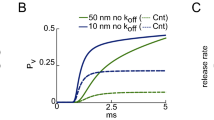
Abstract
The molecular composition of presynaptic and postsynaptic neuronal terminals is dynamic, and yet long-term stabilizations in postsynaptic responses are necessary for synaptic development and long-term plasticity. The need to reconcile these concepts is further complicated by learning- and memory-related plastic changes in the molecular make-up of synapses. Advances in single-particle tracking mean that we can now quantify the number and diffusive properties of specific synaptic molecules, while statistical thermodynamics provides a framework to analyse these molecular fluctuations. In this Review, we discuss the use of these approaches to gain quantitative descriptions of the processes underlying the turnover, long-term stability and plasticity of postsynaptic receptors and show how these can help us to understand the balance between local molecular turnover and synaptic structural identity and integrity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Frank, E. & Fischbach, G. D. Early events in neuromuscular junction formation in vitro. Induction of acetylcholine receptor clusters in the postsynaptic membrane and morphology of newly formed synapses. J. Cell Biol. 83, 143–158 (1979).
Triller, A., Cluzeaud, F., Pfeiffer, F., Betz, H. & Korn, H. Distribution of glycine receptors at central synapses: an immunoelectron microscopy study. J. Cell Biol. 101, 683–688 (1985).
Peters, A., Palay, S. L. & Webster, H. deF. The Fine Structure of the Nervous System: Neurons and Their Supporting Cells (W. B. Sanders, 1976).
Singer, S. J. & Nicolson, G. L. The fluid mosaic model of the structure of cell membranes. Science 175, 720–731 (1972).
Young, S. H. & Poo, M. M. Rapid lateral diffusion of extrajunctional acetylcholine receptors in the developing muscle membrane of Xenopus tadpole. J. Neurosci. 3, 225–231 (1983).
Crick, F. Neurobiology: memory and molecular turnover. Nature 312, 101–101 (1984).
Anderson, M. J. & Cohen, M. W. Nerve‐induced and spontaneous redistribution of acetylcholine receptors on cultured muscle cells. J. Physiol. 268, 757–773 (1977).
Axelrod, D. et al. Lateral motion of fluorescently labeled acetylcholine receptors in membranes of developing muscle fibers. Proc. Natl Acad. Sci. USA 73, 4594–4598 (1976).
Rosenberg, M., Meier, J., Triller, A. & Vannier, C. Dynamics of glycine receptor insertion in the neuronal plasma membrane. J. Neurosci. 21, 5036–5044 (2001).
Passafaro, M., Piëch, V. & Sheng, M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat. Neurosci. 4, 917–926 (2001).
Meier, J., Vannier, C., Sergé, A., Triller, A. & Choquet, D. Fast and reversible trapping of surface glycine receptors by gephyrin. Nat. Neurosci. 4, 253–260 (2001). This was the first study to demonstrate neurotransmitter receptor diffusion in the plasma membrane. It also established that the interaction between receptors and scaffold proteins at the postsynaptic domain is reversible.
Borgdorff, A. J. & Choquet, D. Regulation of AMPA receptor lateral movements. Nature 417, 649–653 (2002).
Sergé, A., Fourgeaud, L., Hémar, A. & Choquet, D. Receptor activation and homer differentially control the lateral mobility of metabotropic glutamate receptor 5 in the neuronal membrane. J. Neurosci. 22, 3910–3920 (2002).
Fornasiero, E. F. et al. Precisely measured protein lifetimes in the mouse brain reveal differences across tissues and subcellular fractions. Nat. Commun. 9, 1–17 (2018).
Ziv, N. E. & Brenner, N. Synaptic tenacity or lack thereof: spontaneous remodeling of synapses. Trends Neurosci. 41, 89–99 (2018).
Kasai, H., Matsuzaki, M., Noguchi, J., Yasumatsu, N. & Nakahara, H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 26, 360–368 (2003).
Mongillo, G., Rumpel, S. & Loewenstein, Y. Intrinsic volatility of synaptic connections — a challenge to the synaptic trace theory of memory. Curr. Opin. Neurobiol. 46, 7–13 (2017).
Ziff, E. B. Enlightening the postsynaptic density. Neuron 19, 1163–1174 (1997).
Chen, X. et al. Organization of the core structure of the postsynaptic density. Proc. Natl Acad. Sci. USA 105, 4453–4458 (2008).
Nusser, Z., Hájos, N., Somogyi, P. & Mody, I. Increased number of synaptic GABAA receptors underlies potentiation at hippocampal inhibitory synapses. Nature 395, 172–177 (1998).
Hayashi, Y. et al. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287, 2262–2267 (2000).
Blanpied, T. A., Scott, D. B. & Ehlers, M. D. Dynamics and regulation of clathrin coats at specialized endocytic zones of dendrites and spines. Neuron 36, 435–449 (2002).
Tardin, C., Cognet, L., Bats, C., Lounis, B. & Choquet, D. Direct imaging of lateral movements of AMPA receptors inside synapses. EMBO J. 22, 4656–4665 (2003).
Dahan, M. et al. Diffusion dynamics of glycine receptors revealed by single-quantum dot tracking. Science 302, 442–445 (2003). Tardin et al. (2003) and Dahan et al. (2003) were the first studies to definitively show neurotransmitter receptor diffusion into and out of synapses.
Choquet, D. & Triller, A. The role of receptor diffusion in the organization of the postsynaptic membrane. Nat. Rev. Neurosci. 4, 251–265 (2003).
Triller, A. & Choquet, D. Surface trafficking of receptors between synaptic and extrasynaptic membranes: and yet they do move! Trends Neurosci. 28, 133–139 (2005).
Charrier, C., Ehrensperger, M.-V., Dahan, M., Levi, S. & Triller, A. Cytoskeleton regulation of glycine receptor number at synapses and diffusion in the plasma membrane. J. Neurosci. 26, 8502–8511 (2006).
Nair, D. et al. Super-resolution imaging reveals that AMPA receptors inside synapses are dynamically organized in nanodomains regulated by PSD95. J. Neurosci. 33, 13204–13224 (2013).
Shinohara, Y. Quantification of postsynaptic density proteins: glutamate receptor subunits and scaffolding proteins. Hippocampus 22, 942–953 (2012).
Specht, C. G. et al. Quantitative nanoscopy of inhibitory synapses: counting gephyrin molecules and receptor binding sites. Neuron 79, 308–321 (2013).
Fukazawa, Y. & Shigemoto, R. Intra-synapse-type and inter-synapse-type relationships between synaptic size and AMPAR expression. Curr. Opin. Neurobiol. 22, 446–452 (2012).
Goncalves, J. et al. Nanoscale co-organization and coactivation of AMPAR, NMDAR, and mGluR at excitatory synapses. Proc. Natl Acad. Sci. USA 117, 14503–14511 (2020).
Ferreira, J. S. et al. Distance-dependent regulation of NMDAR nanoscale organization along hippocampal neuron dendrites. Proc. Natl Acad. Sci. USA 117, 24526–24533 (2020).
Maynard, S. A. et al. Identification of a stereotypic molecular arrangement of endogenous glycine receptors at spinal cord synapses. Elife 10, e74441 (2021).
Ladepeche, L. et al. Single-molecule imaging of the functional crosstalk between surface NMDA and dopamine D1 receptors. Proc. Natl Acad. Sci. USA 110, 18005–18010 (2013).
Chen, X. et al. PSD-95 family MAGUKs are essential for anchoring AMPA and NMDA receptor complexes at the postsynaptic density. Proc. Natl Acad. Sci. USA 112, E6983–E6992 (2015).
Racca, C., Stephenson, F. A., Streit, P., Roberts, J. D. B. & Somogyi, P. NMDA receptor content of synapses in stratum radiatum of the hippocampal CA1 area. J. Neurosci. 20, 2512–2522 (2000).
Helm, M. S. et al. A large-scale nanoscopy and biochemistry analysis of postsynaptic dendritic spines. Nat. Neurosci. 24, 1151–1162 (2021).
Yang, X., Le Corronc, H., Legendre, P., Triller, A. & Specht, C. G. Differential regulation of glycinergic and GABAergic nanocolumns at mixed inhibitory synapses. EMBO Rep. 22, e52154 (2021).
Nakahata, Y. et al. Activation-dependent rapid postsynaptic clustering of glycine receptors in mature spinal cord neurons. eNeuro 4, ENEURO.0194-16.2017 (2017).
Petrini, E. M. et al. Synaptic recruitment of gephyrin regulates surface GABAA receptor dynamics for the expression of inhibitory LTP. Nat. Commun. 5, 3921 (2014).
Ashby, M. C., Maier, S. R., Nishimune, A. & Henley, J. M. Lateral diffusion drives constitutive exchange of AMPA receptors at dendritic spines and is regulated by spine morphology. J. Neurosci. 26, 7046–7055 (2006).
Opazo, P. et al. CaMKII triggers the diffusional trapping of surface AMPARs through phosphorylation of stargazin. Neuron 67, 239–252 (2010).
Constals, A. et al. Glutamate-induced AMPA receptor desensitization increases their mobility and modulates short-term plasticity through unbinding from stargazin. Neuron 85, 787–803 (2015).
Heine, M. et al. Surface mobility of postsynaptic AMPARs tunes synaptic transmission. Science 320, 201–205 (2008). This was the first study to show that AMPAR diffusion at the PSD can shape synaptic transmission via lateral diffusion of desensitized AMPARs out of the synapse to enable recovery from synaptic depression.
Groc, L. et al. NMDA receptor surface trafficking and synaptic subunit composition are developmentally regulated by the extracellular matrix protein reelin. J. Neurosci. 27, 10165–10175 (2007).
Renner, M., Schweizer, C., Bannai, H., Triller, A. & Lévi, S. Diffusion barriers constrain receptors at synapses. PLoS ONE 7, e43032 (2012).
Bannai, H. et al. Activity-dependent tuning of inhibitory neurotransmission based on GABAAR diffusion dynamics. Neuron 62, 670–682 (2009).
Hoze, N. et al. Heterogeneity of AMPA receptor trafficking and molecular interactions revealed by superresolution analysis of live cell imaging. Proc. Natl Acad. Sci. USA 109, 17052–17057 (2012).
Masson, J.-B. et al. Mapping the energy and diffusion landscapes of membrane proteins at the cell surface using high-density single-molecule imaging and Bayesian inference: application to the multiscale dynamics of glycine receptors in the neuronal membrane. Biophys. J. 106, 74–83 (2014). This was the first application of Bayesian inference methods to synapses, where trapping of GlyRs with GPHN scaffolds at inhibitory synapses was analysed.
Choquet, D., Sainlos, M. & Sibarita, J. B. Advanced imaging and labelling methods to decipher brain cell organization and function. Nat. Rev. Neurosci. 22, 237–255 (2021).
Lelek, M. et al. Single-molecule localization microscopy. Nat. Rev. Methods Prim. 1, 1–27 (2021).
Chenouard, N. et al. Objective comparison of particle tracking methods. Nat. Methods 11, 281–289 (2014).
Saxton, M. J. Single-particle tracking: connecting the dots. Nat. Methods 5, 671–672 (2008).
Sergé, A., Bertaux, N., Rigneault, H. & Marguet, D. Dynamic multiple-target tracing to probe spatiotemporal cartography of cell membranes. Nat. Methods 5, 687–694 (2008).
Tinevez, J. Y. et al. TrackMate: an open and extensible platform for single-particle tracking. Methods 115, 80–90 (2017).
Kerr, J. M. & Blanpied, T. A. Subsynaptic AMPA receptor distribution is acutely regulated by actin-driven reorganization of the postsynaptic density. J. Neurosci. 32, 658–673 (2012).
Masson, J. B. et al. Inferring maps of forces inside cell membrane microdomains. Phys. Rev. Lett. 102, 048103 (2009).
Schütz, G. J., Schindler, H. & Schmidt, T. Single-molecule microscopy on model membranes reveals anomalous diffusion. Biophys. J. 73, 1073 (1997).
El Beheiry, M., Dahan, M. & Masson, J.-B. InferenceMAP: mapping of single-molecule dynamics with Bayesian inference. Nat. Methods 12, 594–595 (2015).
Persson, F., Lindén, M., Unoson, C. & Elf, J. Extracting intracellular diffusive states and transition rates from single-molecule tracking data. Nat. Methods 10, 265–269 (2013).
Manzo, C. & Garcia-Parajo, M. F. A review of progress in single particle tracking: from methods to biophysical insights. Rep. Prog. Phys. 78, 124601 (2015).
Türkcan, S., Alexandrou, A. & Masson, J. B. A Bayesian inference scheme to extract diffusivity and potential fields from confined single-molecule trajectories. Biophys. J. 102, 2288–2298 (2012).
Coffey, W. T., Kalmykov, Y. P. & Waldron, J. T. The Langevin Equation (World Scientific, 2004).
Vijayabaskar, M. S. Introduction to hidden Markov models and its applications in biology. Methods Mol. Biol. 1552, 1–12 (2017).
Monnier, N. et al. Inferring transient particle transport dynamics in live cells. Nat. Methods 12, 838–840 (2015).
Verdier, H. et al. Learning physical properties of anomalous random walks using graph neural networks. J. Phys. A Math. Theor. 54, 23 (2021).
Rösch, T. C., Oviedo-Bocanegra, L. M., Fritz, G. & Graumann, P. L. SMTracker: a tool for quantitative analysis, exploration and visualization of single-molecule tracking data reveals highly dynamic binding of B. subtilis global repressor AbrB throughout the genome. Sci. Rep. 8, 15747 (2018).
Hansen, A. S. et al. Robust model-based analysis of single-particle tracking experiments with Spot-On. Elife 7, e33125 (2018).
Laurent, F. et al. TRamWAy: mapping physical properties of individual biomolecule random motion in large-scale single-particle tracking experiments. Bioinformatics 38, 3149–3150 (2022).
Lévi, S. et al. Homeostatic regulation of synaptic GlyR numbers driven by lateral diffusion. Neuron 59, 261–273 (2008).
Morise, J. et al. AMPA receptors in the synapse turnover by monomer diffusion. Nat. Commun. 10, 1–18 (2019).
Lisman, J. & Raghavachari, S. A unified model of the presynaptic and postsynaptic changes during LTP at CA1 synapses. Sci. STKE 2006, re11 (2006).
Chirila, A. M. et al. Long-term potentiation of glycinergic synapses triggered by interleukin 1β. Proc. Natl Acad. Sci. USA 111, 8263–8268 (2014).
Compans, B. et al. NMDAR-dependent long-term depression is associated with increased short term plasticity through autophagy mediated loss of PSD-95. Nat. Commun. 12, 1–18 (2021).
Hausrat, T. J. et al. Radixin regulates synaptic GABAA receptor density and is essential for reversal learning and short-term memory. Nat. Commun. 6, 6872 (2015).
Battaglia, S. et al. Activity-dependent inhibitory synapse scaling is determined by gephyrin phosphorylation and subsequent regulation of GABAA receptor diffusion. eNeuro 5, ENEURO.0203-17.2017 (2018). Using super-resolution imaging and SPT, the authors demonstrate how phosphorylation of a scaffold protein can affect its clustering at synapses and subsequent diffusion trapping of neurotransmitter receptors. This mechanism is shown to be implicated in synaptic scaling.
Ferreira, J. S., Kellermayer, B., Carvalho, A. L. & Groc, L. Interplay between NMDA receptor dynamics and the synaptic proteasome. Eur. J. Neurosci. 54, 6000–6011 (2021).
de Luca, E. et al. Inter-synaptic lateral diffusion of GABAA receptors shapes inhibitory synaptic currents. Neuron 95, 63–69.e5 (2017).
Polenghi, A. et al. Kainate receptor activation shapes short-term synaptic plasticity by controlling receptor lateral mobility at glutamatergic synapses. Cell Rep. 31, 107735 (2020).
Maingret, F. & Groc, L. Characterization of the functional cross-talk between surface GABAA and dopamine D5 receptors. Int. J. Mol. Sci. 22, 4867 (2021).
Cantaut-Belarif, Y. et al. Microglia control the glycinergic but not the GABAergic synapses via prostaglandin E2 in the spinal cord. J. Cell Biol. 216, 2979–2989 (2017).
Aloisi, E. et al. Altered surface mGluR5 dynamics provoke synaptic NMDAR dysfunction and cognitive defects in Fmr1 knockout mice. Nat. Commun. 8, 1–14 (2017).
Penn, A. C. et al. Hippocampal LTP and contextual learning require surface diffusion of AMPA receptors. Nature 549, 384–388 (2017).
Ribeiro, L. F. et al. Ligand-independent activity of the ghrelin receptor modulates AMPA receptor trafficking and supports memory formation. Sci. Signal. 14, eabb1953 (2021).
Saffman, P. G. & Delbrueck, M. Brownian motion in biological membranes. Proc. Natl Acad. Sci. 72, 3111–3113 (1975).
Saxton, M. J. & Jacobson, K. Single-particle tracking:applications to membrane dynamics. Annu. Rev. Biophys. Biomol. Struct. 26, 373–399 (1997). In this seminal review, Saxton and Jacobson comprehensively lay out the arguments for the importance of following single molecules to characterize their diffusion dynamics in the membrane.
Ritchie, K. et al. Detection of non-Brownian diffusion in the cell membrane in single molecule tracking. Biophys. J. 88, 2266–2277 (2005).
Kusumi, A., Sako, Y. & Yamamoto, M. Confined lateral diffusion of membrane receptors as studied by single particle tracking (nanovid microscopy). Effects of calcium-induced differentiation in cultured epithelial cells. Biophys. J. 65, 2021–2040 (1993).
Bouchaud, J. P. & Georges, A. Anomalous diffusion in disordered media: statistical mechanisms, models and physical applications. Phys. Rep. 195, 127–293 (1990).
Metzler, R., Jeon, J. H., Cherstvy, A. G. & Barkai, E. Anomalous diffusion models and their properties: non-stationarity, non-ergodicity, and ageing at the centenary of single particle tracking. Phys. Chem. Chem. Phys. 16, 24128–24164 (2014). This review provides a very thorough and accessible introduction to the theory of anomalous diffusion.
Höfling, F. & Franosch, T. Anomalous transport in the crowded world of biological cells. Rep. Prog. Phys. 76, 046602 (2013).
Santamaria, F., Wils, S., De Schutter, E. & Augustine, G. J. Anomalous diffusion in Purkinje cell dendrites caused by spines. Neuron 52, 635–648 (2006).
Santamaria, F., Wils, S., De Schutter, E. & Augustine, G. J. The diffusional properties of dendrites depend on the density of dendritic spines. Eur. J. Neurosci. 34, 561–568 (2011).
Krapf, D. Mechanisms underlying anomalous diffusion in the plasma membrane. Curr. Top. Membr. 75, 167–207 (2015).
Renner, M. L., Cognet, L., Lounis, B., Triller, A. & Choquet, D. The excitatory postsynaptic density is a size exclusion diffusion environment. Neuropharmacology 56, 30–36 (2009).
Cherstvy, A. G. & Metzler, R. Ergodicity breaking, ageing, and confinement in generalized diffusion processes with position and time dependent diffusivity. J. Stat. Mech. Theory Exp. 2015, P05010 (2015).
Schulz, J. H. P., Barkai, E. & Metzler, R. Aging effects and population splitting in single-particle trajectory averages. Phys. Rev. Lett. 110, 020602 (2013).
MacGillavry, H. D., Song, Y., Raghavachari, S. & Blanpied, T. A. Nanoscale scaffolding domains within the postsynaptic density concentrate synaptic AMPA receptors. Neuron 78, 615–622 (2013).
Pennacchietti, F. et al. Nanoscale molecular reorganization of the inhibitory postsynaptic density is a determinant of GABAergic synaptic potentiation. J. Neurosci. 37, 1747–1756 (2017).
Earnshaw, B. A. & Bressloff, P. C. Biophysical model of AMPA receptor trafficking and its regulation during long-term potentiation/long-term depression. J. Neurosci. 26, 12362–12373 (2006). This article provides the first biophysical model of AMPAR trafficking at dendritic spines, including exocytotic and endocytotic pathways and exchange between synaptic domains and the extrasynaptic membrane via lateral diffusion.
Bressloff, P. C. & Earnshaw, B. A. Diffusion-trapping model of receptor trafficking in dendrites. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 75, 041915 (2007).
Earnshaw, B. A. & Bressloff, P. C. Modeling the role of lateral membrane diffusion in AMPA receptor trafficking along a spiny dendrite. J. Comput. Neurosci. 25, 366–389 (2008).
Holcman, D. & Triller, A. Modeling synaptic dynamics driven by receptor lateral diffusion. Biophys. J. 91, 2405–2415 (2006).
Brown, F. L. H., Leitner, D. M., McCammon, J. A. & Wilson, K. R. Lateral diffusion of membrane proteins in the presence of static and dynamic corrals: suggestions for appropriate observables. Biophys. J. 78, 2257–2269 (2000).
Bressloff, P. C. & Earnshaw, B. A. A dynamic corral model of receptor trafficking at a synapse. Biophys. J. 96, 1786–1802 (2009).
Becker, M. F. P. & Tetzlaff, C. The biophysical basis underlying the maintenance of early phase long-term potentiation. PLoS Comput. Biol. 17, e1008813 (2021).
Santamaria, F., Gonzalez, J., Augustine, G. J. & Raghavachari, S. Quantifying the effects of elastic collisions and non-covalent binding on glutamate receptor trafficking in the post-synaptic density. PLoS Comput. Biol. 6, e1000780 (2010).
Li, T. P., Song, Y., MacGillavry, H. D., Blanpied, T. A. & Raghavachari, S. Protein crowding within the postsynaptic density can impede the escape of membrane proteins. J. Neurosci. 36, 4276–4295 (2016).
Kokolaki, M. L., Fauquier, A. & Renner, M. Molecular crowding and diffusion-capture in synapses. iScience 23, 101382 (2020).
Czöndör, K. et al. Unified quantitative model of AMPA receptor trafficking at synapses. Proc. Natl Acad. Sci. USA 109, 3522–3527 (2012).
Kusters, R., Kapitein, L. C., Hoogenraad, C. C. & Storm, C. Shape-induced asymmetric diffusion in dendritic spines allows efficient synaptic AMPA receptor trapping. Biophys. J. 105, 2743–2750 (2013).
Adrian, M., Kusters, R., Storm, C., Hoogenraad, C. & Kapitein, L. Probing the interplay between dendritic spine morphology and membrane-bound diffusion. Biophys. J. 113, 2261–2270 (2017).
Laurent, F. et al. Mapping spatio-temporal dynamics of single biomolecules in living cells. Phys. Biol. 17, 015003 (2019).
Ferretti, F., Chardès, V., Mora, T., Walczak, A. M. & Giardina, I. Building general Langevin models from discrete datasets. Phys. Rev. X 10, 031018 (2020).
Brückner, D. B., Ronceray, P. & Broedersz, C. P. Inferring the dynamics of underdamped stochastic systems. Phys. Rev. Lett. 125, 058103 (2020).
Frishman, A. & Ronceray, P. Learning force fields from stochastic trajectories. Phys. Rev. X 10, 021009 (2020).
Sekimoto, K. & Triller, A. Compatibility between itinerant synaptic receptors and stable postsynaptic structure. Phys. Rev. E 79, 031905 (2009). In this article, it is proposed for the first time that the difference in synaptic and extrasynaptic receptor concentrations arises by a phase-separation mechanism driven by an effective free energy that takes into account receptor–scaffold protein interactions, highlighting the highly cooperative behaviour of the proteins involved.
Zeng, M. et al. Phase transition in postsynaptic densities underlies formation of synaptic complexes and synaptic plasticity. Cell 166, 1163–1175.e12 (2016).
Bai, G., Wang, Y. & Zhang, M. Gephyrin-mediated formation of inhibitory postsynaptic density sheet via phase separation. Cell Res. 31, 312–325 (2021).
Hosokawa, T. et al. CaMKII activation persistently segregates postsynaptic proteins via liquid phase separation. Nat. Neurosci. 24, 777–785 (2021).
Haselwandter, C. A., Calamai, M., Kardar, M., Triller, A. & Azeredo da Silveira, R. Formation and stability of synaptic receptor domains. Phys. Rev. Lett. 106, 238104 (2011).
Haselwandter, C., Kardar, M., Triller, A. & da Silveira, R. Self-assembly and plasticity of synaptic domains through a reaction-diffusion mechanism. Phys. Rev. E. Stat. Nonlinear Soft Matter Phys. 92, 032705 (2015).
Ranft, J., Almeida, L. G., Rodriguez, P. C., Triller, A. & Hakim, V. An aggregation-removal model for the formation and size determination of post-synaptic scaffold domains. PLoS Comput. Biol. 13, e1005516 (2017).
Hakim, V. & Ranft, J. Lifetime of a structure evolving by cluster aggregation and particle loss, and application to postsynaptic scaffold domains. Phys. Rev. E 101, 012411 (2020).
Turing, A. M. The chemical basis of morphogenesis. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 237, 37–72 (1952).
Turner, M. S., Sens, P. & Socci, N. D. Nonequilibrium raft-like membrane domains under continuous recycling. Phys. Rev. Lett. 95, 168301 (2005).
Weber, C. A., Zwicker, D., Jülicher, F. & Lee, C. F. Physics of active emulsions. Rep. Prog. Phys. 82, 064601 (2019).
Chapdelaine, T., Hakim, V., Triller, A., Ranft, J. & Specht, C. G. Reciprocal stabilization of glycine receptors and gephyrin scaffold proteins at inhibitory synapses. Biophys. J. 120, 805–817 (2021).
Kasai, H., Ziv, N. E., Okazaki, H., Yagishita, S. & Toyoizumi, T. Spine dynamics in the brain, mental disorders and artificial neural networks. Nat. Rev. Neurosci. 22, 407–422 (2021).
Liu, Y. T. et al. Mesophasic organization of GABAA receptors in hippocampal inhibitory synapses. Nat. Neurosci. 23, 1589–1596 (2020).
Markova, O., Alberts, J., Munro, E. & Lenne, P. Bond flexibility and low valence promote finite clusters of self-aggregating particles. Phys. Rev. Lett. 109, 078101 (2012).
Markova, O., Alberts, J., Munro, E. & Lenne, P. Clustering of low-valence particles: structure and kinetics. Phys. Rev. E. Stat. Nonlinear Soft Matter Phys. 90, 022301 (2014).
Magee, J. C. & Grienberger, C. Synaptic plasticity forms and functions. Annu. Rev. Neurosci. 43, 95–117 (2020).
Macpherson, L. J. et al. Dynamic labelling of neural connections in multiple colours by trans-synaptic fluorescence complementation. Nat. Commun. 6, 10024 (2015).
Biermann, B. et al. Imaging of molecular surface dynamics in brain slices using single-particle tracking. Nat. Commun. 5, 3024 (2014).
Hines, A. D. & van Swinderen, B. Tracking single molecule dynamics in the adult drosophila brain. eNeuro 8, ENEURO.0057-21.2021 (2021).
Patching, S. G. Surface plasmon resonance spectroscopy for characterisation of membrane protein-ligand interactions and its potential for drug discovery. Biochim. Biophys. Acta Biomembr. 1838, 43–55 (2014).
Kostrz, D. et al. A modular DNA scaffold to study protein–protein interactions at single-molecule resolution. Nat. Nanotechnol. 14, 988–993 (2019).
Zhu, H. & Gouaux, E. Architecture and assembly mechanism of native glycine receptors. Nature 599, 513–517 (2021).
Ouyang, W., Aristov, A., Lelek, M., Hao, X. & Zimmer, C. Deep learning massively accelerates super-resolution localization microscopy. Nat. Biotechnol. 36, 460–468 (2018).
Nehme, E. et al. DeepSTORM3D: dense 3D localization microscopy and PSF design by deep learning. Nat. Methods 17, 734–740 (2020).
Kellermayer, B. et al. Differential nanoscale topography and functional role of GluN2-NMDA receptor subtypes at glutamatergic synapses. Neuron 100, 106–119.e7 (2018).
Crosby, K. C. et al. Nanoscale subsynaptic domains underlie the organization of the inhibitory synapse. Cell Rep. 26, 3284–3297.e3 (2019).
El Beheiry, M. et al. A primer on the Bayesian approach to high-density single-molecule trajectories analysis. Biophys. J. 110, 1209–1215 (2016).
Tang, A. H. et al. A trans-synaptic nanocolumn aligns neurotransmitter release to receptors. Nature 536, 210–214 (2016).
Haas, K. T. et al. Pre-post synaptic alignment through neuroligin-1 tunes synaptic transmission efficiency. Elife 7, e31755 (2018).
Hruska, M., Henderson, N., Le Marchand, S. J., Jafri, H. & Dalva, M. B. Synaptic nanomodules underlie the organization and plasticity of spine synapses. Nat. Neurosci. 21, 671–682 (2018).
Siddig, S. et al. Super-resolution imaging reveals the nanoscale organization of metabotropic glutamate receptors at presynaptic active zones. Sci. Adv. 6, eaay7193 (2020).
Biederer, T., Kaeser, P. S. & Blanpied, T. A. Transcellular nanoalignment of synaptic function. Neuron 96, 680–696 (2017).
Ehrensperger, M. V., Hanus, C., Vannier, C., Triller, A. & Dahan, M. Multiple association states between glycine receptors and gephyrin identified by SPT analysis. Biophys. J. 92, 3706 (2007).
Calamai, M. et al. Gephyrin oligomerization controls GlyR mobility and synaptic clustering. J. Neurosci. 29, 7639–7648 (2009).
Lee, S. H. et al. Super-resolution imaging of synaptic and extra-synaptic AMPA receptors with different-sized fluorescent probes. Elife 6, e27744 (2017).
Statman, A., Kaufman, M., Minerbi, A., Ziv, N. E. & Brenner, N. Synaptic size dynamics as an effectively stochastic process. PLoS Comput. Biol. 10, e1003846 (2014).
Rubinski, A. & Ziv, N. E. Remodeling and tenacity of inhibitory synapses: relationships with network activity and neighboring excitatory synapses. PLoS Comput. Biol. 11, e1004632 (2015).
Berry, K. P. & Nedivi, E. Spine dynamics: are they all the same? Neuron 96, 43–55 (2017).
Benna, M. K. & Fusi, S. Computational principles of synaptic memory consolidation. Nat. Neurosci. 19, 1697–1706 (2016).
Fusi, S., Drew, P. J. & Abbott, L. F. Cascade models of synaptically stored memories. Neuron 45, 599–611 (2005).
Acknowledgements
The authors thank V. Hakim for discussions on the biophysics of synapses, and for comments on the manuscript. This work was supported by the French Agence Nationale de la Recherche (Syntrack and Synaptune to A.T.) and the European Research Council (ERC Advanced NVS and ERC Synergy MICROCOPS to A.T.).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, contributed substantially to discussion of the content, and reviewed and/or edited the manuscript before submission. S.A.M. and J.R. wrote the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neuroscience thanks J.-B. Masson and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Active zone
-
The site of neurotransmitter release at the presynaptic terminal.
- Affinity trapping
-
The trapping of a molecule in a particular cellular location because of its affinity for its binding partner.
- Bayesian inference
-
A method of statistical inference in which Bayes’s theorem is used to determine the probability that a hypothesis can describe a process (such as the value of a parameter in a model) based on new observations and a given prior probability for the hypothesis (note that a ‘flat’ prior can be used, which assigns equal initial probability to all possible hypotheses).
- Blinking
-
The cycling of fluorophores between light and dark states, enabling the fluorophore to be detected several times.
- Brownian motion
-
The random motion of diffusing particles caused by microscopic collisions with surrounding molecules at finite temperature, first observed by Robert Brown in 1828.
- Confined diffusion
-
Diffusive motion that is confined to a spatial domain of finite size, which manifests itself as a saturation of the mean squared displacement over time.
- Continuous-time random walks
-
A variant of a random walk in which the waiting times between successive steps are drawn from a continuous probability distribution. When the waiting time distribution becomes very broad and heavy tailed, the corresponding continuous-time random walks become subdiffusive.
- Deep learning
-
A class of machine learning that uses multilayer artificial neural networks and that extracts features from a dataset. In imaging, this has been used to reduce imaging time and reconstruct images from undersampled data.
- Desensitization
-
A form of downregulation where an activated receptor decreases its response to an agonist and is hence uncoupled from its signalling cascade.
- Diffusion coefficients
-
The diffusion coefficient is the proportionality constant between the mean squared displacement of a particle and the duration for which the diffusion is observed, as defined for pure diffusion (where the mean squared displacement scales linearly with time).
- DNA-PAINT constructs
-
Oligonucleotides used for single-particle tracking. Reversible hybridization occurs between a docking oligonucleotide strand that is attached to the protein of interest and a fluorophore-conjugated imager strand, enabling single-molecule localization microscopy.
- Dwell times
-
The dwell time is the time for which a receptor stays in the postsynaptic domain after entering from (and before exiting the synapse into) the extrasynaptic space.
- Effective potential energies
-
The effective potential energy is the potential energy that accounts best for the forces inferred (from high-density trajectories) to be acting on the diffusing particles when the forces are proportional to the gradient of the potential energy.
- Ergodicity
-
A property of a dynamical system in which a trajectory visits all of the available phase space such that time averages along trajectories and ensemble averages give the same result.
- Fluorescence recovery after photobleaching
-
A method to determine the kinetics of diffusion of fluorescently labelled molecules by bleaching a small population of the molecules and measuring the time taken for new fluorescent molecules to diffuse into the bleached spot.
- Hidden Markov models
-
(HMMs). Statistical models in which the process being analysed is assumed to be Markovian; that is, its evolution is determined only by the current state of the system but is influenced by an unobserved ‘hidden’ process.
- Inference methods
-
Statistical analyses used to infer the properties of an underlying population that are not directly measurable from a limited number of measurements. This is typically achieved by maximizing the likelihood of the data under the assumption of a given model under certain constraints.
- Lateral diffusion
-
The movement of molecules within the plasma membrane.
- Molecular crowding
-
The situation in which a high concentration of proteins in a localized region of the membrane affects molecule movement in that region by steric hindrance.
- Nanobodies
-
Single-domain antibodies with a molecular mass of 12–15 kDa that are able to selectively bind an antigen.
- Phase separation
-
The generation of two distinct phases from a single homogeneous mixture.
- Point spread function
-
The degree of spreading (blurring) of a point source, such as a fluorophore. Gaussian fitting enables determination of the position of the point source.
- Postsynaptic density
-
(PSD). The electron-dense network of molecules located beneath the postsynaptic membrane of excitatory synapses.
- Proteasome
-
A protein complex that degrades misfolded or damaged proteins.
- Quantum dots
-
Nanometre-sized semiconductors with optoelectronic properties that change as a function of their size. Illuminated by ultraviolet light, they are bright and can be used to track a molecule’s movement for relatively long periods.
- Random walk
-
A conceptualization of diffusive motion in which a ‘walker’ takes sequentially random steps in different directions. For the prototypical random walk, step lengths l are constant and steps occur at constant time steps τ, leading to a diffusion coefficient proportional to l2/τ.
- Scaffold proteins
-
Essential components of the postsynaptic density of excitatory synapses and the postsynaptic domain of inhibitory synapses, involved in binding, clustering and trafficking of postsynaptic receptors.
- SPT photoactivated localization microscopy
-
A fluorescent single-molecule localization microscopy technique using a fluorescent protein attached to a protein of interest.
- Subdiffusion
-
Random, diffusive motion of particles with a sublinear, power-law time dependence of the mean squared displacement. The origins of subdiffusive behaviour can be manifold; for example, subdiffusive behaviour can be caused by transient trapping of particles.
- Super-resolution fluorescence microscopy
-
A series of techniques that enable imaging of fluorescently labelled proteins with resolutions higher than the diffraction limit of light (approximately 200 nm).
- Statistical thermodynamics
-
The description of physical systems that relates the statistics of particles at the microscopic scale (for example, kinetic energies of gas particles) to thermodynamic observables at the macroscopic scale (for example, the pressure of the gas).
- Trajectories
-
The sequences of recorded positions of particles as they move through space.
- Ubiquitination
-
An enzymatic post-translational modification in which a ubiquitin molecule is attached to a substrate protein. This modification regulates the degradation of proteins.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maynard, S.A., Ranft, J. & Triller, A. Quantifying postsynaptic receptor dynamics: insights into synaptic function. Nat Rev Neurosci 24, 4–22 (2023). https://doi.org/10.1038/s41583-022-00647-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41583-022-00647-9
This article is cited by
-
Myosin Va-dependent Transport of NMDA Receptors in Hippocampal Neurons
Neuroscience Bulletin (2024)
-
A dynamic partitioning mechanism polarizes membrane protein distribution
Nature Communications (2023)