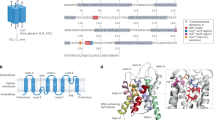
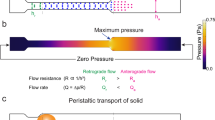
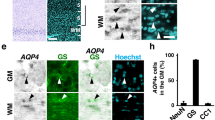
Abstract
Our brains consist of 80% water, which is continuously shifted between different compartments and cell types during physiological and pathophysiological processes. Disturbances in brain water homeostasis occur with pathologies such as brain oedema and hydrocephalus, in which fluid accumulation leads to elevated intracranial pressure. Targeted pharmacological treatments do not exist for these conditions owing to our incomplete understanding of the molecular mechanisms governing brain water transport. Historically, the transmembrane movement of brain water was assumed to occur as passive movement of water along the osmotic gradient, greatly accelerated by water channels termed aquaporins. Although aquaporins govern the majority of fluid handling in the kidney, they do not suffice to explain the overall brain water movement: either they are not present in the membranes across which water flows or they appear not to be required for the observed flow of water. Notably, brain fluid can be secreted against an osmotic gradient, suggesting that conventional osmotic water flow may not describe all transmembrane fluid transport in the brain. The cotransport of water is an unconventional molecular mechanism that is introduced in this Review as a missing link to bridge the gap in our understanding of cellular and barrier brain water transport.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Brightman, M. W. The distribution within the brain of ferritin injected into cerebrospinal fluid compartments. I. Ependymal distribution. J. Cell Biol.26, 99–123 (1965).
Mathiisen, T. M., Lehre, K. P., Danbolt, N. C. & Ottersen, O. P. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia58, 1094–1103 (2010).
Mollgard, K., Balslev, Y., Lauritzen, B. & Saunders, N. R. Cell junctions and membrane specializations in the ventricular zone (germinal matrix) of the developing sheep brain: a CSF-brain barrier. J. Neurocytol.16, 433–444 (1987).
Brightman, M. W. & Reese, T. S. Junctions between intimately apposed cell membranes in the vertebrate brain. J. Cell Biol.40, 648–677 (1969).
Cserr, H. F., Cooper, D. N., Suri, P. K. & Patlak, C. S. Efflux of radiolabeled polyethylene glycols and albumin from rat brain. Am. J. Physiol.240, F319–F328 (1981).
His, W. Über ein perivasculäres canalsystem in den nervösen centralorganen und über dessen beziehungen sum lymphsystem. Z. für wissenschaftliche Zoologie15, 127–141 (1865).
Rennels, M. L., Blaumanis, O. R. & Grady, P. A. Rapid solute transport throughout the brain via paravascular fluid pathways. Adv. Neurol.52, 431–439 (1990).
Iliff, J. J. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci. Transl. Med.4, 147ra111 (2012).
Abbott, N. J., Pizzo, M. E., Preston, J. E., Janigro, D. & Thorne, R. G. The role of brain barriers in fluid movement in the CNS: is there a ‘glymphatic’ system? Acta Neuropathol.135, 387–407 (2018).
Asgari, M., de, Z. D. & Kurtcuoglu, V. Glymphatic solute transport does not require bulk flow. Sci. Rep.6, 38635 (2016).
Faghih, M. M. & Sharp, M. K. Is bulk flow plausible in perivascular, paravascular and paravenous channels? Fluids Barriers CNS15, 17 (2018).
Hladky, S. B. & Barrand, M. A. Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS11, 26 (2014).
Hladky, S. B. & Barrand, M. A. Elimination of substances from the brain parenchyma: efflux via perivascular pathways and via the blood-brain barrier. Fluids Barriers CNS15, 30 (2018).
Holter, K. E. et al. Interstitial solute transport in 3D reconstructed neuropil occurs by diffusion rather than bulk flow. Proc. Natl Acad. Sci. USA114, 9894–9899 (2017).
Jin, B. J., Smith, A. J. & Verkman, A. S. Spatial model of convective solute transport in brain extracellular space does not support a “glymphatic” mechanism. J. Gen. Physiol.148, 489–501 (2016).
Smith, A. J., Yao, X., Dix, J. A., Jin, B. J. & Verkman, A. S. Test of the ‘glymphatic’ hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. eLife6, e27679 (2017).
Smith, A. J. & Verkman, A. S. The “glymphatic” mechanism for solute clearance in Alzheimer’s disease: game changer or unproven speculation? FASEB J.32, 453–551 (2017).
Spector, R., Robert, S. S. & Johanson, C. E. A balanced view of the cerebrospinal fluid composition and functions: focus on adult humans. Exp. Neurol.273, 57–68 (2015).
Dandy, W. E. Experimental hydrocephalus. Ann. Surg.70, 129–142 (1919). This study demonstrated the role of choroid plexus in CSF secretion.
Dietzel, I., Heinemann, U., Hofmeier, G. & Lux, H. D. Transient changes in the size of the extracellular space in the sensorimotor cortex of cats in relation to stimulus-induced changes in potassium concentration. Exp. Brain Res.40, 432–439 (1980). This study was among the first to demonstrate the extracellular space shrinkage occurring with neuronal activity.
Eichling, J. O., Raichle, M. E., Grubb, R. L. Jr. & Ter-Pogossian, M. M. Evidence of the limitations of water as a freely diffusible tracer in brain of the rhesus monkey. Circ. Res.35, 358–364 (1974).
Raichle, M. E. et al. Blood-brain barrier permeability of 11C-labeled alcohols and 15O-labeled water. Am. J. Physiol.230, 543–552 (1976).
Fenstermacher, J. D. & Johnson, J. A. Filtration and reflection coefficients of the rabbit blood-brain barrier. Am. J. Physiol.211, 341–346 (1966).
MacAulay, N., Hamann, S. & Zeuthen, T. in Physiology and Pathology of Chloride Transporters and Channels in the Nervous System (ed Alvarez-Leefmans, F. J. & Delpire, E.) Ch. 28, 547-568 (Academic Press, Elsevier, 2009).
Paulson, O. B., Hertz, M. M., Bolwig, T. G. & Lassen, N. A. Filtration and diffusion of water across the blood-brain barrier in man. Microvasc. Res.13, 113–124 (1977).
Haj-Yasein, N. N. et al. Glial-conditional deletion of aquaporin-4 (Aqp4) reduces blood-brain water uptake and confers barrier function on perivascular astrocyte endfeet. Proc. Natl Acad. Sci. USA108, 17815–17820 (2011). The study demonstrated the lack of AQP4 in brain endothelium.
Agre, P. Molecular physiology of water transport: aquaporin nomenclature workshop. Mammalian aquaporins. Biol. Cell89, 255–257 (1997).
Wang, Y. & Tajkhorshid, E. Molecular mechanisms of conduction and selectivity in aquaporin water channels. J. Nutr.137, 1509S–1515S (2007).
Ho, J. D. et al. Crystal structure of human aquaporin 4 at 1.8A and its mechanism of conductance. Proc. Natl Acad. Sci. USA106, 7437–7442 (2009).
Litman, T., Sogaard, R. & Zeuthen, T. In: Aquaporins. Handbook of Experimental Pharmacology. 190 (ed Beitz, E.) 327-358 (Springer, 2009).
Zeuthen, T. & MacAulay, N. Passive water transport in biological pores. Int. Rev. Cytol.215, 203–230 (2002).
Li, J. et al. Transient formation of water-conducting states in membrane transporters. Proc. Natl Acad. Sci. USA110, 7696–7701 (2013).
MacAulay, N., Gether, U., Klaeke, D. A. & Zeuthen, T. Passive water and urea permeability of a human Na+-glutamate cotransporter expressed in Xenopus oocytes. J. Physiol.542, 817–828 (2002).
Zeuthen, T. & MacAulay, N. Cotransporters as molecular water pumps. Int. Rev. Cytol.215, 259–284 (2002).
Zeuthen, T. Water-transporting proteins. J. Membr. Biol.234, 57–73 (2010).
Tait, M. J., Saadoun, S., Bell, B. A. & Papadopoulos, M. C. Water movements in the brain: role of aquaporins. Trends Neurosci.31, 37–43 (2008).
Nielsen, S., Smith, B. L., Christensen, E. I. & Agre, P. Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc. Natl Acad. Sci. USA90, 7275–7279 (1993).
Speake, T., Freeman, L. J. & Brown, P. D. Expression of aquaporin 1 and aquaporin 4 water channels in rat choroid plexus. Biochim. Biophys. Acta1609, 80–86 (2003).
Nielsen, S. et al. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J. Neurosci.17, 171–180 (1997).
Li, Q. et al. Aquaporin 1 and the Na+/K+/2Cl– cotransporter 1 are present in the leptomeningeal vasculature of the adult rodent central nervous system. Fluids Barriers CNS17, 15 (2020).
Amiry-Moghaddam, M. & Ottersen, O. P. The molecular basis of water transport in the brain. Nat. Rev. Neurosci.4, 991–1001 (2003).
Arcienega, I. I., Brunet, J. F., Bloch, J. & Badaut, J. Cell locations for AQP1, AQP4 and 9 in the non-human primate brain. Neuroscience167, 1103–1114 (2010).
Zhang, Y. et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci.34, 11929–11947 (2014).
Zhang, Y. et al. Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron89, 37–53 (2016).
Misawa, T., Arima, K., Mizusawa, H. & Satoh, J. Close association of water channel AQP1 with amyloid-beta deposition in Alzheimer disease brains. Acta Neuropathol.116, 247–260 (2008).
Nesic, O. et al. Aquaporin 1 - a novel player in spinal cord injury. J. Neurochem.105, 628–640 (2008).
Satoh, J., Tabunoki, H., Yamamura, T., Arima, K. & Konno, H. Human astrocytes express aquaporin-1 and aquaporin-4 in vitro and in vivo. Neuropathology27, 245–256 (2007).
Jung, J. S. et al. Molecular characterization of an aquaporin cDNA from brain: candidate osmoreceptor and regulator of water balance. PNAS91, 13052–13056 (1994).
Hubbard, J. A., Hsu, M. S., Seldin, M. M. & Binder, D. K. Expression of the astrocyte water channel aquaporin-4 in the mouse brain. ASN Neurohttps://doi.org/10.1177/1759091415605486 (2015).
Neely, J. D. et al. Syntrophin-dependent expression and localization of Aquaporin-4 water channel protein. Proc. Natl Acad. Sci. USA98, 14108–14113 (2001).
Ma, T. et al. Generation and phenotype of a transgenic knockout mouse lacking the mercurial-insensitive water channel aquaporin-4. J. Clin. Invest100, 957–962 (1997).
Amiry-Moghaddam, M. et al. Delayed K+ clearance associated with aquaporin-4 mislocalization: phenotypic defects in brains of alpha-syntrophin-null mice. Proc. Natl Acad. Sci. USA100, 13615–13620 (2003).
Assentoft, M., Larsen, B. R. & MacAulay, N. Regulation and function of AQP4 in the central nervous system. Neurochem. Res.40, 2615–2627 (2015).
Nagelhus, E. A., Mathiisen, T. M. & Ottersen, O. P. Aquaporin-4 in the central nervous system: cellular and subcellular distribution and coexpression with KIR4.1. Neuroscience129, 905–913 (2004).
Papadopoulos, M. C. & Verkman, A. S. Aquaporin water channels in the nervous system. Nat. Rev. Neurosci.14, 265–277 (2013).
Yao, X., Hrabetova, S., Nicholson, C. & Manley, G. T. Aquaporin-4-deficient mice have increased extracellular space without tortuosity change. J. Neurosci.28, 5460–5464 (2008).
Eilert-Olsen, M. et al. Deletion of aquaporin-4 changes the perivascular glial protein scaffold without disrupting the brain endothelial barrier. Glia60, 432–440 (2012).
Zeng, X. N. et al. Aquaporin-4 deficiency down-regulates glutamate uptake and GLT-1 expression in astrocytes. Mol. Cell Neurosci.34, 34–39 (2007).
Fenton, R. A. et al. Differential water permeability and regulation of three aquaporin 4 isoforms. Cell Mol. Life Sci.67, 829–840 (2010).
Assentoft, M. et al. Aquaporin 4 as a NH3 channel. J. Biol. Chem.291, 19184–19195 (2016).
Moe, S. E. et al. New isoforms of rat Aquaporin-4. Genomics91, 367–377 (2008).
Neely, J. D., Christensen, B. M., Nielsen, S. & Agre, P. Heterotetrameric composition of aquaporin-4 water channels. Biochemistry38, 11156–11163 (1999).
Furman, C. S. et al. Aquaporin-4 square array assembly: opposing actions of M1 and M23 isoforms. Proc. Natl Acad. Sci. USA100, 13609–13614 (2003).
Yang, B., Brown, D. & Verkman, A. S. The mercurial insensitive water channel (AQP-4) forms orthogonal arrays in stably transfected Chinese hamster ovary cells. J. Biol. Chem.271, 4577–4580 (1996).
Landis, D. M. & Reese, T. S. Arrays of particles in freeze-fractured astrocytic membranes. J. Cell Biol.60, 316–320 (1974).
Neuhaus, J. Orthogonal arrays of particles in astroglial cells: quantitative analysis of their density, size, and correlation with intramembranous particles. Glia3, 241–251 (1990).
Solenov, E., Watanabe, H., Manley, G. T. & Verkman, A. S. Sevenfold-reduced osmotic water permeability in primary astrocyte cultures from AQP-4-deficient mice, measured by a fluorescence quenching method. Am. J. Physiol. Cell Physiol.286, C426–C432 (2004). The study demonstrated the high astrocytic water permeability of AQP4-deficient astrocytes.
Gunnarson, E. et al. Identification of a molecular target for glutamate regulation of astrocyte water permeability. Glia56, 587–596 (2008).
Song, Y. & Gunnarson, E. Potassium dependent regulation of astrocyte water permeability is mediated by cAMP signaling. PLoS ONE7, e34936 (2012).
Zelenina, M., Zelenin, S., Bondar, A. A., Brismar, H. & Aperia, A. Water permeability of aquaporin-4 is decreased by protein kinase C and dopamine. Am. J. Physiol. Ren. Physiol.283, F309–F318 (2002).
Assentoft, M. et al. Phosphorylation of rat aquaporin-4 at Ser(111) is not required for channel gating. Glia61, 1101–1112 (2013).
Assentoft, M., Larsen, B. R., Olesen, E. T., Fenton, R. A. & MacAulay, N. AQP4 plasma membrane trafficking or channel gating is not significantly modulated by phosphorylation at COOH-terminal serine residues. Am. J. Physiol. Cell Physiol.307, C957–C965 (2014).
Sachdeva, R. & Singh, B. Phosphorylation of Ser-180 of rat aquaporin-4 shows marginal affect on regulation of water permeability: molecular dynamics study. J. Biomol. Struct. Dyn.32, 555–566 (2014).
Fischer, M. & Kaldenhoff, R. On the pH regulation of plant aquaporins. J. Biol. Chem.283, 33889–33892 (2008).
Nemeth-Cahalan, K. L. & Hall, J. E. pH and calcium regulate the water permeability of aquaporin 0. J. Biol. Chem.275, 6777–6782 (2000).
Zeuthen, T. & Klaerke, D. A. Transport of water and glycerol in aquaporin 3 is gated by H+. J. Biol. Chem.274, 21631–21636 (1999).
Kaptan, S. et al. H95 is a pH-dependent gate in aquaporin 4. Structure23, 2309–2318 (2015).
Alberga, D. et al. A new gating site in human aquaporin-4: insights from molecular dynamics simulations. Biochim. Biophys. Acta1838, 3052–3060 (2014).
Kraig, R. P. & Chesler, M. Astrocytic acidosis in hyperglycemic and complete ischemia. J. Cereb. Blood Flow Metab.10, 104–114 (1990).
MacAulay, N. & Zeuthen, T. Water transport between CNS compartments: contributions of aquaporins and cotransporters. Neuroscience168, 941–956 (2010).
Zeuthen, T. et al. Water transport by the Na+/glucose cotransporter under isotonic conditions. Biol. Cell89, 307–312 (1997).
Choe, S., Rosenberg, J. M., Abrahamson, J., Wright, E. M. & Grabe, M. Water permeation through the sodium-dependent galactose cotransporter vSGLT. Biophys. J. Biophys. Lett.99, 56–58 (2010).
Hamann, S., Kiilgaard, J. F., la Cour, M., Prause, J. U. & Zeuthen, T. Cotransport of H+, lactate, and H2O in porcine retinal pigment epithelial cells. Exp. Eye Res.76, 493–504 (2003).
Hamann, S., Herrera-Perez, J. J., Zeuthen, T. & Alvarez-Leefmans, F. J. Cotransport of water by the Na+-K+-2Cl− cotransporter NKCC1 in mammalian epithelial cells. J. Physiol.588, 4089–4101 (2010).
MacAulay, N., Gether, U., Klaerke, D. A. & Zeuthen, T. Water transport by the human Na+-coupled glutamate cotransporter expressed in Xenopus oocytes. J. Physiol.530, 367–378 (2001).
MacAulay, N., Zeuthen, T. & Gether, U. Conformational basis for the Li+-induced leak current in the rat gamma-aminobutyric acid (GABA) transporter-1. J. Physiol.544, 447–458 (2002).
Steffensen, A. B. et al. Cotransporter-mediated water transport underlying cerebrospinal fluid formation. Nat. Commun.9, 2167 (2018). This study demonstrated the role of NKCC1 in CSF secretion, showing that it functioned in a manner independent of the osmotic gradient.
Zeuthen, T. Cotransport of K+, Cl− and H2O by membrane proteins from choroid plexus epithelium of Necturus maculosus. J. Physiol.478, 203–219 (1994).
Kimelberg, H. K. Current concepts of brain edema. Review of laboratory investigations. J. Neurosurg.83, 1051–1059 (1995).
Stokum, J. A., Gerzanich, V. & Simard, J. M. Molecular pathophysiology of cerebral edema. J. Cereb. Blood Flow Metab.36, 513–538 (2016).
Davalos, A., Shuaib, A. & Wahlgren, N. G. Neurotransmitters and pathophysiology of stroke: evidence for the release of glutamate and other transmitters/mediators in animals and humans. J. Stroke Cerebrovasc. Dis.9, 2–8 (2000).
Hossmann, K. A., Sakaki, S. & Zimmerman, V. Cation activities in reversible ischemia of the cat brain. Stroke8, 77–81 (1977).
Walz, W. & Mukerji, S. KCl movements during potassium-induced cytotoxic swelling of cultured astrocytes. Exp. Neurol.99, 17–29 (1988).
Benfenati, V. et al. An aquaporin-4/transient receptor potential vanilloid 4 (AQP4/TRPV4) complex is essential for cell-volume control in astrocytes. Proc. Natl Acad. Sci. USA108, 2563–2568 (2011).
Mola, M. G. et al. The speed of swelling kinetics modulates cell volume regulation and calcium signaling in astrocytes: a different point of view on the role of aquaporins. Glia64, 139–154 (2016).
Jo, A. O. et al. TRPV4 and AQP4 channels synergistically regulate cell volume and calcium homeostasis in retinal muller glia. J. Neurosci.35, 13525–13537 (2015).
Toft-Bertelsen, T. L., Krizaj, D. & MacAulay, N. When size matters: transient receptor potential vanilloid 4 channel as a volume-sensor rather than an osmo-sensor. J. Physiol.595, 3287–3302 (2017).
Toft-Bertelsen, T. L., Larsen, B. R. & MacAulay, N. Sensing and regulation of cell volume - we know so much and yet understand so little: TRPV4 as a sensor of volume changes but possibly without a volume-regulatory role? Channels12, 100–108 (2018).
Stokum, J. A. et al. SUR1-TRPM4 and AQP4 form a heteromultimeric complex that amplifies ion/water osmotic coupling and drives astrocyte swelling. Glia66, 108–125 (2018).
Rakers, C., Schmid, M. & Petzold, G. C. TRPV4 channels contribute to calcium transients in astrocytes and neurons during peri-infarct depolarizations in a stroke model. Glia65, 1550–1561 (2017).
Rosic, B. et al. Aquaporin-4-independent volume dynamics of astroglial endfeet during cortical spreading depression. Glia67, 1113–1121 (2019). This study demonstrated that SD-induced glia cell swelling occurred independently of AQP4.
Ballanyi, K., Grafe, P. & Ten Bruggencate, G. Ion activities and potassium uptake mechanisms of glial cells in guinea-pig olfactory cortex slices. J. Physiol.382, 159–174 (1987).
Grafe, P. & Ballanyi, K. Cellular mechanisms of potassium homeostasis in the mammalian nervous system. Can. J. Physiol. Pharmacol.65, 1038–1042 (1987).
Coles, J. A. & Schneider-Picard, G. Increase in glial intracellular K+ in drone retina caused by photostimulation but not mediated by an increase in extracellular K+. Glia2, 213–222 (1989).
Larsen, B. R., Stoica, A. & MacAulay, N. Managing brain extracellular K+ during neuronal activity: the physiological role of the Na+/K+-ATPase subunit isoforms. Front. Physiol.7, 141 (2016).
MacAulay, N. Molecular mechanisms of K+ clearance and extracellular space shrinkage-glia cells as the stars. Gliahttps://doi.org/10.1002/glia.23824 (2020).
Hochman, D. W., Baraban, S. C., Owens, J. W. & Schwartzkroin, P. A. Dissociation of synchronization and excitability in furosemide blockade of epileptiform activity. Science270, 99–102 (1995).
Larsen, B. R. et al. Contributions of the Na+ /K+-ATPase, NKCC1, and Kir4.1 to hippocampal K+ clearance and volume responses. Glia62, 608–622 (2014). This study demonstrated that glial K+-transporting mechanisms do not contribute to extracellular space shrinkage.
MacVicar, B. A. & Hochman, D. Imaging of synaptically evoked intrinsic optical signals in hippocampal slices. J. Neurosci.11, 1458–1469 (1991).
MacVicar, B. A., Feighan, D., Brown, A. & Ransom, B. Intrinsic optical signals in the rat optic nerve: role for K+ uptake via NKCC1 and swelling of astrocytes. Glia37, 114–123 (2002).
Orkand, R. K., Dietzel, I. & Coles, J. A. Light-induced changes in extracellular volume in the retina of the drone, Apis mellifera. Neurosci. Lett.45, 273–278 (1984).
Pal, I., Nyitrai, G., Kardos, J. & Heja, L. Neuronal and astroglial correlates underlying spatiotemporal intrinsic optical signal in the rat hippocampal slice. PLoS ONE8, e57694 (2013).
Ransom, B. R., Yamate, C. L. & Connors, B. W. Activity-dependent shrinkage of extracellular space in rat optic nerve: a developmental study. J. Neurosci.5, 532–535 (1985). This study demonstrated that glial maturation is required for activity-evoked extracellular space shrinkage.
Florence, C. M., Baillie, L. D. & Mulligan, S. J. Dynamic volume changes in astrocytes are an intrinsic phenomenon mediated by bicarbonate ion flux. PLoS ONE7, e51124 (2012).
Larsen, B. R., Stoica, A. & MacAulay, N. Developmental maturation of activity-induced K+ and pH transients and the associated extracellular space dynamics in the rat hippocampus. J. Physiol.597, 583–597 (2019).
Hertz, L. et al. Astrocytic and neuronal accumulation of elevated extracellular K+ with a 2/3K+/Na+ flux ratio-consequences for energy metabolism, osmolarity and higher brain function. Front. Comput. Neurosci.7, 114 (2013).
Kofuji, P. & Newman, E. A. Potassium buffering in the central nervous system. Neuroscience129, 1045–1056 (2004).
MacAulay, N. & Zeuthen, T. Glial K+ clearance and cell swelling: key roles for cotransporters and pumps. Neurochem. Res.37, 2299–2309 (2012).
Nagelhus, E. A. et al. Immunogold evidence suggests that coupling of K+ siphoning and water transport in rat retinal Muller cells is mediated by a co-enrichment of Kir4.1 and AQP4 in specific membrane domains. Glia26, 47–54 (1999).
Nagelhus, E. A. & Ottersen, O. P. Physiological roles of aquaporin-4 in brain. Physiol. Rev.93, 1543–1562 (2013).
Haj-Yasein, N. N. et al. Aquaporin-4 regulates extracellular space volume dynamics during high-frequency synaptic stimulation: a gene deletion study in mouse hippocampus. Glia60, 867–874 (2012).
Toft-Bertelsen, T. L. et al. Clearance of activity-evoked K+ transients and associated glia cell swelling occur independently of AQP4: A study with an isoform-selective AQP4 inhibitor. Glia69, 28–41 (2020). This study demonstrated robust activity-evoked extracellular space shrinkage in the absence of AQP4.
Haj-Yasein, N. N. et al. Evidence that compromised K+ spatial buffering contributes to the epileptogenic effect of mutations in the human kir4.1 gene (KCNJ10). Glia59, 1635–1642 (2011).
Larsen, B. R. & MacAulay, N. Kir4.1-mediated spatial buffering of K+: experimental challenges in determination of its temporal and quantitative contribution to K+ clearance in the brain. Channels8, 544–550 (2014).
Orkand, R. K., Nicholls, J. G. & Kuffler, S. W. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J. Neurophysiol.29, 788–806 (1966).
Ruiz-Ederra, J., Zhang, H. & Verkman, A. S. Evidence against functional interaction between aquaporin-4 water channels and Kir4.1 potassium channels in retinal Muller cells. J. Biol. Chem.282, 21866–21872 (2007).
Binder, D. K. et al. Increased seizure duration and slowed potassium kinetics in mice lacking aquaporin-4 water channels. Glia53, 631–636 (2006).
Haj-Yasein, N. N. et al. Deletion of aquaporin-4 increases extracellular K+ concentration during synaptic stimulation in mouse hippocampus. Brain Struct. Funct.220, 2469–2474 (2015).
Strohschein, S. et al. Impact of aquaporin-4 channels on K+ buffering and gap junction coupling in the hippocampus. Glia59, 973–980 (2011).
Jin, B. J., Zhang, H., Binder, D. K. & Verkman, A. S. Aquaporin-4-dependent K+ and water transport modeled in brain extracellular space following neuroexcitation. J. Gen. Physiol.141, 119–132 (2013).
Binder, D. K., Yao, X., Verkman, A. S. & Manley, G. T. Increased seizure duration in mice lacking aquaporin-4 water channels. Acta Neurochir. Suppl.96, 389–392 (2006).
D’Ambrosio, R., Gordon, D. S. & Winn, H. R. Differential role of KIR channel and Na+/K+-pump in the regulation of extracellular K+ in rat hippocampus. J. Neurophysiol.87, 87–102 (2002).
Bourke, R. S. & Nelson, K. M. Further studies on the K+-dependent swelling of primate cerebral cortex in vivo: the enzymatic basis of the K+-dependent transport of chloride. J. Neurochem.19, 663–685 (1972).
Larsen, B. R. & MacAulay, N. Activity-dependent astrocyte swelling is mediated by pH-regulating mechanisms. Glia65, 1668–1681 (2017). This study demonstrated the importance of pH-regulating transporters in activity-evoked extracellular space shrinkage.
Holthoff, K. & Witte, O. W. Intrinsic optical signals in rat neocortical slices measured with near-infrared dark-field microscopy reveal changes in extracellular space. J. Neurosci.16, 2740–2749 (1996).
Su, G., Kintner, D. B. & Sun, D. Contribution of Na+-K+-Cl– cotransporter to high-[K+]o- induced swelling and EAA release in astrocytes. Am. J. Physiol. Cell Physiol.282, C1136–C1146 (2002).
Walz, W. & Hinks, E. C. Carrier-mediated KCl accumulation accompanied by water movements is involved in the control of physiological K+ levels by astrocytes. Brain Res.343, 44–51 (1985).
Walz, W. Role of Na/K/Cl cotransport in astrocytes. Can. J. Physiol. Pharmacol.70 (Suppl.), S260–S262 (1992).
Zeuthen, T. & MacAulay, N. Cotransport of water by Na+-K+-2Cl– cotransporters expressed in Xenopus oocytes: NKCC1 versus NKCC2. J. Physiol.590, 1139–1154 (2012). This study demonstrated the ability of NKCC1 to cotransport water.
Plotkin, M. D. et al. Expression of the Na+-K+-2Cl− cotransporter BSC2 in the nervous system. Am. J. Physiol.272, C173–C183 (1997). This study demonstrated the lack of NKCC1 expression in glial cells and endothelium and its abundance in the choroid plexus.
Raat, N. J., Delpire, E., Van Os, C. H. & Bindels, R. J. Culturing induced expression of basolateral Na+-K+-2Cl– cotransporter BSC2 in proximal tubule, aortic endothelium, and vascular smooth muscle. Pflug. Arch.431, 458–460 (1996).
Danbolt, N. C. Glutamate uptake. Prog. Neurobiol.65, 1–105 (2001).
Schools, G. P. & Kimelberg, H. K. mGluR3 and mGluR5 are the predominant metabotropic glutamate receptor mRNAs expressed in hippocampal astrocytes acutely isolated from young rats. J. Neurosci. Res.58, 533–543 (1999).
Hansson, E. Metabotropic glutamate receptor activation induces astroglial swelling. J. Biol. Chem.269, 21955–21961 (1994).
Izumi, Y., Kirby, C. O., Benz, A. M., Olney, J. W. & Zorumski, C. F. Muller cell swelling, glutamate uptake, and excitotoxic neurodegeneration in the isolated rat retina. Glia25, 379–389 (1999).
Schneider, G. H., Baethmann, A. & Kempski, O. Mechanisms of glial swelling induced by glutamate. Can. J. Physiol. Pharmacol.70 (Suppl.), S334–S343 (1992).
Makani, S. & Chesler, M. Rapid rise of extracellular pH evoked by neural activity is generated by the plasma membrane calcium ATPase. J. Neurophysiol.103, 667–676 (2010).
Voipio, J. & Kaila, K. Interstitial PCO2 and pH in rat hippocampal slices measured by means of a novel fast CO2/H+-sensitive microelectrode based on a PVC-gelled membrane. Pflug. Arch.423, 193–201 (1993).
Chesler, M. Regulation and modulation of pH in the brain. Physiol. Rev.83, 1183–1221 (2003).
Theparambil, S. M., Ruminot, I., Schneider, H. P., Shull, G. E. & Deitmer, J. W. The electrogenic sodium bicarbonate cotransporter NBCe1 is a high-affinity bicarbonate carrier in cortical astrocytes. J. Neurosci.34, 1148–1157 (2014).
Deitmer, J. W. & Szatkowski, M. Membrane potential dependence of intracellular pH regulation by identified glial cells in the leech central nervous system. J. Physiol.421, 617–631 (1990).
Pappas, C. A. & Ransom, B. R. Depolarization-induced alkalinization (DIA) in rat hippocampal astrocytes. J. Neurophysiol.72, 2816–2826 (1994).
Theparambil, S. M., Naoshin, Z., Thyssen, A. & Deitmer, J. W. Reversed electrogenic sodium bicarbonate cotransporter 1 is the major acid loader during recovery from cytosolic alkalosis in mouse cortical astrocytes. J. Physiol.593, 3533–3547 (2015).
Barros, L. F. Metabolic signaling by lactate in the brain. Trends Neurosci.36, 396–404 (2013).
Mangia, S. et al. The aerobic brain: lactate decrease at the onset of neural activity. Neuroscience118, 7–10 (2003).
Bergersen, L. et al. A novel postsynaptic density protein: the monocarboxylate transporter MCT2 is co-localized with delta-glutamate receptors in postsynaptic densities of parallel fiber-Purkinje cell synapses. Exp. Brain Res.136, 523–534 (2001).
Pierre, K., Pellerin, L., Debernardi, R., Riederer, B. M. & Magistretti, P. J. Cell-specific localization of monocarboxylate transporters, MCT1 and MCT2, in the adult mouse brain revealed by double immunohistochemical labeling and confocal microscopy. Neuroscience100, 617–627 (2000).
Rafiki, A., Boulland, J. L., Halestrap, A. P., Ottersen, O. P. & Bergersen, L. Highly differential expression of the monocarboxylate transporters MCT2 and MCT4 in the developing rat brain. Neuroscience122, 677–688 (2003).
Zeuthen, T., Hamann, S. & la Cour, M. Cotransport of H+, lactate and H2O by membrane proteins in retinal pigment epithelium of bullfrog. J. Physiol.497, 3–17 (1996).
Andrew, R. D., Labron, M. W., Boehnke, S. E., Carnduff, L. & Kirov, S. A. Physiological evidence that pyramidal neurons lack functional water channels. Cereb. Cortex17, 787–802 (2007). This study demonstrated the excessively low neuronal osmotic water permeability.
Risher, W. C., Andrew, R. D. & Kirov, S. A. Real-time passive volume responses of astrocytes to acute osmotic and ischemic stress in cortical slices and in vivo revealed by two-photon microscopy. Glia57, 207–221 (2009).
Steffensen, A. B., Sword, J., Croom, D., Kirov, S. A. & MacAulay, N. Chloride cotransporters as a molecular mechanism underlying spreading depolarization-induced dendritic beading. J. Neurosci.35, 12172–12187 (2015). This study demonstrated the role of cotransporters in SD-induced dendritic beading.
Rash, J. E., Yasumura, T., Hudson, C. S., Agre, P. & Nielsen, S. Direct immunogold labeling of aquaporin-4 in square arrays of astrocyte and ependymocyte plasma membranes in rat brain and spinal cord. Proc. Natl Acad. Sci. USA95, 11981–11986 (1998).
Aitken, P. G. et al. Volume changes induced by osmotic stress in freshly isolated rat hippocampal neurons. Pflug. Arch.436, 991–998 (1998).
Pasantes-Morales, H., Maar, T. E. & Moran, J. Cell volume regulation in cultured cerebellar granule neurons. J. Neurosci. Res.34, 219–224 (1993).
Risher, W. C., Croom, D. & Kirov, S. A. Persistent astroglial swelling accompanies rapid reversible dendritic injury during stroke-induced spreading depolarizations. Glia60, 1709–1720 (2012).
Takano, T. et al. Cortical spreading depression causes and coincides with tissue hypoxia. Nat. Neurosci.10, 754–762 (2007).
Dreier, J. P. & Reiffurth, C. The stroke-migraine depolarization continuum. Neuron86, 902–922 (2015).
Hartings, J. A. et al. Spreading depolarisations and outcome after traumatic brain injury: a prospective observational study. Lancet Neurol.10, 1058–1064 (2011).
Oliveira-Ferreira, A. I. et al. Experimental and preliminary clinical evidence of an ischemic zone with prolonged negative DC shifts surrounded by a normally perfused tissue belt with persistent electrocorticographic depression. J. Cereb. Blood Flow Metab.30, 1504–1519 (2010).
Dreier, J. P. et al. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain129, 3224–3237 (2006).
Lauritzen, M. et al. Clinical relevance of cortical spreading depression in neurological disorders: migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury. J. Cereb. Blood Flow Metab.31, 17–35 (2011).
Hartings, J. A. et al. Spreading depolarizations have prolonged direct current shifts and are associated with poor outcome in brain trauma. Brain134, 1529–1540 (2011).
Dreier, J. P. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat. Med.17, 439–447 (2011).
Leao, A. A. Spreading depression of activity in the cerebral cortex. J. Neurophysiol.7, 359–390 (1944).
Hansen, A. J. & Zeuthen, T. Extracellular ion concentrations during spreading depression and ischemia in the rat brain cortex. Acta Physiol. Scand.113, 437–445 (1981).
Kraig, R. P. & Nicholson, C. Extracellular ionic variations during spreading depression. Neuroscience3, 1045–1059 (1978).
Somjen, G. G. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol. Rev.81, 1065–1096 (2001).
Snow, R. W., Taylor, C. P. & Dudek, F. E. Electrophysiological and optical changes in slices of rat hippocampus during spreading depression. J. Neurophysiol.50, 561–572 (1983).
Muller, M. & Somjen, G. G. Intrinsic optical signals in rat hippocampal slices during hypoxia-induced spreading depression-like depolarization. J. Neurophysiol.82, 1818–1831 (1999).
Zhou, N., Gordon, G. R., Feighan, D. & MacVicar, B. A. Transient swelling, acidification, and mitochondrial depolarization occurs in neurons but not astrocytes during spreading depression. Cereb. Cortex20, 2614–2624 (2010).
Risher, W. C., Ard, D., Yuan, J. & Kirov, S. A. Recurrent spontaneous spreading depolarizations facilitate acute dendritic injury in the ischemic penumbra. J. Neurosci.30, 9859–9868 (2010). This study demonstrated the appearance of dendritic beading during ischaemia.
Rungta, R. L. et al. The cellular mechanisms of neuronal swelling underlying cytotoxic edema. Cell161, 610–621 (2015).
Hoskison, M. M., Yanagawa, Y., Obata, K. & Shuttleworth, C. W. Calcium-dependent NMDA-induced dendritic injury and MAP2 loss in acute hippocampal slices. Neuroscience145, 66–79 (2007).
Gisselsson, L. L., Matus, A. & Wieloch, T. Actin redistribution underlies the sparing effect of mild hypothermia on dendritic spine morphology after in vitro ischemia. J. Cereb. Blood Flow Metab.25, 1346–1355 (2005).
Hoskison, M. M. & Shuttleworth, C. W. Microtubule disruption, not calpain-dependent loss of MAP2, contributes to enduring NMDA-induced dendritic dysfunction in acute hippocampal slices. Exp. Neurol.202, 302–312 (2006).
Sword, J., Croom, D., Wang, P. L., Thompson, R. J. & Kirov, S. A. Neuronal pannexin-1 channels are not molecular routes of water influx during spreading depolarization-induced dendritic beading. J. Cereb. Blood Flow Metab.37, 1626–1633 (2017).
Van, H. A. & Schade, J. P. Chloride movements in cerebral cortex after circulatory arrest and during spreading depression. J. Cell Comp. Physiol.54, 65–84 (1959).
Muller, M. Effects of chloride transport inhibition and chloride substitution on neuron function and on hypoxic spreading-depression-like depolarization in rat hippocampal slices. Neuroscience97, 33–45 (2000).
Kopito, R. R. et al. Regulation of intracellular pH by a neuronal homolog of the erythrocyte anion exchanger. Cell59, 927–937 (1989).
Payne, J. A. Functional characterization of the neuronal-specific K-Cl cotransporter: implications for [K+]o regulation. Am. J. Physiol.273, C1516–C1525 (1997).
Pierre, K., Magistretti, P. J. & Pellerin, L. MCT2 is a major neuronal monocarboxylate transporter in the adult mouse brain. J. Cereb. Blood Flow Metab.22, 586–595 (2002).
Heisey, S. R., Held, D. & Pappenheimer, J. R. Bulk flow and diffusion in the cerebrospinal fluid system of the goat. Am. J. Physiol.203, 775–781 (1962). This study was the first to demonstrate the ability of CSF secretion against an osmotic gradient.
Pollay, M. Formation of cerebrospinal fluid. Relation of studies of isolated choroid plexus to the standing gradient hypothesis. J. Neurosurg.42, 665–673 (1975).
Segal, M. B. & Pollay, M. The secretion of cerebrospinal fluid. Exp. Eye Res.25, 127–148 (1977).
Damkier, H. H., Brown, P. D. & Praetorius, J. Cerebrospinal fluid secretion by the choroid plexus. Physiol. Rev.93, 1847–1892 (2013).
Hochwald, G. M., Wald, A., DiMattio, J. & Malhan, C. The effects of serum osmolarity on cerebrospinal fluid volume flow. Life Sci.15, 1309–1316 (1974).
Javaheri, S. & Wagner, K. R. Bumetanide decreases canine cerebrospinal fluid production. In vivo evidence for NaCl cotransport in the central nervous system. J. Clin. Invest.92, 2257–2261 (1993).
Nilsson, C. et al. Circadian variation in human cerebrospinal fluid production measured by magnetic resonance imaging. Am. J. Physiol.262, R20–R24 (1992).
Pullen, R. G., DePasquale, M. & Cserr, H. F. Bulk flow of cerebrospinal fluid into brain in response to acute hyperosmolality. Am. J. Physiol.253, F538–F545 (1987).
Rubin, R. C., Henderson, E. S., Ommaya, A. K., Walker, M. D. & Rall, D. P. The production of cerebrospinal fluid in man and its modification by acetazolamide. J. Neurosurg.25, 430–436 (1966).
Damkier, H. H., Brown, P. D. & Praetorius, J. Epithelial pathways in choroid plexus electrolyte transport. Physiology25, 239–249 (2010).
de Rougemont, J., Ames, A. III, Nesbett, F. B. & Hofmann, H. F. Fluid formed by choroid plexus; a technique for its collection and a comparison of its electrolyte composition with serum and cisternal fluids. J. Neurophysiol.23, 485–495 (1960). This study demonstrated that newly formed CSF and bulk CSF are similar in electrolyte composition and osmolarity.
Welch, K. Secretion of cerebrospinal fluid by choroid plexus of the rabbit. Am. J. Physiol.205, 617–624 (1963).
Davson, H. & Segal, M. B. The effects of some inhibitors and accelerators of sodium transport on the turnover of 22Na in the cerebrospinal fluid and the brain. J. Physiol.209, 131–153 (1970).
Smith, Q. R., Johanson, C. E. & Woodbury, D. M. Uptake of 36Cl and 22Na by the brain-cerebrospinal fluid system: comparison of the permeability of the blood-brain and blood-cerebrospinal fluid barriers. J. Neurochem.37, 117–124 (1981).
Smith, Q. R. & Rapoport, S. I. Cerebrovascular permeability coefficients to sodium, potassium, and chloride. J. Neurochem.46, 1732–1742 (1986).
Davson, H. A comparative study of the aqueous humour and cerebrospinal fluid in the rabbit. J. Physiol.129, 111–133 (1955).
Ames, A. III, Higashi, K. & NESBETT, F. B. Effects of PCO2 acetazolamide and ouabain on volume and composition of choroid-plexus fluid. J. Physiol.181, 516–524 (1965).
Knuckey, N. W., Fowler, A. G., Johanson, C. E., Nashold, J. R. & Epstein, M. H. Cisterna magna microdialysis of 22Na to evaluate ion transport and cerebrospinal fluid dynamics. J. Neurosurg.74, 965–971 (1991).
Pollay, M. et al. Choroid plexus Na+/K+-activated adenosine triphosphatase and cerebrospinal fluid formation. Neurosurgery17, 768–772 (1985).
DePasquale, M., Patlak, C. S. & Cserr, H. F. Brain ion and volume regulation during acute hypernatremia in Brattleboro rats. Am. J. Physiol.256, F1059–F1066 (1989).
Pollay, M. & Curl, F. Secretion of cerebrospinal fluid by the ventricular ependyma of the rabbit. Am. J. Physiol.213, 1031–1038 (1967).
Bradbury, M. W. & Kleeman, C. R. The effect of chronic osmotic disturbance on the concentrations of cations in cerebrospinal fluid. J. Physiol.204, 181–193 (1969).
Wald, A., Hochwald, G. M. & Malhan, C. The effects of ventricular fluid osmolality on bulk flow of nascent fluid into the cerebral ventricles of cats. Exp. Brain Res.25, 157–167 (1976).
Hendry, E. B. The osmotic pressure and chemical composition of human body fluids. Clin. Chem.8, 246–265 (1962).
Welch, K., Sadler, K. & Gold, G. Volume flow across choroidal ependyma of the rabbit. Am. J. Physiol.210, 232–236 (1966).
Zeuthen, T. Water permeability of ventricular cell membrane in choroid plexus epithelium from Necturus maculosus. J. Physiol.444, 133–151 (1991). This study demonstrated the lack of unstirred layers on the luminal side of the choroid plexus.
Zeuthen, T. & Steffensen, A. B. in Role of the Choroid Plexus in Health and Disease (eds Praetorius, J., Blazer-Yost, B. & Damkier, H.) (Springer, 2020).
Curl, F. D. & Pollay, M. Transport of water and electrolytes between brain and ventricular fluid in the rabbit. Exp. Neurol.20, 558–574 (1968).
Hochwald, G. M., Wald, A. & Malhan, C. The sink action of cerebrospinal fluid volume flow. Effect on brain water content. Arch. Neurol.33, 339–344 (1976).
Sahar, A. & Tsipstein, E. Effects of mannitol and furosemide on the rate of formation of cerebrospinal fluid. Exp. Neurol.60, 584–591 (1978).
Oshio, K., Watanabe, H., Song, Y., Verkman, A. S. & Manley, G. T. Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel Aquaporin-1. FASEB J.19, 76–78 (2005). This study found thatAqp1-/-mice exhibited a 20% reduction in CSF secretion.
Praetorius, J. & Nielsen, S. Distribution of sodium transporters and aquaporin-1 in the human choroid plexus. Am. J. Physiol. Cell Physiol.291, C59–C67 (2006).
Chretien, S. & Catron, J. P. A single mutation inside the NPA motif of aquaporin-1 found in a Colton-null phenotype. Blood93, 4021–4023 (1999).
Preston, G. M., Smith, B. L., Zeidel, M. L., Moulds, J. J. & Agre, P. Mutations in aquaporin-1 in phenotypically normal humans without functional CHIP water channels. Science265, 1585–1587 (1994). This study demonstrated that humans with no AQP1 expression have no neurological deficits.
Ma, T. et al. Severely impaired urinary concentrating ability in transgenic mice lacking aquaporin-1 water channels. J. Biol. Chem.273, 4296–4299 (1998).
Quinton, P. M., Wright, E. M. & Tormey, J. M. Localization of sodium pumps in the choroid plexus epithelium. J. Cell Biol.58, 724–730 (1973).
Bradbury, M. W. & Kleeman, C. R. Stability of the potassium content of cerebrospinal fluid and brain. Am. J. Physiol.213, 519–528 (1967).
Smith, Q. R. & Johanson, C. E. Effect of ouabain and potassium on ion concentrations in the choroidal epithelium. Am. J. Physiol.238, F399–F406 (1980).
Lun, M. P. et al. Spatially heterogeneous choroid plexus transcriptomes encode positional identity and contribute to regional CSF production. J. Neurosci.35, 4903–4916 (2015).
Vates, T. S. Jr., Bonting, S. L. & Oppelt, W. W. Na-K activated adenosine triphosphatase formation of cerebrospinal fluid in the cat. Am. J. Physiol.206, 1165–1172 (1964).
Deng, Q. S. & Johanson, C. E. Cyclic AMP alteration of chloride transport into the choroid plexus-cerebrospinal fluid system. Neurosci. Lett.143, 146–150 (1992).
Lindsey, A. E. et al. Functional expression and subcellular localization of an anion exchanger cloned from choroid plexus. Proc. Natl Acad. Sci. USA87, 5278–5282 (1990).
Praetorius, J., Nejsum, L. N. & Nielsen, S. A SCL4A10 gene product maps selectively to the basolateral plasma membrane of choroid plexus epithelial cells. Am. J. Physiol. Cell Physiol.286, C601–C610 (2004).
Praetorius, J. & Damkier, H. H. Transport across the choroid plexus epithelium. Am. J. Physiol. Cell Physiol.312, C673–C686 (2017).
Deng, Q. S. & Johanson, C. E. Stilbenes inhibit exchange of chloride between blood, choroid plexus and cerebrospinal fluid. Brain Res.501, 183–187 (1989).
Smith, Q. R. & Johanson, C. E. Active transport of chloride by lateral ventricle choroid plexus of the rat. Am. J. Physiol249, F470–F477 (1985).
McCarthy, K. D. & Reed, D. J. The effect of acetazolamide and furosemide on cerebrospinal fluid production and choroid plexus carbonic anhydrase activity. J. Pharmacol. Exp. Ther.189, 194–201 (1974).
Melby, J. M., Miner, L. C. & Reed, D. J. Effect of acetazolamide and furosemide on the production and composition of cerebrospinal fluid from the cat choroid plexus. Can. J. Physiol. Pharmacol.60, 405–409 (1982).
Vogh, B. P. & Langham, M. R. Jr. The effect of furosemide and bumetanide on cerebrospinal fluid formation. Brain Res.221, 171–183 (1981).
Murphy, V. A. & Johanson, C. E. Acidosis, acetazolamide, and amiloride: effects on 22Na transfer across the blood-brain and blood-CSF barriers. J. Neurochem.52, 1058–1063 (1989).
Macri, F. J., Politoff, A., Rubin, R., Dixon, R. & Rall, D. Preferential vasoconstrictor properties of acetazolamide on the arteries of the choroid plexus. Int. J. Neuropharmacol.5, 109–115 (1966).
Swenson, E. R. New insights into carbonic anhydrase inhibition, vasodilation, and treatment of hypertensive-related diseases. Curr. Hypertens. Rep.16, 467 (2014).
Francois, C. & Deprez, C. Ion transport and oxidative metabolism. I. The inhibition of mitochondrial oxidative metabolism by the unsubstituted aromatic sulfonamides (carbonic anhydrase inhibitors). Arch. Int. Physiol. Biochim.79, 993–1007 (1971).
Smith, Q. R. & Johanson, C. E. Chloride efflux from isolated choroid plexus. Brain Res.562, 306–310 (1991).
Osswald, H. & Hawlina, A. Effects of acetazolamide and changes of acid-base balance on the content of cyclic nucleotides in the rat kidney. Pharmacology19, 44–50 (1979).
Jacobs, S. et al. Mice with targeted Slc4a10 gene disruption have small brain ventricles and show reduced neuronal excitability. Proc. Natl Acad. Sci. USA105, 311–316 (2008).
Kao, L. et al. Severe neurologic impairment in mice with targeted disruption of the electrogenic sodium bicarbonate cotransporter NBCe2 (Slc4a5 gene). J. Biol. Chem.286, 32563–32574 (2011).
Damkier, H. H. & Praetorius, J. Genetic ablation of Slc4a10 alters the expression pattern of transporters involved in solute movement in the mouse choroid plexus. Am. J. Physiol. Cell Physiol.302, C1452–C1459 (2012).
Bairamian, D., Johanson, C. E., Parmelee, J. T. & Epstein, M. H. Potassium cotransport with sodium and chloride in the choroid plexus. J. Neurochem.56, 1623–1629 (1991).
Keep, R. F., Xiang, J. & Betz, A. L. Potassium cotransport at the rat choroid plexus. Am. J. Physiol.267, C1616–C1622 (1994). This study demonstrated the outward transport direction of NKCC1 in the choroid plexus.
Kanaka, C. et al. The differential expression patterns of messenger RNAs encoding K-Cl cotransporters (KCC1,2) and Na-K-2Cl cotransporter (NKCC1) in the rat nervous system. Neuroscience104, 933–946 (2001).
Karadsheh, M. F., Byun, N., Mount, D. B. & Delpire, E. Localization of the KCC4 potassium-chloride cotransporter in the nervous system. Neuroscience123, 381–391 (2004).
Pearson, M. M., Lu, J., Mount, D. B. & Delpire, E. Localization of the K+-Cl– cotransporter, KCC3, in the central and peripheral nervous systems: expression in the choroid plexus, large neurons and white matter tracts. Neuroscience103, 481–491 (2001).
Ishikawa, A. et al. Altered electrolyte handling of the choroid plexus in rats with glycerol-induced acute renal failure. Biopharm. Drug Dispos.31, 455–463 (2010).
Miller, T. B., Wilkinson, H. A., Rosenfeld, S. A. & Furuta, T. Intracranial hypertension and cerebrospinal fluid production in dogs: effects of furosemide. Exp. Neurol.94, 66–80 (1986).
Vogh, B. P. & Doyle, A. S. The effect of carbonic anhydrase inhibitors and other drugs on sodium entry to cerebrospinal fluid. J. Pharmacol. Exp. Ther.217, 51–56 (1981).
Johnson, D. C., Singer, S., Hoop, B. & Kazemi, H. Chloride flux from blood to CSF: inhibition by furosemide and bumetanide. J. Appl. Physiol.63, 1591–1600 (1987).
Alvarez-Leefmans, F. J. CrossTalk proposal: apical NKCC1 of choroid plexus epithelial cells works in the net inward flux mode under basal conditions, maintaining intracellular Cl– and cell volume. J. Physiol.598, 4733–4736 (2020).
Alvarez-Leefmans, F. J. Rebuttal from Francisco J. Alvarez-Leefmans. J. Physiol.598, 4741–4742 (2020).
Gregoriades, J. M. C., Madaris, A., Alvarez, F. J. & Alvarez-Leefmans, F. J. Genetic and pharmacological inactivation of apical Na+-K+-2Cl– cotransporter 1 in choroid plexus epithelial cells reveals the physiological function of the cotransporter. Am. J. Physiol. Cell Physiol.316, C525–C544 (2019).
MacAulay, N. & Rose, C. R. CrossTalk opposing view: NKCC1 in the luminal membrane of choroid plexus is outwardly directed under basal conditions and contributes directly to cerebrospinal fluid secretion. J. Physiol.598, 4737–4739 (2020).
MacAulay, N. & Rose, C. R. Rebuttal from Nanna MacAulay and Christine R. Rose. J. Physiol.598, 4743 (2020).
Milhorat, T. H., Hammock, M. K., Fenstermacher, J. D. & Levin, V. A. Cerebrospinal fluid production by the choroid plexus and brain. Science173, 330–332 (1971).
Mokgokong, R., Wang, S., Taylor, C. J., Barrand, M. A. & Hladky, S. B. Ion transporters in brain endothelial cells that contribute to formation of brain interstitial fluid. Pflug. Arch.466, 887–901 (2014).
Oreskovic, D. & Klarica, M. The formation of cerebrospinal fluid: nearly a hundred years of interpretations and misinterpretations. Brain Res. Rev.64, 241–262 (2010).
Butt, A. M., Jones, H. C. & Abbott, N. J. Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study. J. Physiol.429, 47–62 (1990).
Crone, C. & Olesen, S. P. Electrical resistance of brain microvascular endothelium. Brain Res.241, 49–55 (1982).
Betz, A. L. & Goldstein, G. W. Specialized properties and solute transport in brain capillaries. Annu. Rev. Physiol.48, 241–250 (1986).
Smith, Q. R. & Rapoport, S. I. Carrier-mediated transport of chloride across the blood-brain barrier. J. Neurochem.42, 754–763 (1984).
Zador, Z., Stiver, S., Wang, V. & Manley, G. T. In: Aquaporins. Handbook of Experimental Pharmacology. 190 (ed Beitz, E.) 159-170 (Springer, (2009).
O’Donnell, M. E., Tran, L., Lam, T. I., Liu, X. B. & Anderson, S. E. Bumetanide inhibition of the blood-brain barrier Na-K-Cl cotransporter reduces edema formation in the rat middle cerebral artery occlusion model of stroke. J. Cereb. Blood Flow Metab.24, 1046–1056 (2004).
Sun, D., Lytle, C. & O’Donnell, M. E. Astroglial cell-induced expression of Na-K-Cl cotransporter in brain microvascular endothelial cells. Am. J. Physiol.269, C1506–C1512 (1995).
Shawahna, R. et al. Transcriptomic and quantitative proteomic analysis of transporters and drug metabolizing enzymes in freshly isolated human brain microvessels. Mol. Pharm.8, 1332–1341 (2011).
Lane, J. R., Wigham, C. G. & Hodson, S. A. Determination of Na+/Cl-, Na+/HCO3- and Na+/K+/2Cl- co-transporter activity in corneal endothelial cell plasma membrane vesicles. Biochim. Biophys. Acta1328, 237–242 (1997).
Oernbo, E. K. et al. Cerebral influx of Na+ and Cl- as the osmotherapy-mediated rebound response in rats. Fluids Barriers CNS15, 27 (2018).
Simpson, I. A., Carruthers, A. & Vannucci, S. J. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J. Cereb. Blood Flow Metab.27, 1766–1791 (2007).
Helms, H. C. C., Nielsen, C. U., Waagepetersen, H. S. & Brodin, B. Glutamate transporters in the blood-brain barrier. Adv. Neurobiol.16, 297–314 (2017).
Smith, Q. R., Momma, S., Aoyagi, M. & Rapoport, S. I. Kinetics of neutral amino acid transport across the blood-brain barrier. J. Neurochem.49, 1651–1658 (1987).
Smith, Q. R. Transport of glutamate and other amino acids at the blood-brain barrier. J. Nutr.130, 1016S–1022S (2000).
Ohtsuki, S. New aspects of the blood-brain barrier transporters; its physiological roles in the central nervous system. Biol. Pharm. Bull.27, 1489–1496 (2004).
Cornford, E. M. & Hyman, S. Localization of brain endothelial luminal and abluminal transporters with immunogold electron microscopy. NeuroRx.2, 27–43 (2005).
Zeuthen, T., Zeuthen, E. & MacAulay, N. Water transport by GLUT2 expressed in Xenopus laevis oocytes. J. Physiol.579, 345–361 (2007).
Betz, A. L., Firth, J. A. & Goldstein, G. W. Polarity of the blood-brain barrier: distribution of enzymes between the luminal and antiluminal membranes of brain capillary endothelial cells. Brain Res.192, 17–28 (1980).
Lykke, K. et al. Evaluating the involvement of cerebral microvascular endothelial Na+/K+-ATPase and Na+-K+-2Cl- co-transporter in electrolyte fluxes in an in vitro blood-brain barrier model of dehydration. J. Cereb. Blood Flow Metab.39, 497–512 (2019).
Vorbrodt, A. W. & Trowbridge, R. S. Ultracytochemical characteristics of cultured goat brain microvascular endothelial cells [corrected]. J. Histochem. Cytochem.39, 1555–1563 (1991).
Goldstein, G. W. Relation of potassium transport to oxidative metabolism in isolated brain capillaries. J. Physiol.286, 185–195 (1979).
Lin, J. D. Potassium transport in isolated cerebral microvessels from the rat. Jpn. J. Physiol.35, 817–830 (1985).
Katzman, R. Maintenance of a constant brain extracellular potassium. Fed. Proc.35, 1244–1247 (1976).
Al Feteisi, H. et al. Identification and quantification of blood-brain barrier transporters in isolated rat brain microvessels. J. Neurochem.146, 670–685 (2018).
Kubo, Y., Ohtsuki, S., Uchida, Y. & Terasaki, T. Quantitative determination of luminal and abluminal membrane distributions of transporters in porcine brain capillaries by plasma membrane fractionation and quantitative targeted proteomics. J. Pharm. Sci.104, 3060–3068 (2015).
Cserr, H. F., DePasquale, M. & Patlak, C. S. Regulation of brain water and electrolytes during acute hyperosmolality in rats. Am. J. Physiol.253, F522–F529 (1987). This study demonstrated the volume-regulatory ability of the brain barriers.
Wald, A., Hochwald, G. M. & Malhan, C. The relationship between sodium influx and volume flow into the cerebral ventricles of cats. J. Neurochem.25, 151–154 (1975).
Stoica, A. et al. The α2β2 isoform combination dominates the astrocytic Na+/K+-ATPase activity and is rendered nonfunctional by the α2.G301R familial hemiplegic migraine type 2-associated mutation. Glia65, 1777–1793 (2017).
Heo, J., Meng, F. & Hua, S. Z. Contribution of aquaporins to cellular water transport observed by a microfluidic cell volume sensor. Anal. Chem.80, 6974–6980 (2008).
Acknowledgements
I would like to thank all the many great researchers, especially my mentor T. Zeuthen, with whom I have had the pleasure to discuss these topics with at length for the past few decades. I have learned so much from many of you! In addition, I would like to express my gratitude to my research team, past and present, for joining me on our quest to reach an understanding on how water crosses cell membranes in the brain. The work included in this Review was generously funded by the Lundbeck Foundation, the Independent Research Fund Denmark, the Novo Nordic Foundation, Thorberg’s Foundation, the Carlsberg Foundation, Friis’ Foundation, Danielsen’s Foundation, and the Hartmann Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Peer review information
Nature Reviews Neuroscience thanks J. Badaut, R. Enger, who co-reviewed with L. Bordoni, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Dendritic beading
-
The bead-shaped swelling of dendrites during spreading depolarization.
- Blood–brain barrier
-
(BBB). The tight junction-coupled endothelial cell layer that separates the circulating blood from the brain tissue.
- Blood–CSF barrier
-
(BCSFB). The epithelial cell layer that separates the circulating blood from the cerebrospinal fluid (CSF)-filled ventricles.
- Osmotic water permeability
-
The ease with which water crosses a cell membrane with a given transmembrane osmotic challenge.
- Passive water transport
-
Water transport following the osmotic gradient.
- Active water transport
-
Water transport taking place independently of — or even against — a transmembrane osmotic gradient.
- Choroid plexus
-
The epithelial structures placed in the brain ventricles that secrete the majority of the CSF.
- Activity-evoked extracellular space shrinkage
-
The cellular (glial) swelling taking place during neuronal activity, monitored as the size of the extracellular space.
- Cotransport of water
-
The ability of cotransporters to translocate a fixed amount of water molecules in the direction of their transported solutes.
- Elevated intracranial pressure
-
With brain fluid accumulation in pathology, the intracranial pressure increases due to the confinements of the brain within the skull; this condition can be life threatening.
Rights and permissions
About this article
Cite this article
MacAulay, N. Molecular mechanisms of brain water transport. Nat Rev Neurosci 22, 326–344 (2021). https://doi.org/10.1038/s41583-021-00454-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41583-021-00454-8
This article is cited by
-
CSF hyperdynamics in rats mimicking the obesity and androgen excess characteristic of patients with idiopathic intracranial hypertension
Fluids and Barriers of the CNS (2024)
-
Non-coding RNAs and Aquaporin 4: Their Role in the Pathogenesis of Neurological Disorders
Neurochemical Research (2024)
-
Disentangling the impact of cerebrospinal fluid formation and neuronal activity on solute clearance from the brain
Fluids and Barriers of the CNS (2023)
-
Estimates of the permeability of extra-cellular pathways through the astrocyte endfoot sheath
Fluids and Barriers of the CNS (2023)
-
Outcomes of the 2019 hydrocephalus association workshop, "Driving common pathways: extending insights from posthemorrhagic hydrocephalus"
Fluids and Barriers of the CNS (2023)