Abstract
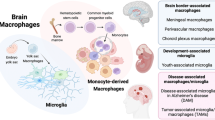
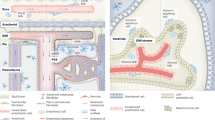
The segregation and limited regenerative capacity of the CNS necessitate a specialized and tightly regulated resident immune system that continuously guards the CNS against invading pathogens and injury. Immunity in the CNS has generally been attributed to neuron-associated microglia in the parenchyma, whose origin and functions have recently been elucidated. However, there are several other specialized macrophage populations at the CNS borders, including dural, leptomeningeal, perivascular and choroid plexus macrophages (collectively known as CNS-associated macrophages (CAMs)), whose origins and roles in health and disease have remained largely uncharted. CAMs are thought to be involved in regulating the fine balance between the proper segregation of the CNS, on the one hand, and the essential exchange between the CNS parenchyma and the periphery, on the other. Recent studies that have been empowered by major technological advances have shed new light on these cells and suggest central roles for CAMs in CNS physiology and in the pathogenesis of diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Fitch, M. T. & Silver, J. CNS injury, glial scars, and inflammation: inhibitory extracellular matrices and regeneration failure. Exp. Neurol. 209, 294–301 (2008).
Ransohoff, R. M. & Brown, M. A. Innate immunity in the central nervous system. J. Clin. Invest. 122, 1164–1171 (2012).
Kierdorf, K. & Prinz, M. Microglia in steady state. J. Clin. Invest. 127, 3201–3209 (2017).
Li, Q. & Barres, B. A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 18, 225–242 (2018).
Galea, I., Bechmann, I. & Perry, V. H. What is immune privilege (not)? Trends Immunol. 28, 12–18 (2007).
Niederkorn, J. Y. See no evil, hear no evil, do no evil: the lessons of immune privilege. Nat. Immunol. 7, 354–359 (2006).
Engelhardt, B. Regulation of immune cell entry into the central nervous system. Results Probl. Cell Differ. 43, 259–280 (2006).
Absinta, M. et al. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. eLife 6, e29738 (2017).
Aspelund, A. et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 212, 991–999 (2015).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015).
Louveau, A. et al. Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J. Clin. Invest. 127, 3210–3219 (2017).
Raper, D., Louveau, A. & Kipnis, J. How do meningeal lymphatic vessels drain the CNS? Trends Neurosci. 39, 581–586 (2016).
Furukawa, M., Shimoda, H., Kajiwara, T., Kato, S. & Yanagisawa, S. Topographic study on nerve-associated lymphatic vessels in the murine craniofacial region by immunohistochemistry and electron microscopy. Biomed. Res. 29, 289–296 (2008).
Gausas, R. E., Daly, T. & Fogt, F. D2-40 expression demonstrates lymphatic vessel characteristics in the dural portion of the optic nerve sheath. Ophthalmic Plast. Reconstr. Surg. 23, 32–36 (2007).
Mascagni, P. Vasorum Lymphaticorum Corporis Humani Historia et Ichnographia (P. Carli, 1787).
Andres, K. H., von Düring, M., Muszynski, K. & Schmidt, R. F. Nerve fibres and their terminals of the dura mater encephali of the rat. Anat. Embryol. 175, 289–301 (1987).
Daneman, R. & Prat, A. The blood–brain barrier. Cold Spring Harb. Perspect. Biol. 7, a020412 (2015).
Erickson, M. A. & Banks, W. A. Neuroimmune axes of the blood–brain barriers and blood–brain interfaces: bases for physiological regulation, disease states, and pharmacological interventions. Pharmacol. Rev. 70, 278–314 (2018).
Mrdjen, D. et al. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity 48, 380–395 (2018). This important study investigates CAM heterogeneity at the single-cell level.
Prinz, M., Erny, D. & Hagemeyer, N. Ontogeny and homeostasis of CNS myeloid cells. Nat. Immunol. 18, 385–392 (2017).
Goldmann, T. et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat. Immunol. 17, 797–805 (2016). Goldmann et al. provide the first description of the origin and kinetics of CNS-associated macrophages in the murine CNS.
Jordão, M. J. C. et al. Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science 363, eaat7554 (2019). This research identifies disease-associated CAM subsets during CNS autoimmunity in mice.
Van Hove, H. et al. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat. Neurosci. 22, 1021–1035 (2019).
McMenamin, P. G., Wealthall, R. J., Deverall, M., Cooper, S. J. & Griffin, B. Macrophages and dendritic cells in the rat meninges and choroid plexus: three-dimensional localisation by environmental scanning electron microscopy and confocal microscopy. Cell Tissue Res. 313, 259–269 (2003).
Schulz, C. et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336, 86–90 (2012).
Kierdorf, K. et al. Microglia emerge from erythromyeloid precursors via Pu. 1- and Irf8-dependent pathways. Nat. Neurosci. 16, 273–280 (2013).
Epelman, S. et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 40, 91–104 (2014).
Gomez Perdiguero, E. et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 518, 547–551 (2015).
Hagemeyer, N. et al. Transcriptome-based profiling of yolk sac-derived macrophages reveals a role for Irf8 in macrophage maturation. EMBO J. 35, 1730–1744 (2016).
Molawi, K. et al. Progressive replacement of embryo-derived cardiac macrophages with age. J. Exp. Med. 211, 2151–2158 (2014).
Mossadegh-Keller, N. et al. Developmental origin and maintenance of distinct testicular macrophage populations. J. Exp. Med. 214, 2829–2841 (2017).
Füger, P. et al. Microglia turnover with aging and in an Alzheimer’s model via long-term in vivo single-cell imaging. Nat. Neurosci. 20, 1371–1376 (2017).
Tay, T. L. et al. A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat. Neurosci. 20, 793–803 (2017). Füger et al. and Tay et al. examine microglia proliferative kinetics in vivo at the single-cell level.
Bechmann, I. et al. Immune surveillance of mouse brain perivascular spaces by blood-borne macrophages. Eur. J. Neurosci. 14, 1651–1658 (2001).
Hickey, W. F., Vass, K. & Lassmann, H. Bone marrow-derived elements in the central nervous system: an immunohistochemical and ultrastructural survey of rat chimeras. J. Neuropathol. Exp. Neurol. 51, 246–256 (1992).
Hickey, W. F. & Kimura, H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science 239, 290–292 (1988).
Mildner, A. et al. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat. Neurosci. 10, 1544–1553 (2007).
Schilling, M., Strecker, J., Ringelstein, E. B., Kiefer, R. & Schäbitz, W. Turn-over of meningeal and perivascular macrophages in the brain of MCP-1-, CCR-2- or double knockout mice. Exp. Neurol. 219, 583–585 (2009).
Hamann, I. et al. Analyses of phenotypic and functional characteristics of CX3CR1-expressing natural killer cells. Immunology 133, 62–73 (2011).
Gerlach, C. et al. The chemokine receptor CX3CR1 defines three antigen-experienced CD8 T cell subsets with distinct roles in immune surveillance and homeostasis. Immunity 45, 1270–1284 (2016).
Yona, S. et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38, 79–91 (2013).
Goldmann, T. et al. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat. Neurosci. 16, 1618–1626 (2013). Yona et al. and Goldmann et al. describe the CX3CR1 ERT2 Cre mouse line, which targets both microglia and CAMs.
Ginhoux, F. et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845 (2010).
Stremmel, C. et al. Yolk sac macrophage progenitors traffic to the embryo during defined stages of development. Nat. Commun. 9, 75 (2018).
Hashimoto, D. et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38, 792–804 (2013).
Matyszak, M. K., Lawson, L. J., Perry, V. H. & Gordon, S. Stromal macrophages of the choroid plexus situated at an interface between the brain and peripheral immune system constitutively express major histocompatibility class II antigens. J. Neuroimmunol. 40, 173–181 (1992).
Liddelow, S. A. Development of the choroid plexus and blood-CSF barrier. Front. Neurosci. 9, 1–13 (2015).
Ghersi-Egea, J.-F. et al. Molecular anatomy and functions of the choroidal blood-cerebrospinal fluid barrier in health and disease. Acta Neuropathol. 135, 337–361 (2018).
Bechmann, I. et al. Turnover of rat brain perivascular cells. Exp. Neurol. 168, 242–249 (2001).
Kida, S., Steart, P. V., Zhang, E. T. & Weller, R. O. Perivascular cells act as scavengers in the cerebral perivascular spaces and remain distinct from pericytes, microglia and macrophages. Acta Neuropathol. 85, 646–652 (1993).
Zhang, E. T., Richards, H. K., Kida, S. & Weller, R. O. Directional and compartmentalised drainage of interstitial fluid and cerebrospinal fluid from the rat brain. Acta Neuropathol. 83, 233–239 (1992).
Nayak, D., Zinselmeyer, B. H., Corps, K. N. & McGavern, D. B. In vivo dynamics of innate immune sentinels in the CNS. Intravital 1, 95–106 (2012).
Zeisel, A. et al. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 347, 1138–1142 (2015). This is the first scRNA-seq study on the rodent CNS, describing the distinct profiles of CAMs versus microglia.
Chinnery, H. R., Ruitenberg, M. J. & McMenamin, P. G. Novel characterization of monocyte-derived cell populations in the meninges and choroid plexus and their rates of replenishment in bone marrow chimeric mice. J. Neuropathol. Exp. Neurol. 69, 896–909 (2010).
Nimmerjahn, A., Kirchhoff, F. & Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318 (2005).
Barkauskas, D. et al. Extravascular CX3CR1+ cells extend intravascular dendritic processes into intact central nervous system vessel lumen. Microsc. Microanal. 19, 778–790 (2013).
Schain, A. J. et al. Activation of pial and dural macrophages and dendritic cells by cortical spreading depression. Ann. Neurol. 83, 508–521 (2018).
Russo, M. V., Latour, L. L. & McGavern, D. B. Distinct myeloid cell subsets promote meningeal remodeling and vascular repair after mild traumatic brain injury. Nat. Immunol. 19, 442–452 (2018).
Ford, A. L. et al. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J. Immunol. 154, 4309–4321 (1995).
Galea, I. et al. Mannose receptor expression specifically reveals perivascular macrophages in normal, injured, and diseased mouse brain. Glia 49, 375–384 (2005).
Ajami, B. et al. Single-cell mass cytometry reveals distinct populations of brain myeloid cells in mouse neuroinflammation and neurodegeneration models. Nat. Neurosci. 21, 541–551 (2018).
Brendecke, S. M. & Prinz, M. Do not judge a cell by its cover—diversity of CNS resident, adjoining and infiltrating myeloid cells in inflammation. Semin. Immunopathol. 37, 591–605 (2015).
Greter, M., Lelios, I. & Croxford, A. L. Microglia versus myeloid cell nomenclature during brain inflammation. Front. Immunol. 6, 249 (2015).
Ginhoux, F. & Jung, S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 14, 392–404 (2014).
Butovsky, O. et al. Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J. Clin. Invest. 122, 3063–3087 (2012).
Gingras, M. C., Lapillonne, H. & Margolin, J. F. CFFM4: a new member of the CD20/FcepsilonRIbeta family. Immunogenetics 53, 468–476 (2001).
Mato, M. et al. Involvement of specific macrophage-lineage cells surrounding arterioles in barrier and scavenger function in brain cortex. Proc. Natl Acad. Sci. USA 93, 3269–3274 (1996).
Régnier-Vigouroux, A. The mannose receptor in the brain. Int. Rev. Cytol. 226, 321–342 (2003).
Kim, W.-K. et al. CD163 identifies perivascular macrophages in normal and viral encephalitic brains and potential precursors to perivascular macrophages in blood. Am. J. Pathol. 168, 822–834 (2006).
Rua, R. & McGavern, D. B. Alternatively activated brain-resident macrophages acquire and retain inflammatory properties following CNS infection while interacting with effector and memory T cells. J. Immunol. 196 (Suppl.), 61.17 (2016).
Mesquita, S. D. et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 560, 185–191 (2018).
Bechmann, I., Galea, I. & Perry, V. H. What is the blood-brain barrier (not)? Trends Immunol. 28, 5–11 (2007).
Claudio, L., Martiney, J. A. & Brosnan, C. F. Ultrastructural studies of the blood-retina barrier after exposure to interleukin-1 beta or tumor necrosis factor-alpha. Lab. Invest. 70, 850–861 (1994).
He, H. et al. Perivascular macrophages limit permeability. Arterioscler. Thromb. Vasc. Biol. 36, 2203–2212 (2016).
Galanternik, M. V. et al. A novel perivascular cell population in the zebrafish brain. eLife 6, e24369 (2017).
Serrats, J. et al. Dual roles for perivascular macrophages in immune-to-brain signaling. Neuron 65, 94–106 (2010).
Matsuwaki, T., Eskilsson, A., Kugelberg, U., Jönsson, J.-I. & Blomqvist, A. Interleukin-1β induced activation of the hypothalamus-pituitary-adrenal axis is dependent on interleukin-1 receptors on non-hematopoietic cells. Brain. Behav. Immun. 40, 166–173 (2014).
Vasilache, A. M., Qian, H. & Blomqvist, A. Immune challenge by intraperitoneal administration of lipopolysaccharide directs gene expression in distinct blood-brain barrier cells toward enhanced prostaglandin E(2) signaling. Brain. Behav. Immun. 48, 31–41 (2015).
Jais, A. et al. Myeloid-cell-derived VEGF maintains brain glucose uptake and limits cognitive impairment in obesity. Cell 165, 882–895 (2016).
Mato, M., Ookawara, S., Sano, M. & Fukuda, S. Uptake of fat by fluorescent granular perithelial cells in cerebral cortex after administration of fat rich chow. Experientia 38, 1496–1498 (1982).
Brück, W. et al. Chapter 14 — macrophages in multiple sclerosis. Immunobiology 195, 588–600 (1996).
Walker-Caulfield, M. E., Hatfield, J. K. & Brown, M. A. Dynamic changes in meningeal inflammation correspond to clinical exacerbations in a murine model of relapsing-remitting multiple sclerosis. J. Neuroimmunol. 278, 112–122 (2015).
Ajami, B., Bennett, J. L., Krieger, C., McNagny, K. M. & Rossi, F. M. V. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat. Neurosci. 14, 1142–1150 (2011).
Brown, D. A. & Sawchenko, P. E. Time course and distribution of inflammatory and neurodegenerative events suggest structural bases for the pathogenesis of experimental autoimmune encephalomyelitis. J. Comp. Neurol. 260, 236–260 (2007).
Howell, O. W., Carassiti, D., Gentleman, S. M. & Nicholas, R. Extensive grey matter pathology in the cerebellum in multiple sclerosis is linked to inflammation in the subarachnoid space. Neuropathol. Appl. Neurobiol. 41, 798–813 (2015).
Boven, L. A. et al. Myelin-laden macrophages are anti-inflammatory, consistent with foam cells in multiple sclerosis. Brain 129, 517–526 (2006).
Greter, M. et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat. Med. 11, 328–334 (2005).
Prinz, M. & Priller, J. The role of peripheral immune cells in the CNS in steady state and disease. Nat. Neurosci. 20, 136–144 (2017).
Bartholomäus, I. et al. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature 462, 94–98 (2009).
Schläger, C. et al. Effector T cell trafficking between the leptomeninges and the cerebrospinal fluid. Nature 530, 349–353 (2016).
Wolf, Y. et al. Microglial MHC class II is dispensable for experimental autoimmune encephalomyelitis and cuprizone-induced demyelination. Eur. J. Immunol. 48, 1308–1318 (2018).
Mundt, S. et al. Conventional DCs sample and present myelin antigens in the healthy CNS and allow parenchymal T cell entry to initiate neuroinflammation. Sci. Immunol. 4, eaau8380 (2019).
Yamasaki, R. et al. Differential roles of microglia and monocytes in the inflamed central nervous system. J. Exp. Med. 211, 1533–1549 (2014).
Kooi, E. et al. Abundant extracellular myelin in the meninges of patients with multiple sclerosis. Neuropathol. Appl. Neurobiol. 35, 283–295 (2009).
Heppner, F. L., Ransohoff, R. M. & Becher, B. Immune attack: the role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 16, 358–372 (2015).
Prinz, M., Priller, J., Sisodia, S. S. & Ransohoff, R. M. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat. Neurosci. 14, 1227–1235 (2011).
Alonso, A., del, C., Grundke-Iqbal, I. & Iqbal, K. Alzheimer’s disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat. Med. 2, 783–787 (1996).
Greenberg, S. M. Cerebral amyloid angiopathy and vessel dysfunction. Cerebrovasc. Dis. 13, 42–47 (2002).
Dierksen, G. A. et al. Spatial relation between microbleeds and amyloid deposits in amyloid angiopathy. Ann. Neurol. 68, 545–548 (2010).
Attems, J., Jellinger, K., Thal, D. R. & Van Nostrand, W. Review: sporadic cerebral amyloid angiopathy. Neuropathol. Appl. Neurobiol. 37, 75–93 (2011).
Hawkes, C. A. et al. Perivascular drainage of solutes is impaired in the ageing mouse brain and in the presence of cerebral amyloid angiopathy. Acta Neuropathol. 121, 431–443 (2011).
Weller, R. O., Subash, M., Preston, S. D., Mazanti, I. & Carare, R. O. Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer’s disease. Brain Pathol. 18, 253–266 (2008).
Mato, M., Ookawara, S. & Kurihara, K. Uptake of exogenous substances and marked infoldings of the fluorescent granular pericyte in cerebral fine vessels. Am. J. Anat. 157, 329–332 (1980).
Mato, M., Ookawara, S., Aikawa, E. & Kawasaki, K. Studies on fluorescent granular perithelium (F.G.P.) of rat cerebral cortex — especially referring to morphological changes in aging. Anat. Anz. 149, 486–501 (1981).
Mato, M. & Ookawara, S. Influences of age and vasopressin on the uptake capacity of fluorescent granular perithelial cells (FGP) of small cerebral vessels of the rat. Am. J. Anat. 162, 45–53 (1981).
Sasaki, A., Nakazato, Y., Ogawa, A. & Sugihara, S. The immunophenotype of perivascular cells in the human brain. Pathol. Int. 46, 15–23 (1996).
Hawkes, C. A. & McLaurin, J. Selective targeting of perivascular macrophages for clearance of β-amyloid in cerebral amyloid angiopathy. Proc. Natl Acad. Sci. USA 106, 1261–1266 (2009). Hawkes and McLaurin describe evidence of a functional role of CAMs in an Alzheimer disease model.
El Khoury, J. et al. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat. Med. 13, 432–438 (2007).
Michaud, J.-P., Bellavance, M.-A., Préfontaine, P. & Rivest, S. Real-time in vivo imaging reveals the ability of monocytes to clear vascular amyloid beta. Cell Rep. 5, 646–653 (2013).
Serbina, N. V. & Pamer, E. G. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 7, 311–317 (2006).
Mildner, A. et al. Distinct and non-redundant roles of microglia and myeloid subsets in mouse models of Alzheimer’s disease. J. Neurosci. 31, 11159–11171 (2011).
Park, L. et al. Brain perivascular macrophages initiate the neurovascular dysfunction of Alzheimer Aβ peptides. Circ. Res. 121, 258–269 (2017).
Park, L. et al. Scavenger receptor CD36 is essential for the cerebrovascular oxidative stress and neurovascular dysfunction induced by amyloid-beta. Proc. Natl Acad. Sci. USA 108, 5063–5068 (2011).
Park, L. et al. Innate immunity receptor CD36 promotes cerebral amyloid angiopathy. Proc. Natl Acad. Sci. USA 110, 3089–3094 (2013).
Thanopoulou, K., Fragkouli, A., Stylianopoulou, F. & Georgopoulos, S. Scavenger receptor class B type I (SR-BI) regulates perivascular macrophages and modifies amyloid pathology in an Alzheimer mouse model. Proc. Natl Acad. Sci. USA 107, 20816–20821 (2010).
Kovacs, G. G. et al. Intracellular processing of disease-associated α-synuclein in the human brain suggests prion-like cell-to-cell spread. Neurobiol. Dis. 69, 76–92 (2014).
Liu, Y. et al. Quantitation of perivascular monocytes and macrophages around cerebral blood vessels of hypertensive and aged rats. J. Cereb. Blood Flow Metab. 14, 348–352 (1994).
Zhou, J. et al. CXCR3-dependent accumulation and activation of perivascular macrophages is necessary for homeostatic arterial remodeling to hemodynamic stresses. J. Exp. Med. 207, 1951–1966 (2010).
Faraco, G. et al. Perivascular macrophages mediate the neurovascular and cognitive dysfunction associated with hypertension. J. Clin. Invest. 126, 4674–4689 (2016).
Yu, Y. et al. Brain perivascular macrophages and the sympathetic response to inflammation in rats after myocardial infarction. Hypertension 55, 652–659 (2010).
Pires, P. W. et al. Improvement in middle cerebral artery structure and endothelial function in stroke-prone spontaneously hypertensive rats after macrophage depletion. Microcirculation 20, 650–661 (2013).
Pedragosa, J. et al. CNS-border associated macrophages respond to acute ischemic stroke attracting granulocytes and promoting vascular leakage. Acta Neuropathol. Commun. 6, 76 (2018).
Holfelder, K. et al. De novo expression of the hemoglobin scavenger receptor CD163 by activated microglia is not associated with hemorrhages in human brain lesions. Histol. Histopathol. 26, 1007–1017 (2011).
Kristensson, K. Microbes’ roadmap to neurons. Nat. Rev. Neurosci. 12, 345–357 (2011).
Lackner, A. A., Dandekar, S. & Gardner, M. B. Neurobiology of simian and feline immunodeficiency virus infections. Brain Pathol. 1, 201–212 (1991).
Allan, J. E., Dixon, J. E. & Doherty, P. C. Nature of the inflammatory process in the central nervous system of mice infected with lymphocytic choriomeningitis virus. Curr. Top. Microbiol. Immunol. 134, 131–143 (1987).
van den Pol, A. N., Mao, G., Yang, Y., Ornaghi, S. & Davis, J. N. Zika virus targeting in the developing brain. J. Neurosci. 37, 2161–2175 (2017).
Thompson, K. A., Cherry, C. L., Bell, J. E. & McLean, C. A. Brain cell reservoirs of latent virus in presymptomatic HIV-infected individuals. Am. J. Pathol. 179, 1623–1629 (2011).
Bragg, D. C. et al. Choroid plexus macrophages proliferate and release toxic factors in response to feline immunodeficiency virus. J. Neurovirol. 8, 225–239 (2002).
Sparger, E. E. et al. Infection of cats with molecularly cloned and biological isolates of the feline immunodeficiency virus. Virology 205, 546–553 (1994).
Joseph, S. B., Arrildt, K. T., Sturdevant, C. B. & Swanstrom, R. HIV-1 target cells in the CNS. J. Neurovirol. 21, 276–289 (2015).
Albright, A. V. et al. Microglia express CCR5, CXCR4, and CCR3, but of these, CCR5 is the principal coreceptor for human immunodeficiency virus type 1 dementia isolates. J. Virol. 73, 205–213 (1999).
González-Scarano, F. & Martín-García, J. The neuropathogenesis of AIDS. Nat. Rev. Immunol. 5, 69–81 (2005).
Glass, J. D., Fedor, H., Wesselingh, S. L. & McArthur, J. C. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann. Neurol. 38, 755–762 (1995).
Kaul, M., Garden, G. A. & Lipton, S. A. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 410, 988–994 (2001).
Filipowicz, A. R. et al. Proliferation of perivascular macrophages contributes to the development of encephalitic lesions in HIV-infected humans and in SIV-infected macaques. Sci. Rep. 6, 32900 (2016).
DiNapoli, S. R. et al. Tissue-resident macrophages can contain replication-competent virus in antiretroviral-naive, SIV-infected Asian macaques. JCI Insight 2, e91214 (2017).
Williams, K. C. et al. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques. J. Exp. Med. 193, 905–916 (2001).
Rua, R. et al. Infection drives meningeal engraftment by inflammatory monocytes that impairs CNS immunity. Nat. Immunol. 20, 407–419 (2019).
Steel, C. D. et al. Distinct macrophage subpopulations regulate viral encephalitis but not viral clearance in the CNS. J. Neuroimmunol. 226, 81–92 (2010).
Elmquist, J. K. et al. Intravenous lipopolysaccharide induces cyclooxygenase 2-like immunoreactivity in rat brain perivascular microglia and meningeal macrophages. J. Comp. Neurol. 381, 119–129 (1997).
Yamate, J., Ishimine, S., Izawa, T., Kumagai, D. & Kuwamura, M. Macrophage populations and expressions of regulatory proinflammatory factors in the rat meninx under lipopolysaccharide treatment in vivo and in vitro. Histol. Histopathol. 24, 13–24 (2009).
Djukic, M. et al. Circulating monocytes engraft in the brain, differentiate into microglia and contribute to the pathology following meningitis in mice. Brain J. Neurol. 129, 2394–2403 (2006).
Mildner, A. et al. Ly-6G+CCR2− myeloid cells rather than Ly-6ChighCCR2+ monocytes are required for the control of bacterial infection in the central nervous system. J. Immunol. 181, 2713–2722 (2008).
Polfliet, M. M. et al. A method for the selective depletion of perivascular and meningeal macrophages in the central nervous system. J. Neuroimmunol. 116, 188–195 (2001).
Polfliet, M. M. et al. Meningeal and perivascular macrophages of the central nervous system play a protective role during bacterial meningitis. J. Immunol. 167, 4644–4650 (2001).
Trostdorf, F. et al. Reduction of meningeal macrophages does not decrease migration of granulocytes into the CSF and brain parenchyma in experimental pneumococcal meningitis. J. Neuroimmunol. 99, 205–210 (1999).
Nau, R. et al. Granulocytes in the subarachnoid space of humans and rabbits with bacterial meningitis undergo apoptosis and are eliminated by macrophages. Acta Neuropathol. 96, 472–480 (1998).
Masuda, T. et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 566, 388–392 (2019). This article describes the first scRNA-seq study on human microglia during health and neuroinflammation.
Najafi, A. R. et al. A limited capacity for microglial repopulation in the adult brain. Glia 66, 2385–2396 (2018).
Pandya, H. et al. Differentiation of human and murine induced pluripotent stem cells to microglia-like cells. Nat. Neurosci. 20, 753–759 (2017).
Muffat, J. et al. Efficient derivation of microglia-like cells from human pluripotent stem cells. Nat. Med. 22, 1358–1367 (2016).
Lun, M. P., Monuki, E. S. & Lehtinen, M. K. Development and functions of the choroid plexus–cerebrospinal fluid system. Nat. Rev. Neurosci. 16, 445–457 (2015).
Ransohoff, R. M. & Engelhardt, B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat. Rev. Immunol. 12, 623–635 (2012).
Pollock, H., Hutchings, M., Weller, R. O. & Zhang, E. T. Perivascular spaces in the basal ganglia of the human brain: their relationship to lacunes. J. Anat. 191, 337–346 (1997).
Lam, M. A. et al. The ultrastructure of spinal cord perivascular spaces: implications for the circulation of cerebrospinal fluid. Sci. Rep. 7, 12924 (2017).
Carare, R. O., Hawkes, C. A. & Weller, R. O. Afferent and efferent immunological pathways of the brain. Anatomy, function and failure. Brain. Behav. Immun. 36, 9–14 (2014).
Engelhardt, B. et al. Vascular, glial, and lymphatic immune gateways of the central nervous system. Acta Neuropathol. 132, 317–338 (2016).
Weller, R. O. Microscopic morphology and histology of the human meninges. Morphol. Bull. Assoc. Anat. 89, 22–34 (2005).
Nabeshima, S., Reese, T. S., Landis, D. M. & Brightman, M. W. Junctions in the meninges and marginal glia. J. Comp. Neurol. 164, 127–169 (1975).
Clarke, A. G. The anatomy of the meninges. Postgrad. Med. J. 20, 74–78 (1944).
Wilson, E. H., Weninger, W. & Hunter, C. A. Trafficking of immune cells in the central nervous system. J. Clin. Invest. 120, 1368–1379 (2010).
Brocklehurst, G. The significance of the evolution of the cerebrospinal fluid system. Ann. R. Coll. Surg. Engl. 61, 349–356 (1979).
Kappers, C. U. A. The meninges in lower vertebrates compared with those in mammals. Arch. Neurol. Psychiatry 15, 281–296 (1926).
Zajícová, A. Comparative morphology of the meninges of amphibians and reptiles. Folia Morphol. 23, 56–64 (1975).
Butler, A. B. & Hodos, W. Comparative Vertebrate Neuroanatomy: Evolution and Adaptation (John Wiley & Sons, 2005).
Mercier, F., Weatherby, T. M. & Hartline, D. K. Meningeal-like organization of neural tissues in calanoid copepods (Crustacea). J. Comp. Neurol. 521, 760–790 (2013).
Cserr, H. F., Bundgaard, M., Ashby, J. K. & Murray, M. On the anatomic relation of choroid plexus to brain: a comparative study. Am. J. Physiol. 238, R76–R81 (1980).
Abbott, N. J., Lane, N. J. & Bundgaard, M. The blood-brain interface in invertebrates. Ann. NY Acad. Sci. 481, 20–42 (2006).
Soulas, C. et al. Genetically modified CD34+ hematopoietic stem cells contribute to turnover of brain perivascular macrophages in long-term repopulated primates. Am. J. Pathol. 174, 1808–1817 (2009).
Fleischhauer, K. Über die Fluoreszenz perivasculärer Zellen im Gehirn der Katze. Z. Zellforsch. Mikrosk. Anat. 64, 140–152 (1964).
Naujoks-Manteuffel, C. & Niemann, U. Microglial cells in the brain of Pleurodeles waltl (Urodela, Salamandridae) after wallerian degeneration in the primary visual system using Bandeiraea simplicifolia isolectin B4-cytochemistry. Glia 10, 101–113 (1994).
Cserr, H. F. & Bundgaard, M. Blood-brain interfaces in vertebrates: a comparative approach. Am. J. Physiol. Regul. Integr. Comp. Physiol. 246, R277–R288 (1984).
Minagar, A., Ragheb, J. & Kelley, R. E. The Edwin Smith surgical papyrus: description and analysis of the earliest case of aphasia. J. Med. Biogr. 11, 114–117 (2003).
Bakay, L. Discovery of the arachnoid membrane. Surg. Neurol. 36, 63–68 (1991).
Dohrmann, G. J. The choroid plexus: a historical review. Brain Res. 18, 197–218 (1970).
Galen, A. Galen on Anatomical Procedures: The Later Books (Cambridge Univ. Press, 2010).
Swanson, L. Neuroanatomical Terminology: A Lexicon of Classical Origins and Historical Foundations (Oxford Univ. Press, 2014).
Wickens, A. P. A History of the Brain: From Stone Age Surgery to Modern Neuroscience (Psychology Press, 2014).
Vesalius, A. De Humani Corporis Fabrica Libri Septem (Johannes Oporinus, 1543).
Liddelow, S. A. Fluids and barriers of the CNS: a historical viewpoint. Fluids Barriers CNS 8, 2 (2011).
Poirier, J. & Derouesné, C. The concept of cerebral lacunae from 1838 to the present [French]. Rev. Neurol. 141, 3–17 (1985).
Deecke, T. On the perivascular spaces in the nervous centers. Am. J. Psychiatry 30, 322–330 (1874).
Obersteiner, H. Über einige Lymphräume im Gehirne. Sitzungsber. Heidelb. Akad. Wiss. Math. Naturwiss. Kl. 61, 57–66 (1870).
Patek, P. R. The perivascular spaces of the mammalian brain. Anat. Rec. 88, 1–24 (1944).
Kubie, L. S. A study of the perivascular tissues of the central nervous system, with the supravital technique. J. Exp. Med. 46, 615–626 (1927).
Rio-Hortega, P. The microglia. Lancet 233, 1023–1026 (1939).
Wislocki, G. B. & Dempsey, E. W. The chemical cytology of the chorioid plexus and blood brain barrier of the rhesus monkey (Macaca mulatta). J. Comp. Neurol. 88, 319–345 (1948).
Mori, S. & Leblond, C. P. Identification of microglia in light and electron microscopy. J. Comp. Neurol. 135, 57–79 (1969).
McLone, D. G. & Bondareff, W. Developmental morphology of the subarachnoid space and contiguous structures in the mouse. Am. J. Anat. 142, 273–293 (1975).
Kivisäkk, P. et al. Localizing CNS immune surveillance: meningeal APCs activate T cells during EAE. Ann. Neurol. 65, 457–469 (2009).
Doran, K. S. et al. Host-pathogen interactions in bacterial meningitis. Acta Neuropathol. 131, 185–209 (2016).
Liu, C. et al. Macrophages mediate the repair of brain vascular rupture through direct physical adhesion and mechanical traction. Immunity 44, 1162–1176 (2016).
Zenaro, E. et al. Neutrophils promote Alzheimer’s disease–like pathology and cognitive decline via LFA-1 integrin. Nat. Med. 21, 880–886 (2015).
Rua, R., Johnson, K. & McGavern, D. B. Discovery of two meningeal macrophage populations with differential roles during homeostasis and inflammation. J. Immunol. 198, 68.6 (2017).
Acknowledgements
The authors apologize to all those colleagues whose work was discussed without proper citation, owing to space constraints. The authors thank C. Gross and A.G. Peres for excellent help in editing the review. This study was supported by the German Research Foundation (DFG) under Germany’s Excellence Strategy (CIBSS EXC-2189, Project ID 390939984). M.P. is supported by the BMBF (Federal Ministry of Education and Research)-funded competence network of multiple sclerosis (KKNMS), the Sobek Foundation, the Ernst-Jung Foundation, the DFG (SFB 992, SFB1160, SFB/TRR167, Reinhart-Koselleck-Grant) and the Ministry of Science, Research and Arts, Baden-Wuerttemberg (Sonderlinie “Neuroinflammation”).
Author information
Authors and Affiliations
Contributions
All authors contributed to researching the data for the article and writing the article. M.P. made substantial contributions to discussion of the content of the article and reviewed/edited the article before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Neuroscience thanks H. Lassmann, S. Rivest and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Microglia
-
The self-renewing population of tissue macrophages in the CNS parenchyma, which serve a plethora of functions during development and homeostasis and are implicated in many neurodegenerative and neuroimmunological diseases of the CNS.
- Lymphatic system
-
A vasculature system throughout the body that consists of low-pressure vessels that drain interstitial fluid from all organs to the heart and also serve important immune functions by allowing immune cell trafficking between organs, the lymph nodes and the spleen.
- Immune surveillance
-
The constant patrol of circulating and resident immune cells throughout the body and within their host tissue. The cells recognize and eliminate invading pathogens, clean up tissue injuries and remove unwanted host cells, such as cancer cells.
- Antigen presentation
-
An essential immune process in which antigen-presenting cells (such as dendritic cells) trigger adaptive T cell responses against specific antigens by presenting antigen epitopes and co-stimulatory signals on their surface.
- Yolk sac
-
A membranous sac present in most developing vertebrate embryos, which provides the embryo with nutrients by a direct connection via blood vessels and also harbours the blood islands, the specialized region within which the first haematopoietic cells of the embryo arise.
- Clonal expansion
-
The extensive proliferation of a group of identical cells that are originally derived from the same ancestor cell.
- Blood–brain barrier
-
(BBB). A multicellular barrier system that separates the CNS parenchyma from the periphery along CNS interfaces and restricts immune cell migration to the CNS.
- Single-cell RNA sequencing
-
(scRNA-seq). A new, unbiased technology using next-generation sequencing to evaluate the gene expression profile of single cells within a whole tissue or an isolated group of cells.
- Astrogliosis
-
Activation of astrocytes by inflammation, injury or an infection, characterized by extensive proliferation, morphological changes and cytokine secretion.
- T helper cells
-
Specialized CD4+ T cells that are involved in the adaptive immune response. T helper cells are activated and expand upon antigen presentation. They secrete important cytokines to support the immune response of macrophages, B cells and also cytotoxic T cells.
- Dendritic cells
-
Professional antigen-presenting cells of the innate immune system that constantly digest and process antigens and present them to T cells to induce an antigen-specific adaptive immune response.
- Induced pluripotent stem cells
-
An artificially generated type of pluripotent stem cell, produced by the reprogramming of adult differentiated cells with defined factors ex vivo.
Rights and permissions
About this article
Cite this article
Kierdorf, K., Masuda, T., Jordão, M.J.C. et al. Macrophages at CNS interfaces: ontogeny and function in health and disease. Nat Rev Neurosci 20, 547–562 (2019). https://doi.org/10.1038/s41583-019-0201-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41583-019-0201-x
This article is cited by
-
Blood–brain borders: a proposal to address limitations of historical blood–brain barrier terminology
Fluids and Barriers of the CNS (2024)
-
CNS-associated macrophages contribute to intracerebral aneurysm pathophysiology
Acta Neuropathologica Communications (2024)
-
Border-associated macrophages in the central nervous system
Journal of Neuroinflammation (2024)
-
IKKβ deletion from CNS macrophages increases neuronal excitability and accelerates the onset of EAE, while from peripheral macrophages reduces disease severity
Journal of Neuroinflammation (2024)
-
Understanding immune microenvironment alterations in the brain to improve the diagnosis and treatment of diverse brain diseases
Cell Communication and Signaling (2024)