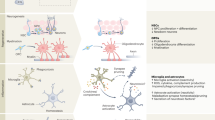
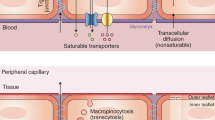
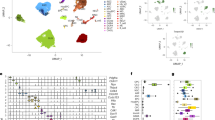
Abstract
A rapidly ageing population and a limited therapeutic toolbox urgently necessitate new approaches to treat neurodegenerative diseases. Brain ageing, the key risk factor for neurodegeneration, involves complex cellular and molecular processes that eventually result in cognitive decline. Although cell-intrinsic defects in neurons and glia may partially explain this decline, cell-extrinsic changes in the systemic environment, mediated by blood, have recently been shown to contribute to brain dysfunction with age. Here, we review the current understanding of how systemic factors mediate brain ageing, how these factors are regulated and how we can translate these findings into therapies for neurodegenerative diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
13 March 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41583-020-0293-3
References
Catani, M. & Sandrone, S. Brain Renaissance: From Vesalius to Modern Neuroscience (Oxford Univ. Press, 2015).
Mazzarello, P. A unifying concept: the history of cell theory. Nat. Cell Biol. 1, E13–E15 (1999).
Hill, A. V. Bayliss and Starling and the happy fellowship of physiologists. J. Physiol. 204, 1–13 (1969).
Junnila, R. K., List, E. O., Berryman, D. E., Murrey, J. W. & Kopchick, J. J. The GH/IGF-1 axis in ageing and longevity. Nat. Rev. Endocrinol. 9, 366–376 (2013).
Zárate, S., Stevnsner, T. & Gredilla, R. Role of estrogen and other sex hormones in brain aging. neuroprotection and DNA repair. Front. Aging Neurosci. 9, 430 (2017).
Menni, C. et al. Circulating proteomic signatures of chronological age. J. Gerontol. A Biol. Sci. Med. Sci. 70, 809–816 (2015).
Hu, W. T. et al. Plasma multianalyte profiling in mild cognitive impairment and Alzheimer disease. Neurology 79, 897–905 (2012).
Ashton, N. J. et al. A plasma protein classifier for predicting amyloid burden for preclinical Alzheimer’s disease. Sci. Adv. 5, eaau7220 (2019).
Tanaka, T. et al. Plasma proteomic signature of age in healthy humans. Aging Cell 17, e12799 (2018).
Sun, B. B. et al. Genomic atlas of the human plasma proteome. Nature 558, 73–79 (2018). Large-scale resource that identified genetic variations associated with plasma protein levels.
Sattlecker, M. et al. Alzheimer’s disease biomarker discovery using SOMAscan multiplexed protein technology. Alzheimers Dement. 10, 724–734 (2014).
Eggel, A. & Wyss-Coray, T. A revival of parabiosis in biomedical research. Swiss Med. Wkly. 144, w13914 (2014).
Villeda, S. A. et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477, 90–94 (2011).
Villeda, S. A. et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat. Med. 20, 659–663 (2014).
Katsimpardi, L. et al. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 344, 630–634 (2014).
Das, M. M. et al. Young bone marrow transplantation preserves learning and memory in old mice. Commun. Biol. 2, 73 (2019).
Smith, L. K. et al. β2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nat. Med. 21, 932–937 (2015).
Castellano, J. M. et al. Human umbilical cord plasma proteins revitalize hippocampal function in aged mice. Nature 544, 488–492 (2017).
Khrimian, L. et al. Gpr158 mediates osteocalcin’s regulation of cognition. J. Exp. Med. 214, 2859–2873 (2017).
Gan, K. J. & Südhof, T. C. Specific factors in blood from young but not old mice directly promote synapse formation and NMDA-receptor recruitment. Proc. Natl Acad. Sci. USA 116, 12524–12533 (2019).
Sasayama, D. et al. Genome-wide quantitative trait loci mapping of the human cerebrospinal fluid proteome. Hum. Mol. Genet. 26, 44–51 (2017).
Yang, A. C. et al. Multiple click-selective tRNA synthetases expand mammalian cell-specific proteomics. J. Am. Chem. Soc. 140, 7046–7051 (2018).
Ngo, J. T., Schuman, E. M. & Tirrell, D. A. Mutant methionyl-tRNA synthetase from bacteria enables site-selective N-terminal labeling of proteins expressed in mammalian cells. Proc. Natl Acad. Sci. USA 110, 4992–4997 (2013).
Mahdavi, A. et al. Engineered aminoacyl-tRNA synthetase for cell-selective analysis of mammalian protein synthesis. J. Am. Chem. Soc. 138, 4278–4281 (2016).
Elliott, T. S. et al. Proteome labeling and protein identification in specific tissues and at specific developmental stages in an animal. Nat. Biotechnol. 32, 465–472 (2014).
Voss, M. W. et al. Exercise and hippocampal memory systems. Trends Cognit. Sci. 23, 318–333 (2019).
Pedersen, B. K. Physical activity and muscle–brain crosstalk. Nat. Rev. Endocrinol. 15, 383–392 (2019).
Morland, C. et al. Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nat. Commun. 8, 15557 (2017).
Sherwood, L. M., Parris, E. E. & Cahill, G. F. Jr. Starvation in Man. N. Engl. J. Med. 282, 668–675 (1970).
Sleiman, S. F. et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. eLife 5, e15092 (2016).
Alves, S., Fol, R. & Cartier, N. Gene therapy strategies for Alzheimer’s disease: an overview. Hum. Gene Ther. 27, 100–107 (2016).
Didonna, A. & Opal, P. The promise and perils of HDAC inhibitors in neurodegeneration. Ann. Clin. Transl. Neurol. 2, 79–101 (2015).
Moon, H. Y. et al. Running-induced systemic cathepsin B secretion is associated with memory function. Cell Metab. 24, 332–340 (2016).
De Miguel, Z. et. al. Exercise conditioned plasma dampens inflammation via clusterin and boosts memory. bioRxiv https://doi.org/10.1101/775288 (2019).
Wrann, C. D. et al. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 18, 649–659 (2013).
Lourenco, M. V. et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 25, 165–175 (2019).
Winchester, J. et al. Walking stabilizes cognitive functioning in Alzheimer’s disease (AD) across one year. Arch. Gerontol. Geriatr. 56, 96–103 (2013).
Whitham, M. et al. Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metab. 27, 237–251.e4 (2018).
Stefano, G. B. et al. Gut, microbiome, and brain regulatory axis: relevance to neurodegenerative and psychiatric disorders. Cell. Mol. Neurobiol. 38, 1197–1206 (2018).
Erny, D. et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18, 965–977 (2015). First study showing the drastic effects of gut microbiota on microglial maturation.
Thion, M. S. et al. Microbiome influences prenatal and adult microglia in a sex-specific manner. Cell 172, 500–516.e16 (2018).
Sampson, T. R. et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167, 1469–1480.e12 (2016).
Harach, T. et al. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci. Rep. 7, 41802 (2017).
Minter, M. R. et al. Antibiotic-induced perturbations in microbial diversity during post-natal development alters amyloid pathology in an aged APPSWE/PS1DeltaE9 murine model of Alzheimer’s disease. Sci. Rep. 7, 10411 (2017).
Heneka, M. T., McManus, R. M. & Latz, E. Inflammasome signalling in brain function and neurodegenerative disease. Nat. Rev. Neurosci. 19, 610–621 (2018).
Derecki, N. C. et al. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J. Exp. Med. 207, 1067–1080 (2010).
Xu, Y. et al. Unexpected role of interferon-γ in regulating neuronal connectivity and social behaviour. Nature 535, 425–429 (2016).
Dulken, B. W. et al. Single-cell analysis reveals T cell infiltration in old neurogenic niches. Nature 571, 205–210 (2019).
Ito, M. et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature 565, 246–250 (2019).
Derecki, N. C., Quinnies, K. M. & Kipnis, J. Alternatively activated myeloid (M2) cells enhance cognitive function in immune compromised mice. Brain Behav. Immun. 25, 379–385 (2011).
Ruckh, J. M. et al. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell 10, 96–103 (2012).
Zenaro, E. et al. Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat. Med. 21, 880–886 (2015).
Hernandez, J. C. et al. Neutrophil adhesion in brain capillaries reduces cortical blood flow and impairs memory function in Alzheimer’s disease mouse models. Nat. Neurosci. 22, 413–420 (2019).
Ruitenberg, A. et al. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam study. Ann. Neurol. 57, 789–794 (2005).
Montagne, A. et al. Blood–brain barrier breakdown in the aging human hippocampus. Neuron 85, 296–302 (2015).
Nation, D. A. et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 25, 270–276 (2019).
Nortley, R. et al. Amyloid β oligomers constrict human capillaries in Alzheimer’s disease via signaling to pericytes. Science 365, eaav9518 (2019).
Bell, R. D. et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68, 409–427 (2010).
Montagne, A. et al. Pericyte degeneration causes white matter dysfunction in the mouse central nervous system. Nat. Med. 24, 326–337 (2018).
Ryu, J. K. et al. Blood coagulation protein fibrinogen promotes autoimmunity and demyelination via chemokine release and antigen presentation. Nat. Commun. 6, 8164 (2015).
Ryu, J. K. et al. Fibrin-targeting immunotherapy protects against neuroinflammation and neurodegeneration. Nat. Immunol. 19, 1212–1223 (2018).
Merlini, M. et al. Fibrinogen induces microglia-mediated spine elimination and cognitive impairment in an Alzheimer’s disease model. Neuron 101, 1099–1108.e6 (2019).
Duan, L. et al. PDGFRβ cells rapidly relay inflammatory signal from the circulatory system to neurons via chemokine CCL2. Neuron 100, 183–200.e8 (2018).
Baruch, K. et al. Aging. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science 346, 89–93 (2014).
Zhu, L. et al. Klotho controls the brain–immune system interface in the choroid plexus. Proc. Natl Acad. Sci. USA 115, E11388–E11396 (2018).
Silva-Vargas, V., Maldonado-Soto, A. R., Mizrak, D., Codega, P. & Doetsch, F. Age-dependent niche signals from the choroid plexus regulate adult neural stem cells. Cell Stem Cell. 19, 643–652 (2016).
Baruch, K. et al. CNS-specific immunity at the choroid plexus shifts toward destructive Th2 inflammation in brain aging. Proc. Natl Acad. Sci. USA 110, 2264 (2013).
Baruch, K. et al. Breaking immune tolerance by targeting Foxp3+ regulatory T cells mitigates Alzheimer’s disease pathology. Nat. Commun. 6, 7967 (2015).
Baruch, K. et al. PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer’s disease. Nat. Med. 22, 135–137 (2016).
Lee, M.-H. et al. Somatic APP gene recombination in Alzheimer’s disease and normal neurons. Nature 563, 639–645 (2018).
Cai, R. et al. Panoptic imaging of transparent mice reveals whole-body neuronal projections and skull-meninges connections. Nat. Neurosci. 22, 317–327 (2019).
Herisson, F. et al. Direct vascular channels connect skull bone marrow and the brain surface enabling myeloid cell migration. Nat. Neurosci. 21, 1209–1217 (2018).
Yao, H. et al. Leukaemia hijacks a neural mechanism to invade the central nervous system. Nature 560, 55–60 (2018).
Park, M. H. et al. Vascular and neurogenic rejuvenation in aging mice by modulation of ASM. Neuron 100, 167–182.e9 (2018).
Liu, X. et al. Cell-type-specific interleukin 1 receptor 1 signaling in the brain regulates distinct neuroimmune activities. Immunity 50, 317–333.e6 (2019).
Blank, T. et al. Brain endothelial- and epithelial-specific interferon receptor chain 1 drives virus-induced sickness behavior and cognitive impairment. Immunity 44, 901–912 (2016).
Vanlandewijck, M. et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature 554, 475–480 (2018).
Sabbagh, M. F. et al. Transcriptional and epigenomic landscapes of CNS and non-CNS vascular endothelial cells. eLife 7, e36187 (2018).
Yousef, H. et al. Aged blood impairs hippocampal neural precursor activity and activates microglia via brain endothelial cell VCAM1. Nat. Med. 25, 988–1000 (2019).
Chen, M. B. et al. Brain endothelial cells are exquisite sensors of age-related circulatory cues. bioRxiv. https://doi.org/10.1101/617258 (2019).
Hickman, S. E. et al. The microglial sensome revealed by direct RNA sequencing. Nat. Neurosci. 16, 1896–1905 (2013).
Deczkowska, A. et al. Disease-associated microglia: a universal immune sensor of neurodegeneration. Cell 173, 1073–1081 (2018).
Pluvinage, J. V. et al. CD22 blockade restores homeostatic microglial phagocytosis in ageing brains. Nature 568, 187–192 (2019).
Marschallinger, J. et al. Lipid droplet accumulating microglia represent a dysfunctional and pro-inflammatory state in the aging brain. bioRxiv https://doi.org/10.1101/722827 (2019).
Bialas, A. R. et al. Microglia-dependent synapse loss in type I interferon-mediated lupus. Nature 546, 539–543 (2017).
Deczkowska, A. et al. Mef2C restrains microglial inflammatory response and is lost in brain ageing in an IFN-I-dependent manner. Nat. Commun. 8, 717 (2017).
Clarke, L. E. et al. Normal aging induces A1-like astrocyte reactivity. Proc. Natl Acad. Sci. USA 115, E1896–E1905 (2018).
Boisvert, M. M., Erikson, G. A., Shokhirev, M. N. & Allen, N. J. The aging astrocyte transcriptome from multiple regions of the mouse brain. Cell Rep. 22, 269–285 (2018).
Liddelow, S. A. et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017).
Banks, W. A., Kastin, A. J. & Broadwell, R. D. Passage of cytokines across the blood–brain barrier. Neuroimmunomodulation 2, 241–248 (1995).
Falcao, A. M. et al. Disease-specific oligodendrocyte lineage cells arise in multiple sclerosis. Nat. Med. 24, 1837–1844 (2018).
Spitzer, S. O. et al. Oligodendrocyte progenitor cells become regionally diverse and heterogeneous with age. Neuron 101, 459–471.e5 (2019).
Gutierrez, E. G., Banks, W. A. & Kastin, A. J. Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. J. Neuroimmunol. 47, 169–176 (1993).
Stellwagen, D. & Malenka, R. C. Synaptic scaling mediated by glial TNF-α. Nature 440, 1054–1059 (2006).
Prieto, G. A. et al. Synapse-specific IL-1 receptor subunit reconfiguration augments vulnerability to IL-1β in the aged hippocampus. Proc. Natl Acad. Sci. USA 112, E5078–E5087 (2015).
Luo, J. et al. Colony-stimulating factor 1 receptor (CSF1R) signaling in injured neurons facilitates protection and survival. J. Exp. Med. 210, 157–172 (2013).
Boyd, T. D. et al. GM-CSF upregulated in rheumatoid arthritis reverses cognitive impairment and amyloidosis in Alzheimer mice. J. Alzheimers Dis. 21, 507–518 (2010).
Rasmussen, M. K., Mestre, H. & Nedergaard, M. The glymphatic pathway in neurological disorders. Lancet Neurol. 17, 1016–1024 (2018).
Da Mesquita, S., Fu, Z. & Kipnis, J. The meningeal lymphatic system: a new player in neurophysiology. Neuron 100, 375–388 (2018).
Iliff, J. J. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 4, 147ra111 (2012).
Iliff, J. J. et al. Cerebral arterial pulsation drives par- avascular CSF-interstitial fluid exchange in the murine brain. J. Neurosci. 33, 18190–18199 (2013).
Kress, B. T. et al. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 76, 845–861 (2014).
Holth, J. K. et al. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science 363, 880–884 (2019).
Hsu, M. et al. Neuroinflammation-induced lymphangiogenesis near the cribriform plate contributes to drainage of CNS-derived antigens and immune cells. Nat. Commun. 10, 229 (2019).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015).
Da Mesquita, S. et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 560, 185–191 (2018).
Ahn, J. H. et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 572, 62–66 (2019).
Lehallier, B. et al. Undulating changes in human plasma proteome across lifespan are linked to disease. Nat. Med. 25, 1843–1850 (2019).
Emilsson, V. et al. Co-regulatory networks of human serum proteins link genetics to disease. Science 361, 769–773 (2018).
Swaminathan, J. et al. Highly parallel single-molecule identification of proteins in zeptomole-scale mixtures. Nat. Biotechnol. 36, 1076–1082 (2018).
Ben-Shaanan, T. L. et al. Activation of the reward system boosts innate and adaptive immunity. Nat. Med. 22, 940–944 (2016).
Middeldorp, J. et al. Preclinical assessment of young blood plasma for Alzheimer disease. JAMA Neurol. 73, 1325–1333 (2016).
Sha, S. J. et al. Safety, tolerability, and feasibility of young plasma infusion in the plasma for Alzheimer symptom Amelioration study: a randomized clinical trial. JAMA Neurol. 76, 35–40 (2019).
Acknowledgements
This work was supported by a National Institutes of Health National Institute on Ageing (NIA) grant F30 AG055255 (J.P.), DP1 AG060638 (T.W.-C.), and the NOMIS Foundation (T.W.-C.).
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
T.W.-C. is a co-founder, shareholder and paid consultant of Alkahest, Inc., a company that develops treatments for CNS diseases based on the concept that circulatory factors regulate brain function. J.V.P. declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Parabionts
-
Animals that share partial blood circulation with another animal via surgical anastomosis.
- Type 2 T helper cell phenotype
-
CD4+ T cells that produce high levels of IL-4, IL-5 and IL-13, but little or no IFNγ, IL-2 or TNF. Canonically, these cells protect against multicellular parasites.
- Caveolae-mediated transcytosis
-
A form of clathrin-independent endocytosis that facilitates non-specific transcytosis of extracellular molecules across the BBB, which are then trafficked across the cell in pH-neutral compartments called caveosomes.
- Aptamer-based proteomics
-
A technique that uses short single-stranded oligonucleotides called aptamers to bind and identify proteins in a complex mixture with high affinity and specificity.
Rights and permissions
About this article
Cite this article
Pluvinage, J.V., Wyss-Coray, T. Systemic factors as mediators of brain homeostasis, ageing and neurodegeneration. Nat Rev Neurosci 21, 93–102 (2020). https://doi.org/10.1038/s41583-019-0255-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41583-019-0255-9
This article is cited by
-
Associations of plasma proteomics and age-related outcomes with brain age in a diverse cohort
GeroScience (2024)
-
Alzheimer’s disease: targeting the peripheral circulation
Molecular Neurodegeneration (2023)
-
Current views on meningeal lymphatics and immunity in aging and Alzheimer’s disease
Molecular Neurodegeneration (2023)
-
Nicotine rebalances NAD+ homeostasis and improves aging-related symptoms in male mice by enhancing NAMPT activity
Nature Communications (2023)
-
Peripheral ApoE4 Leads to Cerebrovascular Dysfunction and Aβ Deposition in Alzheimer's Disease
Neuroscience Bulletin (2023)