. 40, 557–579 (2017).
Blackman, A. V., Abrahamsson, T., Costa, R. P., Lalanne, T. & Sjöström, P. J. Target cell-specific short-term plasticity in local circuits. Front. Synaptic Neurosci. 5, 1–13 (2013).
Costa, R. P., Froemke, R. C., Sjöström, P. J. & van Rossum, M. C. W. Unified pre- and postsynaptic long-term plasticity enables reliable and flexible learning. eLife https://doi.org/10.7554/eLife.09457 (2015).
Chistiakova, M. et al. Distinct heterosynaptic plasticity in fast spiking and non-fast-spiking inhibitory neurons in rat visual cortex. J. Neurosci. 39, 6865–6878 (2019).
Zenke, F., Agnes, E. J. & Gerstner, W. Diverse synaptic plasticity mechanisms orchestrated to form and retrieve memories in spiking neural networks. Nat. Commun. 6, 6922 (2015).
Chen, H. X., Jiang, M., Akakin, D. & Roper, S. N. Long-term potentiation of excitatory synapses on neocortical somatostatin-expressing interneurons. J. Neurophysiol. 102, 3251–3259 (2009).
Castillo, P. E., Weisskopf, M. G. & Nicoll, R. A. The role of Ca2+ channels in hippocampal mossy fiber synaptic transmission and long-term potentiation. Neuron 12, 261–269 (1994).
Seol, G. H. et al. Neuromodulators control the polarity of spike-timing-dependent synaptic plasticity. Neuron 55, 919–929 (2007).
Sjöström, P. J. & Häusser, M. A cooperative switch determines the sign of synaptic plasticity in distal dendrites of neocortical pyramidal neurons. Neuron 51, 227–238 (2006).
Huang, S., Huganir, R. L. & Kirkwood, A. Adrenergic gating of Hebbian spike-timing-dependent plasticity in cortical interneurons. J. Neurosci. 33, 13171–13178 (2013). This study is a prime example of a three-factor plasticity learning rule, showing how neuromodulation controls the polarity of STDP at PC synapses onto BCs and MCs in mouse visual cortex.
Sarihi, A. et al. Metabotropic glutamate receptor type 5-dependent long-term potentiation of excitatory synapses on fast-spiking GABAergic neurons in mouse visual cortex. J. Neurosci. 28, 1224–1235 (2008).
Ho, O. H., Delgado, J. Y. & O’Dell, T. J. Phosphorylation of proteins involved in activity-dependent forms of synaptic plasticity is altered in hippocampal slices maintained in vitro. J. Neurochem. 91, 1344–1357 (2004).
Edelmann, E. & Lessmann, V. Dopamine modulates spike timing-dependent plasticity and action potential properties in CA1 pyramidal neurons of acute rat hippocampal slices. Front. Synaptic Neurosci. 3, 6 (2011).
Lourenço, J. et al. Modulation of coordinated activity across cortical layers by plasticity of inhibitory synapses. Cell Rep. 30, 630–641 e635 (2020). This work shows that potentiation of perisomatic inhibition by L5 PC bursting affects information transfer across cortical layers and determines PC phase locking to cognition-relevant oscillations.
Kullander, K. & Topolnik, L. Cortical disinhibitory circuits: cell types, connectivity and function. Trends Neurosci. 44, 643–657 (2021).
Artinian, J. & Lacaille, J. C. Disinhibition in learning and memory circuits: new vistas for somatostatin interneurons and long-term synaptic plasticity. Brain Res. Bull. 141, 20–26 (2018).
Cunha-Reis, D. & Caulino-Rocha, A. VIP modulation of hippocampal synaptic plasticity: a role for VIP receptors as therapeutic targets in cognitive decline and mesial temporal lobe epilepsy. Front. Cell. Neurosci. https://doi.org/10.3389/fncel.2020.00153 (2020).
Khoshkhoo, S., Vogt, D. & Sohal, V. S. Dynamic, cell-type-specific roles for GABAergic interneurons in a mouse model of optogenetically inducible seizures. Neuron 93, 291–298 (2017).
Froemke, R. C., Merzenich, M. M. & Schreiner, C. E. A synaptic memory trace for cortical receptive field plasticity. Nature 450, 425–429 (2007).
Letzkus, J. J. et al. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature 480, 331–335 (2011). This work shows that associative fear learning in auditory cortex relies on the activation of L1 INs, which in turn inhibit L2/3 PV+ INs for an overall disinhibitory effect in cortical circuits.
Gentet, L. J., Avermann, M., Matyas, F., Staiger, J. F. & Petersen, C. C. Membrane potential dynamics of GABAergic neurons in the barrel cortex of behaving mice. Neuron 65, 422–435 (2010).
Niell, C. M. & Stryker, M. P. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65, 472–479 (2010).
Kaneko, M. & Stryker, M. P. Sensory experience during locomotion promotes recovery of function in adult visual cortex. eLife 3, e02798 (2014).
Fu, Y. et al. A cortical circuit for gain control by behavioral state. Cell 156, 1139–1152 (2014).
Fu, Y., Kaneko, M., Tang, Y., Alvarez-Buylla, A. & Stryker, M. P. A cortical disinhibitory circuit for enhancing adult plasticity. eLife 4, e05558 (2015).
Adler, A., Zhao, R., Shin, M. E., Yasuda, R. & Gan, W. B. Somatostatin-expressing interneurons enable and maintain learning-dependent sequential activation of pyramidal neurons. Neuron https://doi.org/10.1016/j.neuron.2019.01.036 (2019).
Letzkus, J. J., Wolff, S. B. & Luthi, A. Disinhibition, a circuit mechanism for associative learning and memory. Neuron 88, 264–276 (2015).
Leroy, F. et al. Enkephalin release from VIP interneurons in the hippocampal CA2/3a region mediates heterosynaptic plasticity and social memory. Mol. Psychiatry 27, 2879–2900 (2022).
Woodin, M. A., Ganguly, K. & Poo, M. M. Coincident pre- and postsynaptic activity modifies GABAergic synapses by postsynaptic changes in Cl- transporter activity. Neuron 39, 807–820 (2003). Using both hippocampal cultures and acute slices, this influential study shows that coincident presynaptic and postsynaptic activity modifies GABA reversal potential locally by decreasing chloride co-transporter activity.
Ormond, J. & Woodin, M. A. Disinhibition mediates a form of hippocampal long-term potentiation in area CA1. PLoS ONE 4, e7224 (2009).
Ormond, J. & Woodin, M. A. Disinhibition-mediated LTP in the hippocampus is synapse specific. Front. Cell Neurosci. 5, 17 (2011).
Diering, G. H. & Huganir, R. L. The AMPA receptor code of synaptic plasticity. Neuron 100, 314–329 (2018).
Diaz-Alonso, J. & Nicoll, R. A. AMPA receptor trafficking and LTP: carboxy-termini, amino-termini and TARPs. Neuropharmacology 197, 108710 (2021).
Kurotani, T., Yamada, K., Yoshimura, Y., Crair, M. C. & Komatsu, Y. State-dependent bidirectional modification of somatic inhibition in neocortical pyramidal cells. Neuron 57, 905–916 (2008).
Komatsu, Y. GABAB receptors, monoamine receptors, and postsynaptic inositol trisphosphate-induced Ca2+ release are involved in the induction of long-term potentiation at visual cortical inhibitory synapses. J. Neurosci. 16, 6342–6352 (1996).
Sjöström, P. J. & Nelson, S. B. Spike timing, calcium signals and synaptic plasticity. Curr. Opin. Neurobiol. 12, 305–314 (2002).
Marsden, K. C., Shemesh, A., Bayer, K. U. & Carroll, R. C. Selective translocation of Ca2+/calmodulin protein kinase IIα (CaMKIIα) to inhibitory synapses. Proc. Natl Acad. Sci. USA 107, 20559–20564 (2010).
Marsden, K. C., Beattie, J. B., Friedenthal, J. & Carroll, R. C. NMDA receptor activation potentiates inhibitory transmission through GABA receptor-associated protein-dependent exocytosis of GABAA receptors. J. Neurosci. 27, 14326–14337 (2007).
Petrini, E. M. et al. Synaptic recruitment of gephyrin regulates surface GABAA receptor dynamics for the expression of inhibitory LTP. Nat. Commun. 5, 3921 (2014).
Chiu, C. Q. et al. Input-specific NMDAR-dependent potentiation of dendritic GABAergic inhibition. Neuron 97, 368–377 e363 (2018). By combining optogenetics with electrophysiology, this work demonstrates how activation of NMDARs selectively potentiates inhibition from SST+ INs onto neocortical PCs, revealing a candidate mechanism for regulating the E/I balance specifically in PC dendrites.
Pafundo, D. E., Miyamae, T., Lewis, D. A. & Gonzalez-Burgos, G. Presynaptic effects of N-methyl-d-aspartate receptors enhance parvalbumin cell-mediated inhibition of pyramidal cells in mouse prefrontal cortex. Biol. Psychiatry 84, 460–470 (2018).
Wong, H. H., Rannio, S., Jones, V., Thomazeau, A. & Sjöström, P. J. NMDA receptors in axons: there’s no coincidence. J. Physiol. 599, 367–387 (2021).
Bouvier, G., Larsen, R. S., Rodriguez-Moreno, A., Paulsen, O. & Sjöström, P. J. Towards resolving the presynaptic NMDA receptor debate. Curr. Opin. Neurobiol. 51, 1–7 (2018).
Dore, K. et al. Unconventional NMDA receptor signaling. J. Neurosci. 37, 10800–10807 (2017).
Buchanan, K. A. et al. Target-specific expression of presynaptic NMDA receptors in neocortical microcircuits. Neuron 75, 451–466 (2012).
Kullmann, D. M. & Lamsa, K. P. Long-term synaptic plasticity in hippocampal interneurons. Nat. Rev. Neurosci. 8, 687–699 (2007).
Szabo, A. et al. Calcium-permeable AMPA receptors provide a common mechanism for LTP in glutamatergic synapses of distinct hippocampal interneuron types. J. Neurosci. 32, 6511–6516 (2012).
Oren, I., Nissen, W., Kullmann, D. M., Somogyi, P. & Lamsa, K. P. Role of ionotropic glutamate receptors in long-term potentiation in rat hippocampal CA1 oriens-lacunosum moleculare interneurons. J. Neurosci. 29, 939–950 (2009).
Nissen, W., Szabo, A., Somogyi, J., Somogyi, P. & Lamsa, K. P. Cell type-specific long-term plasticity at glutamatergic synapses onto hippocampal interneurons expressing either parvalbumin or CB1 cannabinoid receptor. J. Neurosci. 30, 1337–1347 (2010).
Camiré, O. & Topolnik, L. Dendritic calcium nonlinearities switch the direction of synaptic plasticity in fast-spiking interneurons. J. Neurosci. 34, 3864–3877 (2014). This ground-breaking study shows how, in the mouse hippocampal CA1 region, TBS of PC–BC connections elicits LTP when paired with subthreshold BC activation but evokes LTD when paired with BC spiking.
Toth, K. & McBain, C. J. Target-specific expression of pre- and postsynaptic mechanisms. J. Physiol. 525, 41–51 (2000).
Maccaferri, G., Toth, K. & McBain, C. J. Target-specific expression of presynaptic mossy fiber plasticity. Science 279, 1368–1370 (1998).
Lalanne, T., Oyrer, J., Farrant, M. & Sjöström, P. J. Synapse type-dependent expression of calcium-permeable AMPA receptors. Front. Synaptic Neurosci. 10, 34 (2018).
Lalanne, T. et al. Synapse-specific expression of calcium-permeable AMPA receptors in neocortical layer 5. J. Physiol. 594, 837–861 (2016).
Vasuta, C. et al. Metaplastic regulation of CA1 schaffer collateral pathway plasticity by hebbian MGluR1a-mediated plasticity at excitatory synapses onto somatostatin-expressing interneurons. eNeuro https://doi.org/10.1523/ENEURO.0051-15.2015 (2015).
Perez, Y., Morin, F. & Lacaille, J. C. A Hebbian form of long-term potentiation dependent on mGluR1a in hippocampal inhibitory interneurons. Proc. Natl Acad. Sci. USA 98, 9401–9406 (2001).
Lapointe, V. et al. Synapse-specific mGluR1-dependent long-term potentiation in interneurones regulates mouse hippocampal inhibition. J. Physiol. 555, 125–135 (2004).
Topolnik, L., Azzi, M., Morin, F., Kougioumoutzakis, A. & Lacaille, J. C. mGluR1/5 subtype-specific calcium signalling and induction of long-term potentiation in rat hippocampal oriens/alveus interneurones. J. Physiol. 575, 115–131 (2006).
Hasselmo, M. E. The role of acetylcholine in learning and memory. Curr. Opin. Neurobiol. 16, 710–715 (2006).
Bear, M. F. & Singer, W. Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature 320, 172–176 (1986).
Takkala, P. & Woodin, M. A. Muscarinic acetylcholine receptor activation prevents disinhibition-mediated LTP in the hippocampus. Front. Cell Neurosci. 7, 16 (2013).
Mitsushima, D., Sano, A. & Takahashi, T. A cholinergic trigger drives learning-induced plasticity at hippocampal synapses. Nat. Commun. 4, 2760 (2013). This work shows that contextual fear learning enhances the strength of inhibitory inputs onto hippocampal PCs through nAChR activation but not mAChR activation.
Morales-Weil, K. et al. Priming of GABAergic long-term potentiation by muscarinic receptors. Neuroscience 428, 242–251 (2020).
Griguoli, M. & Cherubini, E. Regulation of hippocampal inhibitory circuits by nicotinic acetylcholine receptors. J. Physiol. 590, 655–666 (2012).
Castillo, P. E., Younts, T. J., Chavez, A. E. & Hashimotodani, Y. Endocannabinoid signaling and synaptic function. Neuron 76, 70–81 (2012).
Castillo, P. E., Chiu, C. Q. & Carroll, R. C. Long-term plasticity at inhibitory synapses. Curr. Opin. Neurobiol. 21, 328–338 (2011).
Piette, C., Cui, Y., Gervasi, N. & Venance, L. Lights on endocannabinoid-mediated synaptic potentiation. Front. Mol. Neurosci. 13, 132 (2020).
Sjöström, P. J., Turrigiano, G. G. & Nelson, S. B. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron 39, 641–654 (2003).
Chevaleyre, V. & Castillo, P. E. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron 38, 461–472 (2003).
Jiang, B. et al. The maturation of GABAergic transmission in visual cortex requires endocannabinoid-mediated LTD of inhibitory inputs during a critical period. Neuron 66, 248–259 (2010).
Gibson, J. R., Bartley, A. F. & Huber, K. M. Role for the subthreshold currents ILeak and IH in the homeostatic control of excitability in neocortical somatostatin-positive inhibitory neurons. J. Neurophysiol. 96, 420–432 (2006). This work shows that an increase in excitability of SST+ INs in somatosensory cortex following a 2.5-day pharmacological blockade of spiking, which ultimately increases inhibitory drive, is an example of a non-homeostatic response to reduced circuit activity.
Vogels, T. P., Rajan, K. & Abbott, L. F. Neural network dynamics. Annu. Rev. Neurosci. 28, 357–376 (2005).
Turrigiano, G. G. & Nelson, S. B. Homeostatic plasticity in the developing nervous system. Nat. Rev. Neurosci. 5, 97–107 (2004).
Mongillo, G., Curti, E., Romani, S. & Amit, D. J. Learning in realistic networks of spiking neurons and spike-driven plastic synapses. Eur. J. Neurosci. 21, 3143–3160 (2005).
Royer, S. & Paré, D. Conservation of total synaptic weight through balanced synaptic depression and potentiation. Nature 422, 518–522 (2003).
Turrigiano, G., Abbott, L. F. & Marder, E. Activity-dependent changes in the intrinsic properties of cultured neurons. Science 264, 974–977 (1994).
Turrigiano, G. G., Leslie, K. R., Desai, N. S., Rutherford, L. C. & Nelson, S. B. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature 391, 892–896 (1998).
Kilman, V., van Rossum, M. C. & Turrigiano, G. G. Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABAA receptors clustered at neocortical synapses. J. Neurosci. 22, 1328–1337 (2002).
Echegoyen, J., Neu, A., Graber, K. D. & Soltesz, I. Homeostatic plasticity studied using in vivo hippocampal activity-blockade: synaptic scaling, intrinsic plasticity and age-dependence. PLoS ONE 2, e700 (2007).
Wetmore, C., Olson, L. & Bean, A. J. Regulation of brain-derived neurotrophic factor (BDNF) expression and release from hippocampal neurons is mediated by non-NMDA type glutamate receptors. J. Neurosci. 14, 1688–1700 (1994).
Rutherford, L. C., Nelson, S. B. & Turrigiano, G. G. BDNF has opposite effects on the quantal amplitude of pyramidal neuron and interneuron excitatory synapses. Neuron 21, 521–530 (1998).
Hengen, K. B., Lambo, M. E., Van Hooser, S. D., Katz, D. B. & Turrigiano, G. G. Firing rate homeostasis in visual cortex of freely behaving rodents. Neuron 80, 335–342 (2013).
Keck, T., Hubener, M. & Bonhoeffer, T. Interactions between synaptic homeostatic mechanisms: an attempt to reconcile BCM theory, synaptic scaling, and changing excitation/inhibition balance. Curr. Opin. Neurobiol. 43, 87–93 (2017).
Chen, J. L. et al. Structural basis for the role of inhibition in facilitating adult brain plasticity. Nat. Neurosci. 14, 587–594 (2011).
Chen, J. L. et al. Clustered dynamics of inhibitory synapses and dendritic spines in the adult neocortex. Neuron 74, 361–373 (2012).
van Versendaal, D. et al. Elimination of inhibitory synapses is a major component of adult ocular dominance plasticity. Neuron 74, 374–383 (2012).
Debanne, D., Inglebert, Y. & Russier, M. Plasticity of intrinsic neuronal excitability. Curr. Opin. Neurobiol. 54, 73–82 (2019).
Desai, N. S., Rutherford, L. C. & Turrigiano, G. G. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat. Neurosci. 2, 515–520 (1999).
MacLean, J. N., Zhang, Y., Johnson, B. R. & Harris-Warrick, R. M. Activity-independent homeostasis in rhythmically active neurons. Neuron 37, 109–120 (2003).
Marder, E. & Prinz, A. A. Current compensation in neuronal homeostasis. Neuron 37, 2–4 (2003).
Ross, S. T. & Soltesz, I. Long-term plasticity in interneurons of the dentate gyrus. Proc. Natl Acad. Sci. USA 98, 8874 (2001).
Dasgupta, D. & Sikdar, S. K. Calcium permeable AMPA receptor-dependent long lasting plasticity of intrinsic excitability in fast spiking interneurons of the dentate gyrus decreases inhibition in the granule cell layer. Hippocampus 25, 269–285 (2015).
Dasgupta, D. & Sikdar, S. K. Heterogeneous network dynamics in an excitatory–inhibitory network model by distinct intrinsic mechanisms in the fast spiking interneurons. Brain Res. 1714, 27–44 (2019).
Campanac, E. et al. Enhanced intrinsic excitability in basket cells maintains excitatory–inhibitory balance in hippocampal circuits. Neuron 77, 712–722 (2013).
Mansvelder, H. D., Verhoog, M. B. & Goriounova, N. A. Synaptic plasticity in human cortical circuits: cellular mechanisms of learning and memory in the human brain? Curr. Opin. Neurobiol. 54, 186–193 (2019).
Chittajallu, R. et al. Activity-dependent tuning of intrinsic excitability in mouse and human neurogliaform cells. eLife https://doi.org/10.7554/eLife.57571 (2020).
Suzuki, N., Tang, C. & Bekkers, J. Persistent barrage firing in cortical interneurons can be induced in vivo and may be important for the suppression of epileptiform activity. Front. Cell. Neurosci. https://doi.org/10.3389/fncel.2014.00076 (2014).
Desai, N. S., Nelson, S. B. & Turrigiano, G. G. Activity-dependent regulation of excitability in rat visual cortical neurons. Neurocomputing 26–27, 101–106 (1999).
Desai, N. S., Rutherford, L. C. & Turrigiano, G. G. BDNF regulates the intrinsic excitability of cortical neurons. Learn. Mem. 6, 284–291 (1999).
Lee, S.-H., Land, P. W. & Simons, D. J. Layer- and cell-type-specific effects of neonatal whisker-trimming in adult rat barrel cortex. J. Neurophysiol. 97, 4380–4385 (2007).
Bartley, A. F., Huang, Z. J., Huber, K. M. & Gibson, J. R. Differential activity-dependent, homeostatic plasticity of two neocortical inhibitory circuits. J. Neurophysiol. 100, 1983–1994 (2008).
Sun, Q.-Q. Experience-dependent intrinsic plasticity in interneurons of barrel cortex layer IV. J. Neurophysiol. 102, 2955–2973 (2009).
Dehorter, N. et al. Tuning of fast-spiking interneuron properties by an activity-dependent transcriptional switch. Science 349, 1216 (2015).
Gainey, M. A., Aman, J. W. & Feldman, D. E. Rapid disinhibition by adjustment of PV intrinsic excitability during whisker map plasticity in mouse S1. J. Neurosci. 38, 4749 (2018). This work shows that whisker deprivation reduces the intrinsic excitability of mouse barrel cortex PV+ INs, which leads to disinhibition and to homeostatic stabilization of feedforward E/I balance in PCs.
Miller, M. N., Okaty, B. W., Kato, S. & Nelson, S. B. Activity-dependent changes in the firing properties of neocortical fast-spiking interneurons in the absence of large changes in gene expression. Dev. Neurobiol. 71, 62–70 (2011).
Zhong, P. & Yan, Z. Differential regulation of the excitability of prefrontal cortical fast-spiking interneurons and pyramidal neurons by serotonin and fluoxetine. PLoS ONE 6, e16970 (2011).
Takesian, A. E., Kotak, V. C. & Sanes, D. H. Age-dependent effect of hearing loss on cortical inhibitory synapse function. J. Neurophysiol. 107, 937–947 (2012).
Turrigiano, G. G. & Nelson, S. B. Hebb and homeostasis in neuronal plasticity. Curr. Opin. Neurobiol. 10, 358–364 (2000).
Itami, C., Kimura, F. & Nakamura, S. Brain-derived neurotrophic factor regulates the maturation of layer 4 fast-spiking cells after the second postnatal week in the developing barrel cortex. J. Neurosci. 27, 2241–2252 (2007).
Okaty, B. W., Miller, M. N., Sugino, K., Hempel, C. M. & Nelson, S. B. Transcriptional and electrophysiological maturation of neocortical fast-spiking GABAergic interneurons. J. Neurosci. 29, 7040–7052 (2009).
Doischer, D. et al. Postnatal differentiation of basket cells from slow to fast signaling devices. J. Neurosci. 28, 12956–12968 (2008).
Chattopadhyaya, B. et al. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J. Neurosci. 24, 9598–9611 (2004).
Takesian, A. E., Kotak, V. C., Sharma, N. & Sanes, D. H. Hearing loss differentially affects thalamic drive to two cortical interneuron subtypes. J. Neurophysiol. 110, 999–1008 (2013).
Goldberg, E. M. et al. K+ channels at the axon initial segment dampen near-threshold excitability of neocortical fast-spiking GABAergic interneurons. Neuron 58, 387–400 (2008).
Wonders, C. P. & Anderson, S. A. The origin and specification of cortical interneurons. Nat. Rev. Neurosci. 7, 687–696 (2006).
Flames, N. et al. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J. Neurosci. 27, 9682–9695 (2007).
Vullhorst, D. et al. Selective expression of ErbB4 in interneurons, but not pyramidal cells, of the rodent hippocampus. J. Neurosci. 29, 12255 (2009).
Wen, L. et al. Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. Proc. Natl Acad. Sci. USA 107, 1211 (2010).
Li, K.-X. et al. Neuregulin 1 regulates excitability of fast-spiking neurons through Kv1.1 and acts in epilepsy. Nat. Neurosci. 15, 267–273 (2012).
Zhang, W. & Linden, D. J. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat. Rev. Neurosci. 4, 885–900 (2003).
Wang, Z., Xu, N. L., Wu, C. P., Duan, S. & Poo, M. M. Bidirectional changes in spatial dendritic integration accompanying long-term synaptic modifications. Neuron 37, 463–472 (2003).
Frick, A., Magee, J. & Johnston, D. LTP is accompanied by an enhanced local excitability of pyramidal neuron dendrites. Nat. Neurosci. 7, 126–135 (2004).
Campanac, E., Daoudal, G., Ankri, N. & Debanne, D. Downregulation of dendritic Ih in CA1 pyramidal neurons after LTP. J. Neurosci. 28, 8635–8643 (2008).
Daoudal, G. & Debanne, D. Long-term plasticity of intrinsic excitability: learning rules and mechanisms. Learn. Mem. 10, 456–465 (2003).
Hensch, T. K. & Quinlan, E. M. Critical periods in amblyopia. Vis. Neurosci. 35, E014 (2018).
Sjöström, P. J., Turrigiano, G. G. & Nelson, S. B. Rate, timing, and cooperativity jointly determine cortical synaptic plasticity. Neuron 32, 1149–1164 (2001).
Pawlak, V., Wickens, J. R., Kirkwood, A. & Kerr, J. N. D. Timing is not everything: neuromodulation opens the STDP gate. Front. Synaptic Neurosci. 2, 146 (2010).
Larsen, R. S., Rao, D., Manis, P. B. & Philpot, B. D. STDP in the developing sensory neocortex. Front. Synaptic Neurosci. 2, 9 (2010).
Larsen, R. S. et al. Synapse-specific control of experience-dependent plasticity by presynaptic NMDA receptors. Neuron 83, 879–893 (2014).
Stent, G. S. A physiological mechanism for Hebb’s postulate of learning. Proc. Natl Acad. Sci. Usa. 70, 997–1001 (1973).
Kilb, W. When are depolarizing GABAergic responses excitatory? Front. Mol. Neurosci. https://doi.org/10.3389/fnmol.2021.747835 (2021).
Ben-Ari, Y., Gaiarsa, J. L., Tyzio, R. & Khazipov, R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol. Rev. 87, 1215–1284 (2007).
Bonifazi, P. et al. GABAergic hub neurons orchestrate synchrony in developing hippocampal networks. Science 326, 1419–1424 (2009).
van Welie, I., Smith, I. T. & Watt, A. J. The metamorphosis of the developing cerebellar microcircuit. Curr. Opin. Neurobiol. 21, 245–253 (2011).
Zilberter, M. Reality of inhibitory GABA in neonatal brain: time to rewrite the textbooks? J. Neurosci. 36, 10242–10244 (2016).
Haam, J. et al. GABA is excitatory in adult vasopressinergic neuroendocrine cells. J. Neurosci. 32, 572–582 (2012).
Mongillo, G., Rumpel, S. & Loewenstein, Y. Inhibitory connectivity defines the realm of excitatory plasticity. Nat. Neurosci. 21, 1463–1470 (2018).
Minsky, M. L. in Computers and Thought (eds E. A. Feigenbaum & J. Feldman) 406-450 (McGraw-Hill, 1963).
Sacramento, J., Costa, R. P., Bengio, Y. & Senn, W. in Advances in Neural Information Processing Systems 31 (eds Bengio, S., et al.) 8721–8732 (Curran Associates, Inc., 2018).
Greedy, W., Zhu, H. W., Pemberton, J., Mellor, J. & Costa, R. P. Single-phase deep learning in cortico-cortical networks. Preprint at arXiv arXiv:2206.11769 (2022).
Sejnowski, T. J. & Rosenberg, C. R. in Neurocomputing: Foundations of Research 661–672 (Bradford Books, 1988).
Lecun, Y. et al. Handwritten digit recognition with a back-propagation network. In Advances in Neural Information Processing Systems 1989 (1990).
Rumelhart, D. E., Hinton, G. E. & Williams, R. J. Learning representations by back-propagating errors. Nature 323, 533 (1986).
Roelfsema, P. R. & Holtmaat, A. Control of synaptic plasticity in deep cortical networks. Nat. Rev. Neurosci. 19, 166–180 (2018).
Halvagal, M. S. & Zenke, F. The combination of Hebbian and predictive plasticity learns invariant object representations in deep sensory networks. Preprint at bioRxiv https://doi.org/10.1101/2022.03.17.484712 (2022).
Journé, A., Rodriguez, H. G., Guo, Q. & Moraitis, T. Hebbian deep learning without feedback. Preprint at arXiv arXiv:2209.11883 (2022).
Illing, B., Ventura, J., Bellec, G. & Gerstner, W. Local plasticity rules can learn deep representations using self-supervised contrastive predictions. In Advances in Neural Information Processing Systems 34, 30365–30379 (2021).
Whittington, J. C. R. & Bogacz, R. An approximation of the error backpropagation algorithm in a predictive coding network with local Hebbian synaptic plasticity. Neural Comput. 29, 1229–1262 (2017).
Crick, F. The recent excitement about neural networks. Nature 337, 129–132 (1989).
Grossberg, S. Competitive learning: from interactive activation to adaptive resonance. Cogn. Sci. 11, 23–63 (1987).
Whittington, J. C. R. & Bogacz, R. Theories of error back-propagation in the brain. Trends Cogn. Sci. 23, 235–250 (2019).
Richards, B. A. & Lillicrap, T. P. Dendritic solutions to the credit assignment problem. Curr. Opin. Neurobiol. 54, 28–36 (2018).
Richards, B. A. et al. A deep learning framework for neuroscience. Nat. Neurosci. 22, 1761–1770 (2019).
Marblestone, A. H., Wayne, G. & Kording, K. P. Toward an integration of deep learning and neuroscience. Front. Comput. Neurosci. 10, 94 (2016).
Tripp, B. & Eliasmith, C. Function approximation in inhibitory networks. Neural Netw. 77, 95–106 (2016).
Payeur, A., Guerguiev, J., Zenke, F., Richards, B. A. & Naud, R. Burst-dependent synaptic plasticity can coordinate learning in hierarchical circuits. Nat. Neurosci. 24, 1010–1019 (2021).
Mercier, M. S., Magloire, V., Cornford, J. H. & Kullmann, D. M. Long-term potentiation in neurogliaform interneurons modulates excitation-inhibition balance in the temporoammonic pathway. J. Physiol. https://doi.org/10.1113/JP282753 (2022).
Khan, A. G. et al. Distinct learning-induced changes in stimulus selectivity and interactions of GABAergic interneuron classes in visual cortex. Nat. Neurosci. 21, 851–859 (2018).
Williams, L. E. & Holtmaat, A. Higher-order thalamocortical inputs gate synaptic long-term potentiation via disinhibition. Neuron 101, 91–102.e4 (2019).
Jasper, P. et al. Learning and attention increase visual response selectivity through distinct mechanisms. Neuron 110, 686–697.e6 (2022).
Markram, H., Wang, Y. & Tsodyks, M. Differential signaling via the same axon of neocortical pyramidal neurons. Proc. Natl Acad. Sci. USA 95, 5323–5328 (1998).
Campagnola, L. et al. Local connectivity and synaptic dynamics in mouse and human neocortex. Science 375, eabj5861 (2022).
Kodandaramaiah, S. B., Franzesi, G. T., Chow, B. Y., Boyden, E. S. & Forest, C. R. Automated whole-cell patch-clamp electrophysiology of neurons in vivo. Nat. Methods 9, 585–587 (2012).
Annecchino, L. A. & Schultz, S. R. Progress in automating patch clamp cellular physiology. Brain Neurosci. Adv. 2, 2398212818776561 (2018).
Perin, R. & Markram, H. A computer-assisted multi-electrode patch-clamp system. JoVE https://doi.org/10.3791/50630 (2013).
Lalanne, T., Abrahamsson, T. & Sjöström, P. J. Using multiple whole-cell recordings to study spike-timing-dependent plasticity in acute neocortical slices. CSH Protoc. 6, 573–583 (2016).
Song, S., Sjöström, P. J., Reigl, M., Nelson, S. & Chklovskii, D. B. Highly nonrandom features of synaptic connectivity in local cortical circuits. PLoS Biol. 3, e68 (2005).
Zhang, Y. P. & Oertner, T. G. Optical induction of synaptic plasticity using a light-sensitive channel. Nat. Methods 4, 139–141 (2007).
Emiliani, V., Cohen, A. E., Deisseroth, K. & Häusser, M. All-optical interrogation of neural circuits. J. Neurosci. 35, 13917–13926 (2015).
Cela, E. & Sjöström, P. J. Novel optogenetic approaches in epilepsy research. Front. Neurosci. https://doi.org/10.3389/fnins.2019.00947 (2019).
Cela, E. et al. An optogenetic kindling model of neocortical epilepsy. Sci. Rep. 9, 5236 (2019).
Marin, O. Interneuron dysfunction in psychiatric disorders. Nat. Rev. Neurosci. 13, 107–120 (2012).
Cao, W. et al. Gamma oscillation dysfunction in mPFC leads to social deficits in neuroligin 3 R451C knockin mice. Neuron 98, 670 (2018).
Chao, H. T. et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature 468, 263–269 (2010).
Tabuchi, K. et al. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science 318, 71–76 (2007).
Lewis, D. A., Hashimoto, T. & Volk, D. W. Cortical inhibitory neurons and schizophrenia. Nat. Rev. Neurosci. 6, 312–324 (2005).
Del Pino, I. et al. Erbb4 deletion from fast-spiking interneurons causes schizophrenia-like phenotypes. Neuron 79, 1152–1168 (2013).
Mukherjee, A., Carvalho, F., Eliez, S. & Caroni, P. Long-lasting rescue of network and cognitive dysfunction in a genetic Schizophrenia model. Cell 178, 1387–1402.e14 (2019).
Kempter, R., Gerstner, W. & van Hemmen, J. L. Hebbian learning and spiking neurons. Phys. Rev. E 59, 4498–4514 (1999).
Gerstner, W., Kempter, R., van Hemmen, J. L. & Wagner, H. A neuronal learning rule for sub-millisecond temporal coding. Nature 383, 76–81 (1996).
Barack, D. L. et al. A call for more clarity around causality in neuroscience. Trends Neurosci. https://doi.org/10.1016/j.tins.2022.06.003 (2022).
Chistiakova, M., Bannon, N. M., Bazhenov, M. & Volgushev, M. Heterosynaptic plasticity: multiple mechanisms and multiple roles. Neuroscientist 20, 483–498 (2014).
Andersen, P., Sundberg, S. H., Sveen, O. & Wigstrom, H. Specific long-lasting potentiation of synaptic transmission in hippocampal slices. Nature 266, 736–737 (1977).
Schuman, E. M. Synapse specificity and long-term information storage. Neuron 18, 339–342 (1997).
Alemi, A., Baldassi, C., Brunel, N. & Zecchina, R. A three-threshold learning rule approaches the maximal capacity of recurrent neural networks. PLoS Comput. Biol. 11, e1004439 (2015).
Engert, F. & Bonhoeffer, T. Synapse specificity of long-term potentiation breaks down at short distances. Nature 388, 279–284 (1997).
Harvey, C. D. & Svoboda, K. Locally dynamic synaptic learning rules in pyramidal neuron dendrites. Nature 450, 1195–1200 (2007).
Schuman, E. M. & Madison, D. V. Locally distributed synaptic potentiation in the hippocampus. Science 263, 532–536 (1994).
Lynch, G. S., Dunwiddie, T. & Gribkoff, V. Heterosynaptic depression: a postsynaptic correlate of long-term potentiation. Nature 266, 737–739 (1977).
Bramham, C. R. & Srebro, B. Induction of long-term depression and potentiation by low- and high-frequency stimulation in the dentate area of the anesthetized rat: magnitude, time course and EEG. Brain Res. 405, 100–107 (1987).
Dudek, S. M. & Bear, M. F. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-d-aspartate receptor blockade. Proc. Natl Acad. Sci. Usa. 89, 4363–4367 (1992).
Mulkey, R. M. & Malenka, R. C. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron 9, 967–975 (1992).
Naraghi, M. & Neher, E. Linearized buffered Ca2+ diffusion in microdomains and its implications for calculation of [Ca2+] at the mouth of a calcium channel. J. Neurosci. 17, 6961–6973 (1997).
Kaiser, K. M., Lübke, J., Zilberter, Y. & Sakmann, B. Postsynaptic calcium influx at single synaptic contacts between pyramidal neurons and bitufted interneurons in layer 2/3 of rat neocortex is enhanced by backpropagating action potentials. J. Neurosci. 24, 1319–1329 (2004).
Nicoll, R. A. & Schmitz, D. Synaptic plasticity at hippocampal mossy fibre synapses. Nat. Rev. Neurosci. 6, 863–876 (2005).
Foncelle, A. et al. Modulation of spike-timing dependent plasticity: towards the inclusion of a third factor in computational models. Front. Comput. Neurosci. https://doi.org/10.3389/fncom.2018.00049 (2018).
Sjöström, P. J., Turrigiano, G. G. & Nelson, S. B. Endocannabinoid-dependent neocortical layer-5 LTD in the absence of postsynaptic spiking. J. Neurophysiol. 92, 3338–3343 (2004).
Gambino, F. et al. Sensory-evoked LTP driven by dendritic plateau potentials in vivo. Nature 515, 116–119 (2014).
Lisman, J., Grace, A. A. & Duzel, E. A neoHebbian framework for episodic memory; role of dopamine-dependent late LTP. Trends Neurosci. 34, 536–547 (2011).
Kuśmierz, L., Isomura, T. & Toyoizumi, T. Learning with three factors: modulating Hebbian plasticity with errors. Curr. Opin. Neurobiol. 46, 170–177 (2017).
Frémaux, N. & Gerstner, W. Neuromodulated spike-timing-dependent plasticity, and theory of three-factor learning rules. Front. Neural Circuits 9, 85 (2015).
Destexhe, A., Rudolph, M. & Pare, D. The high-conductance state of neocortical neurons in vivo. Nat. Rev. Neurosci. 4, 739–751 (2003).
Ganguly, K., Kiss, L. & Poo, M. Enhancement of presynaptic neuronal excitability by correlated presynaptic and postsynaptic spiking. Nat. Neurosci. 3, 1018–1026 (2000).
Cudmore, R. H. & Turrigiano, G. G. Long-term potentiation of intrinsic excitability in LV visual cortical neurons. J. Neurophysiol. 92, 341–348 (2004).
Losonczy, A., Makara, J. K. & Magee, J. C. Compartmentalized dendritic plasticity and input feature storage in neurons. Nature 452, 436–441 (2008).
Grubb, M. S. & Burrone, J. Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature 465, 1070–1074 (2010).
Acknowledgements
The authors thank A. Watt, W. Gerstner, R. P. Costa, A. Suvrathan, C. Bourque, H. Wong, O. Camiré, S. Rannio and Sjöström laboratory members for help and useful discussions. P.J.S. was supported by Fonds de Recherche du Québec - Santé (FRQS) CB 254033 and Natural Sciences and Engineering Research Council of Canada (NSERC) DG 2017-04730. A.R.M. was supported by doctoral awards from FRQS (287520) and Healthy Brains for Healthy Lives (HBHL). C.Y.C.C. was in receipt of doctoral awards NSERC D3-534171-2019 and Fonds de Recherche du Québec - Nature et technologies (FRQNT) 275075. A.W. was a recipient of HBHL, Integrated Program in Neuroscience(IPN) and Quebec Bio-Imaging Network (QBIN) fellowships. N.C. is an NSERC USRA (552184-2020) and FRQNT BRPC Supplement (298265) recipient. M.H. was funded by Canada Summer Jobs (CSJ).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, contributed substantially to discussion of the content and wrote the article. A.R.M, C.Y.C.C, A.W. and P.J.S. reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neuroscience thanks Anthony Holtmaat, and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Anti-Hebbian
-
A rule that disobeys Hebb’s postulate, such as synaptic strengthening resulting from asynchronous firing in connected cells or, conversely, coincident firing eliciting synaptic weakening.
- Coincidence detection
-
A process by which a neuron or a neuronal circuit can detect the occurrence of temporally close but spatially distributed input signals to form associations between distinct events.
- Disinhibition
-
Reduction of inhibitory drive onto an excitatory neuron.
- E → I plasticity
-
Plasticity at synapses from excitatory to inhibitory cells.
- E/I balance
-
The relative contributions of excitatory and inhibitory synaptic input to an individual neuron or in a local circuit.
- Excitatory postsynaptic potential (EPSP)-spike potentiation
-
The ability of long-term potentiation (LTP) to additionally increase the potentiated input’s capacity to drive postsynaptic spiking by altering postsynaptic excitability.
- Expression of plasticity
-
The mechanisms that alter the strength of a synaptic connection, such as the addition or removal of neurotransmitter receptor channels postsynaptically, or changes of release probability presynaptically.
- Homeostatic plasticity
-
The capacity of neurons to regulate their own excitability and synaptic drive slowly over many hours in the face of changes in network structure and activity.
- I → E plasticity
-
Plasticity at synapses from inhibitory to excitatory cells, which has often been called inhibitory long-term potentiation (LTP) or inhibitory long-term depression (LTD).
- Induction of plasticity
-
The processes that trigger the expression of long-term plasticity; typically a repeated activity pattern, but could also be chemical or pharmacological.
- Miniature excitatory postsynaptic current
-
A depolarizing current elicited by excitatory neurotransmitters such as glutamate that promotes spiking in the postsynaptic neuron.
- Miniature inhibitory postsynaptic current
-
A hyperpolarizing current elicited by inhibitory neurotransmitters such as GABA that reduces spiking in the postsynaptic neuron.
- Negative feedback
-
A mechanism that acts similar to a thermostat to keep a parameter such as temperature or activity within reasonable bounds by reducing it if too high and increasing it if too low.
- Positive feedback
-
A mechanism that achieves run-away regenerative events, such as voltage-dependent sodium channels driving action potential rise; the more they depolarize, the more they open and promote further depolarization.
- Quantal amplitude
-
The release of one synaptic vesicle containing a stereotyped amount of neurotransmitter — a quantum — elicits a postsynaptic response of one quantal amplitude.
- Reversal potential
-
The membrane potential at which an ion channel current reverses its sign.
- Rheobase
-
The minimal current amplitude needed to be injected into a cell to elicit an action potential. It is a measure of membrane potential excitability.
- Synapse type-specific plasticity
-
The activity requirements that determine plasticity depend on the synapse type, which in turn is related to the presynaptic and the postsynaptic cell types.
- Theta burst stimulation
-
(TBS). Short bursts of stimulation at high frequency, typically 100 Hz, with the bursts themselves applied at 5–8 Hz, to mimic hippocampal theta rhythm and to achieve pre-priming disinhibition, which yields more long-term potentiation (LTP) while improving biological realism.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
McFarlan, A.R., Chou, C.Y.C., Watanabe, A. et al. The plasticitome of cortical interneurons. Nat Rev Neurosci 24, 80–97 (2023). https://doi.org/10.1038/s41583-022-00663-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41583-022-00663-9