Abstract
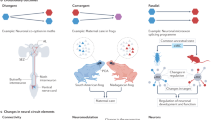
Almost 60 years have passed since the initial discovery by Hubel and Wiesel that changes in neuronal activity can elicit developmental rewiring of the central nervous system (CNS). Over this period, we have gained a more comprehensive picture of how both spontaneous neural activity and sensory experience-induced changes in neuronal activity guide CNS circuit development. Here we review activity-dependent synaptic pruning in the mammalian CNS, which we define as the removal of a subset of synapses, while others are maintained, in response to changes in neural activity in the developing nervous system. We discuss the mounting evidence that immune and cell-death molecules are important mechanistic links by which changes in neural activity guide the pruning of specific synapses, emphasizing the role of glial cells in this process. Finally, we discuss how these developmental pruning programmes may go awry in neurodevelopmental disorders of the human CNS, focusing on autism spectrum disorder and schizophrenia. Together, our aim is to give an overview of how the field of activity-dependent pruning research has evolved, led to exciting new questions and guided the identification of new, therapeutically relevant mechanisms that result in aberrant circuit development in neurodevelopmental disorders.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Riccomagno, M. M. & Kolodkin, A. L. Sculpting neural circuits by axon and dendrite pruning. Annu. Rev. Cell Dev. Biol. 31, 779–805 (2015).
Schuldiner, O. & Yaron, A. Mechanisms of developmental neurite pruning. Cell Mol. Life Sci. 72, 101–119 (2015).
Luo, L. & O’Leary, D. D. Axon retraction and degeneration in development and disease. Annu. Rev. Neurosci. 28, 127–156 (2005).
Sanes, J. R. & Lichtman, J. W. Development of the vertebrate neuromuscular junction. Annu. Rev. Neurosci. 22, 389–442 (1999).
Katz, L. C. & Shatz, C. J. Synaptic activity and the construction of cortical circuits. Science 274, 1133–1138 (1996).
Hua, J. Y. & Smith, S. J. Neural activity and the dynamics of central nervous system development. Nat. Neurosci. 7, 327–332 (2004).
Kano, M. & Hashimoto, K. Synapse elimination in the central nervous system. Curr. Opin. Neurobiol. 19, 154–161 (2009).
Shatz, C. & Stryker, M. Prenatal tetrodotoxin infusion blocks segregation of retinogeniculate afferents. Science 242, 87–89 (1988).
Cang, J. et al. Development of precise maps in visual cortex requires patterned spontaneous activity in the retina. Neuron 48, 797–809 (2005).
Muir-Robinson, G., Hwang, B. J. & Feller, M. B. Retinogeniculate axons undergo eye-specific segregation in the absence of eye-specific layers. J. Neurosci. 22, 5259–5264 (2002).
Penn, A., Riquelme, P., Feller, M. B. & Shatz, C. Competition in retinogeniculate patterning driven by spontaneous activity. Science 279, 2108–2112 (1998).
Torborg, C. L. & Feller, M. B. Spontaneous patterned retinal activity and the refinement of retinal projections. Prog. Neurobiol. 76, 213–235 (2005).
Butts, D. A., Kanold, P. O. & Shatz, C. J. A burst-based “Hebbian” learning rule at retinogeniculate synapses links retinal waves to activity-dependent refinement. PLoS Biol. 5, e61 (2007).
Huberman, A. D. et al. Eye-specific retinogeniculate segregation independent of normal neuronal activity. Science 300, 994–998 (2003).
McLaughlin, T., Torborg, C. L., Feller, M. B. & O’Leary, D. D. Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron 40, 1147–1160 (2003).
Grubb, M. S., Rossi, F. M., Changeux, J. P. & Thompson, I. D. Abnormal functional organization in the dorsal lateral geniculate nucleus of mice lacking the beta 2 subunit of the nicotinic acetylcholine receptor. Neuron 40, 1161–1172 (2003).
Burbridge, T. J. et al. Visual circuit development requires patterned activity mediated by retinal acetylcholine receptors. Neuron 84, 1049–1064 (2014).
Ziburkus, J. & Guido, W. Loss of binocular responses and reduced retinal convergence during the period of retinogeniculate axon segregation. J. Neurophysiol. 96, 2775–2784 (2006).
Rossi, F. M. et al. Requirement of the nicotinic acetylcholine receptor beta 2 subunit for the anatomical and functional development of the visual system. Proc. Natl Acad. Sci. USA 98, 6453–6458 (2001).
Stellwagen, D. & Shatz, C. An instructive role for retinal waves in the development of retinogeniculate connectivity. Neuron 33, 357–367 (2002).
Zhang, J., Ackman, J. B., Xu, H. P. & Crair, M. C. Visual map development depends on the temporal pattern of binocular activity in mice. Nat. Neurosci. 15, 298–307 (2011).
Kano, M. & Watanabe, T. Developmental synapse remodeling in the cerebellum and visual thalamus. F1000Res. https://doi.org/10.12688/f1000research.18903.1 (2019).
Watanabe, M. & Kano, M. Climbing fiber synapse elimination in cerebellar Purkinje cells. Eur. J. Neurosci. 34, 1697–1710 (2011).
Andjus, P. R., Zhu, L., Cesa, R., Carulli, D. & Strata, P. A change in the pattern of activity affects the developmental regression of the Purkinje cell polyinnervation by climbing fibers in the rat cerebellum. Neuroscience 121, 563–572 (2003).
Hashimoto, K. et al. Postsynaptic P/Q-type Ca2+ channel in Purkinje cell mediates synaptic competition and elimination in developing cerebellum. Proc. Natl Acad. Sci. USA 108, 9987–9992 (2011).
Miyazaki, T. et al. Cav2.1 in cerebellar Purkinje cells regulates competitive excitatory synaptic wiring, cell survival, and cerebellar biochemical compartmentalization. J. Neurosci. 32, 1311–1328 (2012).
Mikuni, T. et al. Arc/Arg3.1 is a postsynaptic mediator of activity-dependent synapse elimination in the developing cerebellum. Neuron 78, 1024–1035 (2013).
Lorenzetto, E. et al. Genetic perturbation of postsynaptic activity regulates synapse elimination in developing cerebellum. Proc. Natl Acad. Sci. USA 106, 16475–16480 (2009).
Kawamura, Y. et al. Spike timing-dependent selective strengthening of single climbing fibre inputs to Purkinje cells during cerebellar development. Nat. Commun. 4, 2732 (2013).
Nakayama, H. et al. GABAergic inhibition regulates developmental synapse elimination in the cerebellum. Neuron 74, 384–396 (2012).
Kano, M. et al. Impaired synapse elimination during cerebellar development in PKC gamma mutant mice. Cell 83, 1223–1231 (1995).
Ichikawa, R. et al. Territories of heterologous inputs onto Purkinje cell dendrites are segregated by mGluR1-dependent parallel fiber synapse elimination. Proc. Natl Acad. Sci. USA 113, 2282–2287 (2016).
Ichise, T. et al. mGluR1 in cerebellar Purkinje cells essential for long-term depression, synapse elimination, and motor coordination. Science 288, 1832–1835 (2000).
Kano, M. et al. Persistent multiple climbing fiber innervation of cerebellar Purkinje cells in mice lacking mGluR1. Neuron 18, 71–79 (1997).
Kano, M., Hashimoto, K. & Tabata, T. Type-1 metabotropic glutamate receptor in cerebellar Purkinje cells: a key molecule responsible for long-term depression, endocannabinoid signalling and synapse elimination. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 2173–2186 (2008).
Kano, M. et al. Phospholipase cbeta4 is specifically involved in climbing fiber synapse elimination in the developing cerebellum. Proc. Natl Acad. Sci. USA 95, 15724–15729 (1998).
Uesaka, N. et al. Retrograde semaphorin signaling regulates synapse elimination in the developing mouse brain. Science 344, 1020–1023 (2014).
Uesaka, N. et al. Retrograde signaling for climbing fiber synapse elimination. Cerebellum 14, 4–7 (2015).
Choo, M. et al. Retrograde BDNF to TrkB signaling promotes synapse elimination in the developing cerebellum. Nat. Commun. 8, 195 (2017).
Uesaka, N. et al. Retrograde signaling from progranulin to Sort1 counteracts synapse elimination in the developing cerebellum. Neuron 97, 796–805 e795 (2018).
Miyazaki, T. et al. Glutamate transporter GLAST controls synaptic wrapping by Bergmann glia and ensures proper wiring of Purkinje cells. Proc. Natl Acad. Sci. USA 114, 7438–7443 (2017).
Nakayama, H. et al. Microglia permit climbing fiber elimination by promoting GABAergic inhibition in the developing cerebellum. Nat. Commun. 9, 2830 (2018).
Wiesel, T. N. & Hubel, D. H. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J. Neurophysiol. 26, 1003–1017 (1963).
Antonini, A., Fagiolini, M. & Stryker, M. P. Anatomical correlates of functional plasticity in mouse visual cortex. J. Neurosci. 19, 4388–4406 (1999).
Antonini, A. & Stryker, M. P. Plasticity of geniculocortical afferents following brief or prolonged monocular occlusion in the cat. J. Comp. Neurol. 369, 64–82 (1996).
Antonini, A. & Stryker, M. P. Effect of sensory disuse on geniculate afferents to cat visual cortex. Vis. Neurosci. 15, 401–409 (1998).
Zhou, Y., Lai, B. & Gan, W. B. Monocular deprivation induces dendritic spine elimination in the developing mouse visual cortex. Sci. Rep. 7, 4977 (2017).
Sun, Y. J., Espinosa, J. S., Hoseini, M. S. & Stryker, M. P. Experience-dependent structural plasticity at pre- and postsynaptic sites of layer 2/3 cells in developing visual cortex. Proc. Natl Acad. Sci. USA 116, 21812–21820 (2019).
Huh, C. Y. L. et al. Long-term monocular deprivation during juvenile critical period disrupts binocular integration in mouse visual thalamus. J. Neurosci. 40, 585–604 (2020).
Yu, H., Majewska, A. K. & Sur, M. Rapid experience-dependent plasticity of synapse function and structure in ferret visual cortex in vivo. Proc. Natl Acad. Sci. USA 108, 21235–21240 (2011).
Tremblay, M. E., Lowery, R. L. & Majewska, A. K. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 8, e1000527 (2010).
Zhou, Y. et al. REM sleep promotes experience-dependent dendritic spine elimination in the mouse cortex. Nat. Commun. 11, 4819 (2020).
Rittenhouse, C. D., Shouval, H. Z., Paradiso, M. A. & Bear, M. F. Monocular deprivation induces homosynaptic long-term depression in visual cortex. Nature 397, 347–350 (1999).
Sidorov, M. S., Kaplan, E. S., Osterweil, E. K., Lindemann, L. & Bear, M. F. Metabotropic glutamate receptor signaling is required for NMDA receptor-dependent ocular dominance plasticity and LTD in visual cortex. Proc. Natl Acad. Sci. USA 112, 12852–12857 (2015).
Zhou, Q., Homma, K. J. & Poo, M. M. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron 44, 749–757 (2004).
Shinoda, Y., Tanaka, T., Tominaga-Yoshino, K. & Ogura, A. Persistent synapse loss induced by repetitive LTD in developing rat hippocampal neurons. PLoS ONE 5, e10390 (2010).
Wiegert, J. S. & Oertner, T. G. Long-term depression triggers the selective elimination of weakly integrated synapses. Proc. Natl Acad. Sci. USA 110, E4510–E4519 (2013).
Hensch, T. K. et al. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science 282, 1504–1508 (1998).
Mataga, N., Mizuguchi, Y. & Hensch, T. K. Experience-dependent pruning of dendritic spines in visual cortex by tissue plasminogen activator. Neuron 44, 1031–1041 (2004).
Hooks, B. M. & Chen, C. Vision triggers an experience-dependent sensitive period at the retinogeniculate synapse. J. Neurosci. 28, 4807–4817 (2008).
Hong, Y. K. et al. Refinement of the retinogeniculate synapse by bouton clustering. Neuron 84, 332–339 (2014).
Cheadle, L. et al. Sensory experience engages microglia to shape neural connectivity through a non-phagocytic mechanism. Neuron 108, 451–468 e459 (2020).
Wimmer, V. C., Broser, P. J., Kuner, T. & Bruno, R. M. Experience-induced plasticity of thalamocortical axons in both juveniles and adults. J. Comp. Neurol. 518, 4629–4648 (2010).
Sadaka, Y., Weinfeld, E., Lev, D. L. & White, E. L. Changes in mouse barrel synapses consequent to sensory deprivation from birth. J. Comp. Neurol. 457, 75–86 (2003).
Gunner, G. et al. Sensory lesioning induces microglial synapse elimination via ADAM10 and fractalkine signaling. Nat. Neurosci. 22, 1075–1088 (2019).
Chen, C. C., Bajnath, A. & Brumberg, J. C. The impact of development and sensory deprivation on dendritic protrusions in the mouse barrel cortex. Cereb. Cortex 25, 1638–1653 (2015).
Zuo, Y., Yang, G., Kwon, E. & Gan, W. B. Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature 436, 261–265 (2005).
Bian, W. J., Miao, W. Y., He, S. J., Qiu, Z. & Yu, X. Coordinated spine pruning and maturation mediated by inter-spine competition for cadherin/catenin complexes. Cell 162, 808–822 (2015).
Midorikawa, M. & Miyata, M. Distinct functional developments of surviving and eliminated presynaptic terminals. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.2022423118 (2021).
Yang, J. et al. Astrocytes contribute to synapse elimination via type 2 inositol 1,4,5-trisphosphate receptor-dependent release of ATP. eLife 5, e15043 (2016).
Morimoto, K. & Nakajima, K. Role of the immune system in the development of the central nervous system. Front. Neurosci. 13, 916 (2019).
Corriveau, R. A., Huh, G. S. & Shatz, C. J. Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron 21, 505–520 (1998).
Lee, H. et al. Synapse elimination and learning rules co-regulated by MHC class I H2-Db. Nature 509, 195–200 (2014).
Huh, G. S. et al. Functional requirement for class I MHC in CNS development and plasticity. Science 290, 2155–2159 (2000).
Syken, J., Grandpre, T., Kanold, P. O. & Shatz, C. J. PirB restricts ocular-dominance plasticity in visual cortex. Science 313, 1795–1800 (2006).
Adelson, J. D. et al. Developmental sculpting of intracortical circuits by MHC class I H2-Db and H2-Kb. Cereb. Cortex 26, 1453–1463 (2016).
Glynn, M. W. et al. MHCI negatively regulates synapse density during the establishment of cortical connections. Nat. Neurosci. 14, 442–451 (2011).
McAllister, A. K. Major histocompatibility complex I in brain development and schizophrenia. Biol. Psychiatry 75, 262–268 (2014).
Du Clos, T. W. Pentraxins: structure, function, and role in inflammation. ISRN Inflamm. 2013, 379040 (2013).
Schlimgen, A. K., Helms, J. A., Vogel, H. & Perin, M. S. Neuronal pentraxin, a secreted protein with homology to acute phase proteins of the immune system. Neuron 14, 519–526 (1995).
Tsui, C. C. et al. Narp, a novel member of the pentraxin family, promotes neurite outgrowth and is dynamically regulated by neuronal activity. J. Neurosci. 16, 2463–2478 (1996).
Dodds, D. C., Omeis, I. A., Cushman, S. J., Helms, J. A. & Perin, M. S. Neuronal pentraxin receptor, a novel putative integral membrane pentraxin that interacts with neuronal pentraxin 1 and 2 and taipoxin-associated calcium-binding protein 49. J. Biol. Chem. 272, 21488–21494 (1997).
Xu, D. et al. Narp and NP1 form heterocomplexes that function in developmental and activity-dependent synaptic plasticity. Neuron 39, 513–528 (2003).
Figueiro-Silva, J. et al. Neuronal pentraxin 1 negatively regulates excitatory synapse density and synaptic plasticity. J. Neurosci. 35, 5504–5521 (2015).
Bjartmar, L. et al. Neuronal pentraxins mediate synaptic refinement in the developing visual system. J. Neurosci. 26, 6269–6281 (2006).
Ma, Y. J. & Garred, P. Pentraxins in complement activation and regulation. Front. Immunol. 9, 3046 (2018).
Lu, J., Mold, C., Du Clos, T. W. & Sun, P. D. Pentraxins and Fc receptor-mediated immune responses. Front. Immunol. 9, 2607 (2018).
Kovacs, R. A. et al. Identification of neuronal pentraxins as synaptic binding partners of C1q and the involvement of NP1 in synaptic pruning in adult mice. Front. Immunol. 11, 599771 (2020).
Paolicelli, R. C. et al. Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–1458 (2011).
Schafer, D. P. et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705 (2012).
Sipe, G. O. et al. Microglial P2Y12 is necessary for synaptic plasticity in mouse visual cortex. Nat. Commun. 7, 10905 (2016).
Milinkeviciute, G. et al. Microglia regulate pruning of specialized synapses in the auditory brainstem. Front. Neural Circuits 13, 55 (2019).
Mallya, A. P., Wang, H. D., Lee, H. N. R. & Deutch, A. Y. Microglial pruning of synapses in the prefrontal cortex during adolescence. Cereb. Cortex 29, 1634–1643 (2019).
Kopec, A. M., Smith, C. J., Ayre, N. R., Sweat, S. C. & Bilbo, S. D. Microglial dopamine receptor elimination defines sex-specific nucleus accumbens development and social behavior in adolescent rats. Nat. Commun. 9, 3769 (2018).
Kim, H. J. et al. Deficient autophagy in microglia impairs synaptic pruning and causes social behavioral defects. Mol. Psychiatry https://doi.org/10.1038/mp.2016.103 (2016).
Weinhard, L. et al. Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat. Commun. 9, 1228 (2018).
Lim, T. K. & Ruthazer, E. S. Microglial trogocytosis and the complement system regulate axonal pruning in vivo. eLife https://doi.org/10.7554/eLife.62167 (2021).
Stevens, B. et al. The classical complement cascade mediates CNS synapse elimination. Cell 131, 1164–1178 (2007).
Zhan, Y. et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 17, 400–406 (2014).
Bialas, A. R. & Stevens, B. TGF-beta signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat. Neurosci. 16, 1773–1782 (2013).
Jung, S. et al. Analysis of fractalkine receptor CX3CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell Biol. 20, 4106–4114 (2000).
Lee, M., Lee, Y., Song, J., Lee, J. & Chang, S. Y. Tissue-specific role of CX3CR1 expressing immune cells and their relationships with human disease. Immune Netw. 18, e5 (2018).
Zhao, W., Lu, H., Wang, X., Ransohoff, R. M. & Zhou, L. CX3CR1 deficiency delays acute skeletal muscle injury repair by impairing macrophage functions. FASEB J. 30, 380–393 (2016).
Blomster, L. V. et al. CX3CR1 deficiency exacerbates neuronal loss and impairs early regenerative responses in the target-ablated olfactory epithelium. Mol. Cell. Neurosci. 48, 236–245 (2011).
Ingram, G., Hakobyan, S., Robertson, N. P. & Morgan, B. P. Complement in multiple sclerosis: its role in disease and potential as a biomarker. Clin. Exp. Immunol. 155, 128–139 (2009).
Barrington, R., Zhang, M., Fischer, M. & Carroll, M. C. The role of complement in inflammation and adaptive immunity. Immunol. Rev. 180, 5–15 (2001).
Chu, Y. et al. Enhanced synaptic connectivity and epilepsy in C1q knockout mice. Proc. Natl Acad. Sci. USA 107, 7975–7980 (2010).
Lehrman, E. K. et al. CD47 protects synapses from excess microglia-mediated pruning during development. Neuron 100, 120–134 e126 (2018).
Cong, Q., Soteros, B. M., Wollet, M., Kim, J. H. & Sia, G. M. The endogenous neuronal complement inhibitor SRPX2 protects against complement-mediated synapse elimination during development. Nat. Neurosci. 23, 1067–1078 (2020).
Linnartz, B., Kopatz, J., Tenner, A. J. & Neumann, H. Sialic acid on the neuronal glycocalyx prevents complement C1 binding and complement receptor-3-mediated removal by microglia. J. Neurosci. 32, 946–952 (2012).
Gyorffy, B. A. et al. Local apoptotic-like mechanisms underlie complement-mediated synaptic pruning. Proc. Natl Acad. Sci. USA 115, 6303–6308 (2018).
Scott-Hewitt, N. et al. Local externalization of phosphatidylserine mediates developmental synaptic pruning by microglia. EMBO J. 39, e105380 (2020).
Schecter, R. W. et al. Experience-dependent synaptic plasticity in V1 occurs without microglial CX3CR1. J. Neurosci. 37, 10541–10553 (2017).
Kaiser, N., Patz, C., Brachtendorf, S., Eilers, J. & Bechmann, I. Undisturbed climbing fiber pruning in the cerebellar cortex of CX3CR1-deficient mice. Glia 68, 2316–2329 (2020).
Shi, Q. et al. Complement C3-deficient mice fail to display age-related hippocampal decline. J. Neurosci. 35, 13029–13042 (2015).
Lowery, R. L., Tremblay, M. E., Hopkins, B. E. & Majewska, A. K. The microglial fractalkine receptor is not required for activity-dependent plasticity in the mouse visual system. Glia 65, 1744–1761 (2017).
Welsh, C. A., Stephany, C. E., Sapp, R. W. & Stevens, B. Ocular dominance plasticity in binocular primary visual cortex does not require C1q. J. Neurosci. 40, 769–783 (2020).
Badimon, A. et al. Negative feedback control of neuronal activity by microglia. Nature 586, 417–423 (2020).
Peng, J. et al. Microglial P2Y12 receptor regulates ventral hippocampal CA1 neuronal excitability and innate fear in mice. Mol. Brain 12, 71 (2019).
Favuzzi, E. et al. GABA-receptive microglia selectively sculpt developing inhibitory circuits. Cell 184, 4048–4063 (2021).
Chung, W. S. et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504, 394–400 (2013).
Lee, J. H. et al. Astrocytes phagocytose adult hippocampal synapses for circuit homeostasis. Nature https://doi.org/10.1038/s41586-020-03060-3 (2020).
Byun, Y. G. & Chung, W. S. A novel in vitro live-imaging assay of astrocyte-mediated phagocytosis using pH indicator-conjugated synaptosomes. J. Vis. Exp. https://doi.org/10.3791/56647 (2018).
Risher, W. C. et al. Astrocytes refine cortical connectivity at dendritic spines. eLife https://doi.org/10.7554/eLife.04047 (2014).
Vainchtein, I. D. et al. Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science 359, 1269–1273 (2018).
Filipello, F. et al. The microglial innate immune receptor TREM2 is required for synapse elimination and normal brain connectivity. Immunity 48, 979–991 e978 (2018).
Jay, T. R. et al. TREM2 is required for microglial instruction of astrocytic synaptic engulfment in neurodevelopment. Glia 67, 1873–1892 (2019).
Yasuda, M., Nagappan-Chettiar, S., Johnson-Venkatesh, E. M. & Umemori, H. An activity-dependent determinant of synapse elimination in the mammalian brain. Neuron 109, 1333–1349 e1336 (2021).
Nagata, S. Apoptosis and clearance of apoptotic cells. Annu. Rev. Immunol. 36, 489–517 (2018).
Li, Z. et al. Caspase-3 activation via mitochondria is required for long-term depression and AMPA receptor internalization. Cell 141, 859–871 (2010).
Jiao, S. & Li, Z. Nonapoptotic function of BAD and BAX in long-term depression of synaptic transmission. Neuron 70, 758–772 (2011).
Erturk, A., Wang, Y. & Sheng, M. Local pruning of dendrites and spines by caspase-3-dependent and proteasome-limited mechanisms. J. Neurosci. 34, 1672–1688 (2014).
Xu, Z. X. et al. Caspase-2 promotes AMPA receptor internalization and cognitive flexibility via mTORC2-AKT-GSK3beta signaling. Nat. Commun. 10, 3622 (2019).
Creamer, T. P. Calcineurin. Cell Commun. Signal. 18, 137 (2020).
Flavell, S. W. et al. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science 311, 1008–1012 (2006).
Tian, X., Kai, L., Hockberger, P. E., Wokosin, D. L. & Surmeier, D. J. MEF-2 regulates activity-dependent spine loss in striatopallidal medium spiny neurons. Mol. Cell. Neurosci. 44, 94–108 (2010).
Wilkerson, J. R. et al. A role for dendritic mGluR5-mediated local translation of Arc/Arg3.1 in MEF2-dependent synapse elimination. Cell Rep. 7, 1589–1600 (2014).
Zagorska, A., Traves, P. G., Lew, E. D., Dransfield, I. & Lemke, G. Diversification of TAM receptor tyrosine kinase function. Nat. Immunol. 15, 920–928 (2014).
Li, T. et al. A splicing isoform of GPR56 mediates microglial synaptic refinement via phosphatidylserine binding. EMBO J. 39, e104136 (2020).
Paidassi, H. et al. C1q binds phosphatidylserine and likely acts as a multiligand-bridging molecule in apoptotic cell recognition. J. Immunol. 180, 2329–2338 (2008).
Owen, K. L., Brockwell, N. K. & Parker, B. S. JAK-STAT signaling: a double-edged sword of immune regulation and cancer progression. Cancers (Basel) https://doi.org/10.3390/cancers11122002 (2019).
Li, Z., Okamoto, K., Hayashi, Y. & Sheng, M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell 119, 873–887 (2004).
Lees, R. M., Johnson, J. D. & Ashby, M. C. Presynaptic boutons that contain mitochondria are more stable. Front. Synaptic Neurosci. 11, 37 (2019).
Sun, T., Qiao, H., Pan, P. Y., Chen, Y. & Sheng, Z. H. Motile axonal mitochondria contribute to the variability of presynaptic strength. Cell Rep. 4, 413–419 (2013).
Koyama, R. & Ikegaya, Y. Microglia in the pathogenesis of autism spectrum disorders. Neurosci. Res. 100, 1–5 (2015).
Pardo, C. A., Vargas, D. L. & Zimmerman, A. W. Immunity, neuroglia and neuroinflammation in autism. Int. Rev. Psychiatry 17, 485–495 (2005).
Vargas, D. L., Nascimbene, C., Krishnan, C., Zimmerman, A. W. & Pardo, C. A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 57, 67–81 (2005).
Belmonte, M. K. et al. Autism and abnormal development of brain connectivity. J. Neurosci. 24, 9228–9231 (2004).
Peca, J. & Feng, G. Cellular and synaptic network defects in autism. Curr. Opin. Neurobiol. 22, 866–872 (2012).
Thomas, M. S., Davis, R., Karmiloff-Smith, A., Knowland, V. C. & Charman, T. The over-pruning hypothesis of autism. Dev. Sci. 19, 284–305 (2016).
Waites, C. L. & Garner, C. C. Presynaptic function in health and disease. Trends Neurosci. 34, 326–337 (2011).
Melom, J. E. & Littleton, J. T. Synapse development in health and disease. Curr. Opin. Genet. Dev. 21, 256–261 (2011).
Penzes, P., Cahill, M. E., Jones, K. A., VanLeeuwen, J. E. & Woolfrey, K. M. Dendritic spine pathology in neuropsychiatric disorders. Nat. Neurosci. 14, 285–293 (2011).
Maenner, M. J. et al. Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2016. MMWR Surveill. Summ. 69, 1–12 (2020).
Tang, G. et al. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron 83, 1131–1143 (2014).
Hutsler, J. J. & Zhang, H. Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res. 1309, 83–94 (2010).
Voineagu, I. et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 474, 380–384 (2011).
Nardone, S. et al. DNA methylation analysis of the autistic brain reveals multiple dysregulated biological pathways. Transl. Psychiatry 4, e433 (2014).
Bourgeron, T. From the genetic architecture to synaptic plasticity in autism spectrum disorder. Nat. Rev. Neurosci. 16, 551–563 (2015).
De Rubeis, S. et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 515, 209–215 (2014).
Darnell, J. C. & Richter, J. D. Cytoplasmic RNA-binding proteins and the control of complex brain function. Cold Spring Harb. Perspect. Biol. 4, a012344 (2012).
Antar, L. N., Afroz, R., Dictenberg, J. B., Carroll, R. C. & Bassell, G. J. Metabotropic glutamate receptor activation regulates fragile x mental retardation protein and FMR1 mRNA localization differentially in dendrites and at synapses. J. Neurosci. 24, 2648–2655 (2004).
Weiler, I. J. et al. Fragile X mental retardation protein is translated near synapses in response to neurotransmitter activation. Proc. Natl Acad. Sci. USA 94, 5395–5400 (1997).
Huber, K. M., Gallagher, S. M., Warren, S. T. & Bear, M. F. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc. Natl Acad. Sci. USA 99, 7746–7750 (2002).
Li, J., Pelletier, M. R., Perez Velazquez, J. L. & Carlen, P. L. Reduced cortical synaptic plasticity and GluR1 expression associated with fragile X mental retardation protein deficiency. Mol. Cell. Neurosci. 19, 138–151 (2002).
Le Meur, N. et al. MEF2C haploinsufficiency caused by either microdeletion of the 5q14.3 region or mutation is responsible for severe mental retardation with stereotypic movements, epilepsy and/or cerebral malformations. J. Med. Genet. 47, 22–29 (2010).
Nowakowska, B. A. et al. Severe mental retardation, seizures, and hypotonia due to deletions of MEF2C. Am. J. Med. Genet. B Neuropsychiatric Genet. 153B, 1042–1051 (2010).
Paciorkowski, A. R. et al. MEF2C haploinsufficiency features consistent hyperkinesis, variable epilepsy, and has a role in dorsal and ventral neuronal developmental pathways. Neurogenetics 14, 99–111 (2013).
Pfeiffer, B. E. et al. Fragile X mental retardation protein is required for synapse elimination by the activity-dependent transcription factor MEF2. Neuron 66, 191–197 (2010).
Tsai, N. P., Wilkerson, J. R., Guo, W. & Huber, K. M. FMRP-dependent Mdm2 dephosphorylation is required for MEF2-induced synapse elimination. Hum. Mol. Genet. 26, 293–304 (2017).
Tsai, N. P. et al. Multiple autism-linked genes mediate synapse elimination via proteasomal degradation of a synaptic scaffold PSD-95. Cell 151, 1581–1594 (2012).
Hinton, V. J., Brown, W. T., Wisniewski, K. & Rudelli, R. D. Analysis of neocortex in three males with the fragile X syndrome. Am. J. Med. Genet. 41, 289–294 (1991).
Rudelli, R. D. et al. Adult fragile X syndrome. Clinico-neuropathologic findings. Acta Neuropathol. 67, 289–295 (1985).
He, C. X. & Portera-Cailliau, C. The trouble with spines in fragile X syndrome: density, maturity and plasticity. Neuroscience 251, 120–128 (2013).
Jawaid, S. et al. Alterations in CA1 hippocampal synapses in a mouse model of fragile X syndrome. Glia 66, 789–800 (2018).
Dolen, G. et al. Correction of fragile X syndrome in mice. Neuron 56, 955–962 (2007).
Cruz-Martin, A., Crespo, M. & Portera-Cailliau, C. Delayed stabilization of dendritic spines in fragile X mice. J. Neurosci. 30, 7793–7803 (2010).
Pan, F., Aldridge, G. M., Greenough, W. T. & Gan, W. B. Dendritic spine instability and insensitivity to modulation by sensory experience in a mouse model of fragile X syndrome. Proc. Natl Acad. Sci. USA 107, 17768–17773 (2010).
Patel, A. B., Loerwald, K. W., Huber, K. M. & Gibson, J. R. Postsynaptic FMRP promotes the pruning of cell-to-cell connections among pyramidal neurons in the L5A neocortical network. J. Neurosci. 34, 3413–3418 (2014).
Isshiki, M. et al. Enhanced synapse remodelling as a common phenotype in mouse models of autism. Nat. Commun. 5, 4742 (2014).
Darnell, J. C. et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 146, 247–261 (2011).
Gabrielli, A. P., Manzardo, A. M. & Butler, M. G. GeneAnalytics pathways and profiling of shared autism and cancer genes. Int. J. Mol. Sci. https://doi.org/10.3390/ijms20051166 (2019).
Kwon, C. H. et al. Pten regulates neuronal arborization and social interaction in mice. Neuron 50, 377–388 (2006).
Tsai, P. T. et al. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature 488, 647–651 (2012).
Ebrahimi-Fakhari, D. et al. Impaired mitochondrial dynamics and mitophagy in neuronal models of tuberous sclerosis complex. Cell Rep. 17, 1053–1070 (2016).
Wang, T. et al. Flux of signalling endosomes undergoing axonal retrograde transport is encoded by presynaptic activity and TrkB. Nat. Commun. 7, 12976 (2016).
Wang, T. et al. Control of autophagosome axonal retrograde flux by presynaptic activity unveiled using botulinum neurotoxin type a. J. Neurosci. 35, 6179–6194 (2015).
Binotti, B. et al. The GTPase Rab26 links synaptic vesicles to the autophagy pathway. eLife 4, e05597 (2015).
Hernandez, D. et al. Regulation of presynaptic neurotransmission by macroautophagy. Neuron 74, 277–284 (2012).
Shehata, M., Matsumura, H., Okubo-Suzuki, R., Ohkawa, N. & Inokuchi, K. Neuronal stimulation induces autophagy in hippocampal neurons that is involved in AMPA receptor degradation after chemical long-term depression. J. Neurosci. 32, 10413–10422 (2012).
Nikoletopoulou, V., Sidiropoulou, K., Kallergi, E., Dalezios, Y. & Tavernarakis, N. Modulation of autophagy by BDNF underlies synaptic plasticity. Cell Metab. 26, 230–242 e235 (2017).
Xu, Z. X. et al. Elevated protein synthesis in microglia causes autism-like synaptic and behavioral aberrations. Nat. Commun. 11, 1797 (2020).
Gkogkas, C. G. et al. Autism-related deficits via dysregulated eIF4E-dependent translational control. Nature 493, 371–377 (2013).
Santini, E. et al. Exaggerated translation causes synaptic and behavioural aberrations associated with autism. Nature 493, 411–415 (2013).
American Psychiatric Association DSM-5 Task Force. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th Edn. (American Psychiatric Association, 2020).
Fusar-Poli, P. et al. The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry 70, 107–120 (2013).
Feinberg, I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J. Psychiatr. Res. 17, 319–334 (1982).
Zipursky, R. B., Lim, K. O., Sullivan, E. V., Brown, B. W. & Pfefferbaum, A. Widespread cerebral gray matter volume deficits in schizophrenia. Arch. Gen. Psychiatry 49, 195–205 (1992).
Andreasen, N. C. et al. Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biol. Psychiatry 70, 672–679 (2011).
Glantz, L. A. & Lewis, D. A. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch. Gen. Psychiatry 57, 65–73 (2000).
Kolluri, N., Sun, Z., Sampson, A. R. & Lewis, D. A. Lamina-specific reductions in dendritic spine density in the prefrontal cortex of subjects with schizophrenia. Am. J. Psychiatry 162, 1200–1202 (2005).
Glantz, L. A. & Lewis, D. A. Reduction of synaptophysin immunoreactivity in the prefrontal cortex of subjects with schizophrenia. Regional and diagnostic specificity. Arch. Gen. Psychiatry 54, 943–952 (1997).
Davidsson, P. et al. The synaptic-vesicle-specific proteins rab3a and synaptophysin are reduced in thalamus and related cortical brain regions in schizophrenic brains. Schizophr. Res. 40, 23–29 (1999).
Bitanihirwe, B. K., Lim, M. P., Kelley, J. F., Kaneko, T. & Woo, T. U. Glutamatergic deficits and parvalbumin-containing inhibitory neurons in the prefrontal cortex in schizophrenia. BMC Psychiatry 9, 71 (2009).
Onwordi, E. C. et al. Synaptic density marker SV2A is reduced in schizophrenia patients and unaffected by antipsychotics in rats. Nat. Commun. 11, 246 (2020).
Fromer, M. et al. De novo mutations in schizophrenia implicate synaptic networks. Nature 506, 179–184 (2014).
Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 511, 421–427 (2014).
Mukai, J. et al. Recapitulation and reversal of schizophrenia-related phenotypes in setd1a-deficient mice. Neuron 104, 471–487 e412 (2019).
Nagahama, K. et al. Setd1a insufficiency in mice attenuates excitatory synaptic function and recapitulates schizophrenia-related behavioral abnormalities. Cell Rep. 32, 108126 (2020).
Jones, C. A., Watson, D. J. & Fone, K. C. Animal models of schizophrenia. Br. J. Pharmacol. 164, 1162–1194 (2011).
Stefansson, H. et al. Common variants conferring risk of schizophrenia. Nature 460, 744–747 (2009).
Mokhtari, R. & Lachman, H. M. The major histocompatibility complex (MHC) in schizophrenia: a review. J. Clin. Cell Immunol. https://doi.org/10.4172/2155-9899.1000479 (2016).
Sekar, A. et al. Schizophrenia risk from complex variation of complement component 4. Nature https://doi.org/10.1038/nature16549 (2016).
Comer, A. L. et al. Increased expression of schizophrenia-associated gene C4 leads to hypoconnectivity of prefrontal cortex and reduced social interaction. PLoS Biol. 18, e3000604 (2020).
Yilmaz, M. et al. Overexpression of schizophrenia susceptibility factor human complement C4A promotes excessive synaptic loss and behavioral changes in mice. Nature Neurosci. https://doi.org/10.1038/s41593-020-00763-8 (2020).
Sellgren, C. M. et al. Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat. Neurosci. 22, 374–385 (2019).
Kim, M. et al. Brain gene co-expression networks link complement signaling with convergent synaptic pathology in schizophrenia. Nature Neurosci. https://doi.org/10.1038/s41593-021-00847-z (2021).
Al-Haddad, B. J. S. et al. The fetal origins of mental illness. Am. J. Obstet. Gynecol. 221, 549–562 (2019).
Bayer, T. A., Falkai, P. & Maier, W. Genetic and non-genetic vulnerability factors in schizophrenia: the basis of the “two hit hypothesis”. J. Psychiatr. Res. 33, 543–548 (1999).
Bolte, S., Girdler, S. & Marschik, P. B. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell Mol. Life Sci. 76, 1275–1297 (2019).
Brown, A. S. The environment and susceptibility to schizophrenia. Prog. Neurobiol. 93, 23–58 (2011).
van Os, J., Kenis, G. & Rutten, B. P. The environment and schizophrenia. Nature 468, 203–212 (2010).
Patterson, P. H. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav. Brain Res. 204, 313–321 (2009).
Fernandez de Cossio, L., Guzman, A., van der Veldt, S. & Luheshi, G. N. Prenatal infection leads to ASD-like behavior and altered synaptic pruning in the mouse offspring. Brain Behav. Immun. 63, 88–98 (2017).
Andoh, M. et al. Exercise reverses behavioral and synaptic abnormalities after maternal inflammation. Cell Rep. 27, 2817–2825 e2815 (2019).
Ikezu, S. et al. Inhibition of colony stimulating factor 1 receptor corrects maternal inflammation-induced microglial and synaptic dysfunction and behavioral abnormalities. Mol. Psychiatry https://doi.org/10.1038/s41380-020-0671-2 (2020).
Cao, P. et al. Early-life inflammation promotes depressive symptoms in adolescence via microglial engulfment of dendritic spines. Neuron 109, 2573–2589.e9 (2021).
Pekala, M., Doliwa, M. & Kalita, K. Impact of maternal immune activation on dendritic spine development. Dev. Neurobiol. (2020).
McNamara, R. K., Vannest, J. J. & Valentine, C. J. Role of perinatal long-chain omega-3 fatty acids in cortical circuit maturation: Mechanisms and implications for psychopathology. World J. Psychiatry 5, 15–34 (2015).
Madore, C. et al. Essential omega-3 fatty acids tune microglial phagocytosis of synaptic elements in the mouse developing brain. Nat. Commun. 11, 6133 (2020).
Choi, G. B. et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 351, 933–939 (2016).
Kim, S. et al. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature 549, 528–532 (2017).
Lammert, C. R. et al. Cutting edge: critical roles for microbiota-mediated regulation of the immune system in a prenatal immune activation model of autism. J. Immunol. 201, 845–850 (2018).
Logan, M. A. & Freeman, M. R. The scoop on the fly brain: glial engulfment functions in Drosophila. Neuron Glia Biol. 3, 63–74 (2007).
Awasaki, T. et al. Essential role of the apoptotic cell engulfment genes draper and ced-6 in programmed axon pruning during Drosophila metamorphosis. Neuron 50, 855–867 (2006).
Fuentes-Medel, Y. et al. Glia and muscle sculpt neuromuscular arbors by engulfing destabilized synaptic boutons and shed presynaptic debris. PLoS Biol. 7, e1000184 (2009).
Hakim, Y., Yaniv, S. P. & Schuldiner, O. Astrocytes play a key role in Drosophila mushroom body axon pruning. PLoS ONE 9, e86178 (2014).
Cherra, S. J. III & Jin, Y. A two-immunoglobulin-domain transmembrane protein mediates an epidermal-neuronal interaction to maintain synapse density. Neuron 89, 325–336 (2016).
Boulanger, A. et al. Axonal chemokine-like Orion induces astrocyte infiltration and engulfment during mushroom body neuronal remodeling. Nat. Commun. 12, 1849 (2021).
Yu, X. M. et al. Plum, an immunoglobulin superfamily protein, regulates axon pruning by facilitating TGF-beta signaling. Neuron 78, 456–468 (2013).
Ellis, H. M. & Horvitz, H. R. Genetic control of programmed cell death in the nematode C. elegans. Cell 44, 817–829 (1986).
Nakajima, Y. I. & Kuranaga, E. Caspase-dependent non-apoptotic processes in development. Cell Death Differ. 24, 1422–1430 (2017).
Meng, L. et al. The cell death pathway regulates synapse elimination through cleavage of gelsolin in caenorhabditis elegans neurons. Cell Rep. 11, 1737–1748 (2015).
Miller-Fleming, T. W. et al. The DEG/ENaC cation channel protein UNC-8 drives activity-dependent synapse removal in remodeling GABAergic neurons. eLife https://doi.org/10.7554/eLife.14599 (2016).
Raiders, S. et al. Glia actively sculpt sensory neurons by controlled phagocytosis to tune animal behavior. eLife https://doi.org/10.7554/eLife.63532 (2021).
Acknowledgements
The authors thank P. Feinberg for his careful review of the manuscript. This work was supported by grant numbers NIMH-R01MH113743 and NINDS-R01NS117533 (D.P.S.) and NINDS-F31NS117053 (G.G.).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Neuroscience thanks M. Freeman, M. Kano, M. Matteoli and A. Schaeffer for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Synaptic pruning
-
Developmental elimination of elements that comprise a bona fide structural synapse (presynaptic terminal and postsynaptic membranes), which might also include some pruning of small branches of axonal arbors and dendrites, while remaining synapses are maintained and strengthened.
- Spontaneous neural activity
-
Neuronal activity that is not driven by an external stimulus.
- Experience-driven neural activity
-
Neuronal activity driven by external changes affecting sensory experience.
- Eye-specific segregation
-
A process involving synaptic pruning by which spontaneous retinal activity drives presynaptic inputs from retinal ganglion cells to segregate and synapse in discrete, non-overlapping territories within the lateral geniculate nucleus during postnatal development.
- Monocular deprivation
-
The loss of sensory input to one eye, typically performed by suturing one eye closed for a defined period.
- Ocular dominance plasticity
-
A process by which monocular deprivation results in strengthening of synaptic inputs from the open eye and weakening and elimination of synapses corresponding to the sutured, deprived eye.
- Long-term depression
-
(LTD). A process by which changes in neuronal activity, such as sustained low-frequency stimulation, induce a reduction in synaptic strength.
- Engulfment
-
The internalization or phagocytosis of material by a cell for degradation.
- Trogocytosis
-
Partial phagocytosis of membrane material (trogo means ‘nibble’) while leaving the remaining membrane intact.
- Apoptosis
-
A canonical highly regulated process of programmed cell death that occurs in multiple contexts, including during development, and involves membrane blebbing, cell shrinkage and DNA fragmentation.
Rights and permissions
About this article
Cite this article
Faust, T.E., Gunner, G. & Schafer, D.P. Mechanisms governing activity-dependent synaptic pruning in the developing mammalian CNS. Nat Rev Neurosci 22, 657–673 (2021). https://doi.org/10.1038/s41583-021-00507-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41583-021-00507-y
This article is cited by
-
Common and distinct cortical thickness alterations in youth with autism spectrum disorder and attention-deficit/hyperactivity disorder
BMC Medicine (2024)
-
Unraveling the intercellular communication disruption and key pathways in Alzheimer’s disease: an integrative study of single-nucleus transcriptomes and genetic association
Alzheimer's Research & Therapy (2024)
-
Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases
Signal Transduction and Targeted Therapy (2024)
-
Understanding neural circuit function through synaptic engineering
Nature Reviews Neuroscience (2024)
-
Erythropoietin restrains the inhibitory potential of interneurons in the mouse hippocampus
Molecular Psychiatry (2024)