Abstract
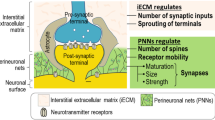
Perineuronal nets (PNNs) are extracellular matrix (ECM) chondroitin sulfate proteoglycan (CSPG)-containing structures that surround the soma and dendrites of various mammalian neuronal cell types. PNNs appear during development around the time that the critical periods for developmental plasticity end and are important for both their onset and closure. A similar structure — the perinodal ECM — surrounds the axonal nodes of Ranvier and appears as myelination is completed, acting as an ion-diffusion barrier that affects axonal conduction speed. Recent work has revealed the importance of PNNs in controlling plasticity in the CNS. Digestion, blocking or removal of PNNs influences functional recovery after a variety of CNS lesions. PNNs have further been shown to be involved in the regulation of memory and have been implicated in a number of psychiatric disorders.
This is a preview of subscription content, access via your institution
Access options



Similar content being viewed by others
References
Golgi, C. Bollettino Della Società Medico-Chirurgica di Pavia 1898: Intorno Alla Struttura Delle Cellule Nervose (Premiata Tipografia Fratelli Fusi, Pavia, Italy, 1898).
Celio, M. R., Spreafico, R., De Biasi, S. & Vitellaro-Zuccarello, L. Perineuronal nets: past and present. Trends Neurosci. 21, 510–514 (1998).
Shen, H. H. Core Concept: Perineuronal nets gain prominence for their role in learning, memory, and plasticity. Proc. Natl Acad. Sci. USA 115, 9813–9815 (2018).
Blosa, M. et al. Unique features of extracellular matrix in the mouse medial nucleus of trapezoid body—implications for physiological functions. Neuroscience 228, 215–234 (2013).
Vo, T. et al. The chemorepulsive axon guidance protein semaphorin3A is a constituent of perineuronal nets in the adult rodent brain. Mol. Cell. Neurosci. 56, 186–200 (2013). SEMA3A binds specifically to the chondroitin sulfates in PNNs, which localizes it to the synapses that connect to PV-expressing interneurons.
Bruckner, G. et al. Extracellular matrix organization in various regions of rat brain grey matter. J. Neurocytol. 25, 333–346 (1996).
Seeger, G., Brauer, K., Hartig, W. & Bruckner, G. Mapping of perineuronal nets in the rat brain stained by colloidal iron hydroxide histochemistry and lectin cytochemistry. Neuroscience 58, 371–388 (1994).
Koppe, G., Bruckner, G., Brauer, K., Hartig, W. & Bigl, V. Developmental patterns of proteoglycan-containing extracellular matrix in perineuronal nets and neuropil of the postnatal rat brain. Cell Tissue Res. 288, 33–41 (1997).
Bruckner, G., Grosche, J., Hartlage-Rubsamen, M., Schmidt, S. & Schachner, M. Region and lamina-specific distribution of extracellular matrix proteoglycans, hyaluronan and tenascin-R in the mouse hippocampal formation. J. Chem. Neuroanat. 26, 37–50 (2003).
Jager, C. et al. Perineuronal and perisynaptic extracellular matrix in the human spinal cord. Neuroscience 238, 168–184 (2013).
Hendry, S. H., Hockfield, S., Jones, E. G. & McKay, R. Monoclonal antibody that identifies subsets of neurones in the central visual system of monkey and cat. Nature 307, 267–269 (1984).
Hendry, S. H., Jones, E. G., Hockfield, S. & McKay, R. D. Neuronal populations stained with the monoclonal antibody Cat-301 in the mammalian cerebral cortex and thalamus. J. Neurosci. 8, 518–542 (1988).
Zaremba, S., Naegele, J. R., Barnstable, C. J. & Hockfield, S. Neuronal subsets express multiple high-molecular-weight cell-surface glycoconjugates defined by monoclonal antibodies Cat-301 and VC1.1. J. Neurosci. 10, 2985–2995 (1990).
Galtrey, C. M., Kwok, J. C., Carulli, D., Rhodes, K. E. & Fawcett, J. W. Distribution and synthesis of extracellular matrix proteoglycans, hyaluronan, link proteins and tenascin-R in the rat spinal cord. Eur. J. Neurosci. 27, 1373–1390 (2007).
Matthews, R. T. et al. Aggrecan glycoforms contribute to the molecular heterogeneity of perineuronal nets. J. Neurosci. 22, 7536–7547 (2002). Antibodies recognizing different glycanation isoforms of aggrecan bind to particular classes of PNNs, demonstrating that the CSPGs in different types of PNN are differently modified by glycanation.
Dauth, S. et al. Extracellular matrix protein expression is brain region dependent. J. Comp. Neurol. 524, 1309–1336 (2016).
Morikawa, S., Ikegaya, Y., Narita, M. & Tamura, H. Activation of perineuronal net-expressing excitatory neurons during associative memory encoding and retrieval. Sci. Rep. 7, 46024 (2017).
Rasband, M. N. The axon initial segment and the maintenance of neuronal polarity. Nat. Rev. Neurosci. 11, 552–562 (2010).
Oohashi, T. et al. Bral1, a brain-specific link protein, colocalizing with the versican V2 isoform at the nodes of Ranvier in developing and adult mouse central nervous systems. Mol. Cell. Neurosci. 19, 43–57 (2002).
Ferrer-Ferrer, M. & Dityatev, A. Shaping synapses by the neural extracellular matrix. Front. Neuroanat. 12, 40 (2018).
Tsilibary, E. et al. Neural ECM proteases in learning and synaptic plasticity. Prog. Brain Res. 214, 135–157 (2014).
Bruckner, G., Morawski, M. & Arendt, T. Aggrecan-based extracellular matrix is an integral part of the human basal ganglia circuit. Neuroscience 151, 489–504 (2008).
Sorg, B. A. et al. Casting a wide net: role of perineuronal nets in neural plasticity. J. Neurosci. 36, 11459–11468 (2016).
Deepa, S. S. et al. Composition of perineuronal net extracellular matrix in rat brain: a different disaccharide composition for the net-associated proteoglycans. J. Biol. Chem. 281, 17789–17800 (2006). The pattern of sulfation of CSPGs in PNNs is different from that of the diffuse matrix, and the sulfation pattern changes during brain maturation.
Kwok, J. C., Foscarin, S. & Fawcett, J. W. Perineuronal nets: a special structure in the central nervous system extracellular matrix. Neuromethods 93, 32 (2015).
Oohashi, T., Edamatsu, M., Bekku, Y. & Carulli, D. The hyaluronan and proteoglycan link proteins: organizers of the brain extracellular matrix and key molecules for neuronal function and plasticity. Exp. Neurol. 274, 134–144 (2015).
Gao, R. et al. Spatiotemporal expression patterns of chondroitin sulfate proteoglycan mRNAs in the developing rat brain. Neuroreport 29, 517–523 (2018).
Carulli, D. et al. Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain 133, 2331–2347 (2010). Chondrotinase experiments had shown that digestion of CSPGs reactivates plasticity; in this study, PNNs are specifically removed through the knockout of link proteins, whereas CSPGs are present (but diffuse). The animals show the same changes in plasticity as those seen in chondroitinase-treated animals, demonstrating that PNNs regulate plasticity.
Rogers, S. L., Rankin-Gee, E., Risbud, R. M., Porter, B. E. & Marsh, E. D. Normal development of the perineuronal net in humans; in patients with and without epilepsy. Neuroscience 384, 350–360 (2018).
Kwok, J. C., Carulli, D. & Fawcett, J. W. In vitro modeling of perineuronal nets: hyaluronan synthase and link protein are necessary for their formation and integrity. J. Neurochem. 114, 1447–1459 (2010).
Giamanco, K. A., Morawski, M. & Matthews, R. T. Perineuronal net formation and structure in aggrecan knockout mice. Neuroscience 170, 1314–1327 (2010).
Morawski, M. et al. Tenascin-R promotes assembly of the extracellular matrix of perineuronal nets via clustering of aggrecan. Phil. Trans. R. Soc. B 369, 20140046 (2014).
Rowlands, D. et al. Aggrecan directs extracellular matrix mediated neuronal plasticity. J. Neurosci. 38, 10102–10113 (2018). Knockout of the PNN component aggrecan leads to absence of PNNs and persistent plasticity, implicating aggrecan in PNN construction and plasticity control.
Giamanco, K. A. & Matthews, R. T. Deconstructing the perineuronal net: cellular contributions and molecular composition of the neuronal extracellular matrix. Neuroscience 218, 367–384 (2012).
Brakebusch, C. et al. Brevican-deficient mice display impaired hippocampal CA1 long-term potentiation but show no obvious deficits in learning and memory. Mol. Cell. Biol. 22, 7417–7427 (2002).
Zhou, X. H. et al. Neurocan is dispensable for brain development. Mol. Cell. Biol. 21, 5970–5978 (2001).
Geissler, M. et al. Primary hippocampal neurons, which lack four crucial extracellular matrix molecules, display abnormalities of synaptic structure and function and severe deficits in perineuronal net formation. J. Neurosci. 33, 7742–7755 (2013).
Arranz, A. M. et al. Hyaluronan deficiency due to Has3 knock-out causes altered neuronal activity and seizures via reduction in brain extracellular space. J. Neurosci. 34, 6164–6176 (2014).
Bekku, Y. et al. Bral2 is indispensable for the proper localization of brevican and the structural integrity of the perineuronal net in the brainstem and cerebellum. J. Comp. Neurol. 520, 1721–1736 (2012).
Bruckner, G. et al. Postnatal development of perineuronal nets in wild-type mice and in a mutant deficient in tenascin-R. J. Comp. Neurol. 428, 616–629 (2000). Knockout of CNS tenascin-C leads to partly formed PNNs and persistent plasticity, implicating tenascin-C in PNN construction and control of plasticity.
Miyata, S., Komatsu, Y., Yoshimura, Y., Taya, C. & Kitagawa, H. Persistent cortical plasticity by upregulation of chondroitin 6-sulfation. Nat. Neurosci. 15, 414–422 (2012). Transgenic overexpression of C6ST leads to persistent plasticity, showing that 6-sulfated CSPGs are permissive for plasticity.
Pizzorusso, T. et al. Reactivation of ocular dominance plasticity in the adult visual cortex with chondroitinase ABC. Science 298, 1248–1251 (2002). PNNs in the visual cortex develop in a light-dependent fashion as critical period plasticity ends; treatment of the visual cortex with chondroitinase to remove PNNs restores plasticity.
Kind, P. C. et al. The development and activity-dependent expression of aggrecan in the cat visual cortex. Cereb. Cortex 23, 349–360 (2013).
McRae, P. A., Rocco, M. M., Kelly, G., Brumberg, J. C. & Matthews, R. T. Sensory deprivation alters aggrecan and perineuronal net expression in the mouse barrel cortex. J. Neurosci. 27, 5405–5413 (2007).
Lander, C., Kind, P., Maleski, M. & Hockfield, S. A family of activity-dependent neuronal cell-surface chondroitin sulfate proteoglycans in cat visual cortex. J. Neurosci. 17, 1928–1939 (1997).
Spatazza, J. et al. Choroid-plexus-derived Otx2 homeoprotein constrains adult cortical plasticity. Cell Rep. 3, 1815–1823 (2013).
Beurdeley, M. et al. Otx2 binding to perineuronal nets persistently regulates plasticity in the mature visual cortex. J. Neurosci. 32, 9429–9437 (2012). The diffusible transcription factor OTX2 binds to CSPGs in PNNs, enabling maturation of PV interneurons and termination of plasticity.
Sugiyama, S. et al. Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell 134, 508–520 (2008). OTX2 is produced in the retina and visual system, binding to PV interneurons and enabling activation and termination of the critical period for plasticity.
Lee, H. H. C. et al. Genetic Otx2 mis-localization delays critical period plasticity across brain regions. Mol. Psychiatry 22, 785 (2017).
Mikami, T. & Kitagawa, H. Biosynthesis and function of chondroitin sulfate. Biochim. Biophys. Acta 1830, 4719–4733 (2013).
Mikami, T., Yasunaga, D. & Kitagawa, H. Contactin-1 is a functional receptor for neuroregulatory chondroitin sulfate-E. J. Biol. Chem. 284, 4494–4499 (2009).
Gama, C. I. et al. Sulfation patterns of glycosaminoglycans encode molecular recognition and activity. Nat. Chem. Biol. 2, 467–473 (2006).
Sugahara, K. & Mikami, T. Chondroitin/dermatan sulfate in the central nervous system. Curr. Opin. Struct. Biol. 17, 536–545 (2007).
Morawski, M. et al. Ion exchanger in the brain: Quantitative analysis of perineuronally fixed anionic binding sites suggests diffusion barriers with ion sorting properties. Sci. Rep. 5, 16471 (2015).
Frischknecht, R. et al. Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat. Neurosci. 12, 897–904 (2009). A hyaluronan-based matrix around hippocampal neurons affects the mobility of AMPA receptors and their localization to synaptic or extrasynaptic locations.
Favuzzi, E. et al. Activity-dependent gating of parvalbumin interneuron function by the perineuronal net protein brevican. Neuron 95, 639–655 (2017). Brevican in PNNs controls the location of AMPA and potassium channels and thus affects excitability. Brevican level is affected by experience, enabling learning and memory.
Klueva, J., Gundelfinger, E. D., Frischknecht, R. R. & Heine, M. Intracellular Ca(2)(+) and not the extracellular matrix determines surface dynamics of AMPA-type glutamate receptors on aspiny neurons. Phil. Trans. R. Soc. B 369, 20130605 (2014).
Sullivan, C. S. et al. Perineuronal net protein neurocan inhibits NCAM/EphA3 repellent signaling in GABAergic interneurons. Sci. Rep. 8, 6143 (2018).
Xu, D. et al. Narp and NP1 form heterocomplexes that function in developmental and activity-dependent synaptic plasticity. Neuron 39, 513–528 (2003).
Chang, M. C. et al. Narp regulates homeostatic scaling of excitatory synapses on parvalbumin-expressing interneurons. Nat. Neurosci. 13, 1090–1097 (2010).
Lee, S. J. et al. Presynaptic neuronal pentraxin receptor organizes excitatory and inhibitory synapses. J. Neurosci. 37, 1062–1080 (2017).
Lang, B. T. et al. Modulation of the proteoglycan receptor PTPsigma promotes recovery after spinal cord injury. Nature 518, 404–408 (2015).
Yi, J. H. et al. Receptor protein tyrosine phosphatase sigma binds to neurons in the adult mouse brain. Exp. Neurol. 255, 12–18 (2014).
Dickendesher, T. L. et al. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat. Neurosci. 15, 703–712 (2012).
Orlando, c., Ster, J., Gerber, U., Fawcett, J. W. & Raineteau, O. Peridendritic chondroitin sulfate proteoglycans restrict structural plasticity in an integrin-dependent manner. J. Neurosci. 32, 18009–18017 (2012).
Tan, C. L. et al. Integrin activation promotes axon growth on inhibitory chondroitin sulfate proteoglycans by enhancing integrin signaling. J. Neurosci. 31, 6289–6295 (2011).
de Winter, F. et al. The chemorepulsive protein semaphorin 3A and perineuronal net-mediated plasticity. Neural Plast. 2016, 3679545 (2016).
Carulli, D., Foscarin, S., Faralli, A., Pajaj, E. & Rossi, F. Modulation of semaphorin3A in perineuronal nets during structural plasticity in the adult cerebellum. Mol. Cell. Neurosci. 57, 10–22 (2013).
Dick, G. et al. Semaphorin 3A binds to the perineuronal nets via chondroitin sulfate type E motifs in rodent brains. J. Biol. Chem. 288, 27384–27395 (2013).
Nadanaka, S., Kinouchi, H., Taniguchi-Morita, K., Tamura, J. & Kitagawa, H. Down-regulation of chondroitin 4-O-sulfotransferase-1 by Wnt signaling triggers diffusion of Wnt-3a. J. Biol. Chem. 286, 4199–4208 (2011).
Miller, G. M. & Hsieh-Wilson, L. C. Sugar-dependent modulation of neuronal development, regeneration, and plasticity by chondroitin sulfate proteoglycans. Exp. Neurol. 274, 115–125 (2015).
Purushothaman, A., Sugahara, K. & Faissner, A. Chondroitin sulfate “wobble motifs” modulate maintenance and differentiation of neural stem cells and their progeny. J. Biol. Chem. 287, 2935–2942 (2012).
Yabuno, K. et al. A sulfated glycosaminoglycan linkage region is a novel type of human natural killer-1 (HNK-1) epitope expressed on aggrecan in perineuronal nets. PLOS ONE 10, e0144560 (2015).
Dwyer, C. A., Katoh, T., Tiemeyer, M. & Matthews, R. T. Neurons and glia modify receptor protein-tyrosine phosphatase zeta (RPTPzeta)/phosphacan with cell-specific O-mannosyl glycans in the developing brain. J. Biol. Chem. 290, 10256–10273 (2015).
Kalb, R. G. & Hockfield, S. Electrical activity in the neuromuscular unit can influence the molecular development of motor neurons. Dev. Biol. 162, 539–548 (1994).
Myers, A. K., Ray, J. & Kulesza, R. J. Jr. Neonatal conductive hearing loss disrupts the development of the Cat-315 epitope on perineuronal nets in the rat superior olivary complex. Brain Res. 1465, 34–47 (2012).
Carulli, D., Foscarin, S. & Rossi, F. Activity-dependent plasticity and gene expression modifications in the adult CNS. Front. Mol. Neurosci. 4, 50 (2011).
Balmer, T. S., Carels, V. M., Frisch, J. L. & Nick, T. A. Modulation of perineuronal nets and parvalbumin with developmental song learning. J. Neurosci. 29, 12878–12885 (2009).
Dityatev, A. et al. Activity-dependent formation and functions of chondroitin sulfate-rich extracellular matrix of perineuronal nets. Dev. Neurobiol. 67, 570–588 (2007).
Yin, Z. Q., Crewther, S. G., Wang, C. & Crewther, D. P. Pre- and post-critical period induced reduction of Cat-301 immunoreactivity in the lateral geniculate nucleus and visual cortex of cats Y-blocked as adults or made strabismic as kittens. Mol. Vis. 12, 858–866 (2006).
Kalb, R. G. & Hockfield, S. Large diameter primary afferent input is required for expression of the Cat-301 proteoglycan on the surface of motor neurons. Neuroscience 34, 391–401 (1990). PNN formation around motor neurons is dependent on electrical activity via sensory input.
Zaremba, S., Guimaraes, A., Kalb, R. G. & Hockfield, S. Characterization of an activity-dependent, neuronal surface proteoglycan identified with monoclonal antibody Cat-301. Neuron 2, 1207–1219 (1989).
Harauzov, A. et al. Reducing intracortical inhibition in the adult visual cortex promotes ocular dominance plasticity. J. Neurosci. 30, 361–371 (2010).
Foscarin, S. et al. Experience-dependent plasticity and modulation of growth regulatory molecules at central synapses. PLOS ONE 6, e16666 (2011).
Sale, A. et al. Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat. Neurosci. 10, 679–681 (2007).
Banerjee, S. B. et al. Perineuronal nets in the adult sensory cortex are necessary for fear learning. Neuron 95, 169–179 (2017).
Faralli, A. et al. Modifications of perineuronal nets and remodelling of excitatory and inhibitory afferents during vestibular compensation in the adult mouse. Brain Struct. Funct. 22, 3193–3209 (2016).
Favuzzi, E. & Rico, B. Molecular diversity underlying cortical excitatory and inhibitory synapse development. Curr. Opin. Neurobiol. 53, 8–15 (2018).
Wang, D., Ichiyama, R. M., Zhao, R., Andrews, M. R. & Fawcett, J. W. Chondroitinase combined with rehabilitation promotes recovery of forelimb function in rats with chronic spinal cord injury. J. Neurosci. 31, 9332–9344 (2011).
Smith, C. C. et al. Differential regulation of perineuronal nets in the brain and spinal cord with exercise training. Brain Res. Bull. 111, 20–26 (2015).
Miyata, S., Akagi, A., Hayashi, N., Watanabe, K. & Oohira, A. Activity-dependent regulation of a chondroitin sulfate proteoglycan 6B4 phosphacan/RPTPbeta in the hypothalamic supraoptic nucleus. Brain Res. 1017, 163–171 (2004).
Okamoto, M. et al. Kainic acid-induced convulsions cause prolonged changes in the chondroitin sulfate proteoglycans neurocan and phosphacan in the limbic structures. Exp. Neurol. 184, 179–195 (2003).
McRae, P. A., Baranov, E., Rogers, S. L. & Porter, B. E. Persistent decrease in multiple components of the perineuronal net following status epilepticus. Eur. J. Neurosci. 36, 3471–3482 (2012).
Yutsudo, N. & Kitagawa, H. Involvement of chondroitin 6-sulfation in temporal lobe epilepsy. Exp. Neurol. 274, 126–133 (2015).
Donato, F., Rompani, S. B. & Caroni, P. Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature 504, 272–276 (2013). Learning involves formation of new inhibitory synapses onto PV-expressing interneurons, leading to decreased GABA production. Chondroitinase also causes the formation of new inhibitory synapses on PV-expressing interneurons and lowered GABA production.
Bernard, C. & Prochiantz, A. Otx2-PNN interaction to regulate cortical plasticity. Neural Plast. 2016, 7931693 (2016).
Wlodarczyk, J., Mukhina, I., Kaczmarek, L. & Dityatev, A. Extracellular matrix molecules, their receptors, and secreted proteases in synaptic plasticity. Dev. Neurobiol. 71, 1040–1053 (2011).
Murase, S., Lantz, C. L. & Quinlan, E. M. Light reintroduction after dark exposure reactivates plasticity in adults via perisynaptic activation of MMP-9. eLife 6, e27345 (2017). Light exposure after dark exposure in adult mice reactivates ocular dominance plasticity through release of metalloproteinases and degradation of ECM.
Kelly, E. A., Russo, A. S., Jackson, C. D., Lamantia, C. E. & Majewska, A. K. Proteolytic regulation of synaptic plasticity in the mouse primary visual cortex: analysis of matrix metalloproteinase 9 deficient mice. Front. Cell Neurosci. 9, 369 (2015).
Ganguly, K. et al. Matrix metalloproteinase (MMP) 9 transcription in mouse brain induced by fear learning. J. Biol. Chem. 288, 20978–20991 (2013).
Rossier, J. et al. Cortical fast-spiking parvalbumin interneurons enwrapped in the perineuronal net express the metallopeptidases Adamts8, Adamts15 and Neprilysin. Mol. Psychiatry 20, 154–161 (2015).
Rankin-Gee, E. K. et al. Perineuronal net degradation in epilepsy. Epilepsia 56, 1124–1133 (2015).
Yuan, W., Matthews, R. T., Sandy, J. D. & Gottschall, P. E. Association between protease-specific proteolytic cleavage of brevican and synaptic loss in the dentate gyrus of kainate-treated rats. Neuroscience 114, 1091–1101 (2002).
Gottschall, P. E. & Howell, M. D. ADAMTS expression and function in central nervous system injury and disorders. Matrix Biol. 44–46, 70–76 (2015).
Levy, C., Brooks, J. M., Chen, J., Su, J. & Fox, M. A. Cell-specific and developmental expression of lectican-cleaving proteases in mouse hippocampus and neocortex. J. Comp. Neurol. 523, 629–648 (2015).
Dubey, D. et al. Increased metalloproteinase activity in the hippocampus following status epilepticus. Epilepsy Res. 132, 50–58 (2017).
Lin, R., Kwok, J. C., Crespo, D., & Fawcett, J. W. Chondroitinase, ABC has a long lasting effect on chondroitin sulphate glycosaminoglycan content in the injured rat brain. J. Neurochem. 104, 400–408 (2008).
Senkov, O., Andjus, P., Radenovic, L., Soriano, E. & Dityatev, A. Neural ECM molecules in synaptic plasticity, learning, and memory. Prog. Brain Res. 214, 53–80 (2014).
Dityatev, A., Schachner, M. & Sonderegger, P. The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat. Rev. Neurosci. 11, 735–746 (2010).
Srinivasan, J., Schachner, M. & Catterall, W. A. Interaction of voltage-gated sodium channels with the extracellular matrix molecules tenascin-C and tenascin-R. Proc. Natl Acad. Sci. USA 95, 15753–15757 (1998).
Xiao, Z. C. et al. Tenascin-R is a functional modulator of sodium channel beta subunits. J. Biol. Chem. 274, 26511–26517 (1999).
Weber, P. et al. Mice deficient for tenascin-R display alterations of the extracellular matrix and decreased axonal conduction velocities in the CNS. J. Neurosci. 19, 4245–4262 (1999).
Saghatelyan, A. K. et al. The extracellular matrix molecule tenascin-R and its HNK-1 carbohydrate modulate perisomatic inhibition and long-term potentiation in the CA1 region of the hippocampus. Eur. J. Neurosci. 12, 3331–3342 (2000).
Saghatelyan, A. K. et al. Reduced perisomatic inhibition, increased excitatory transmission, and impaired long-term potentiation in mice deficient for the extracellular matrix glycoprotein tenascin-R. Mol. Cell. Neurosci. 17, 226–240 (2001).
Bukalo, O., Schachner, M. & Dityatev, A. Hippocampal metaplasticity induced by deficiency in the extracellular matrix glycoprotein tenascin-R. J. Neurosci. 27, 6019–6028 (2007). Tenascin deficiency has widespread effects on hippocampal plasticity.
Lensjo, K. K., Lepperod, M. E., Dick, G., Hafting, T. & Fyhn, M. Removal of perineuronal nets unlocks juvenile plasticity through network mechanisms of decreased inhibition and increased gamma activity. J. Neurosci. 37, 1269–1283 (2017).
Hou, X. et al. Chondroitin sulfate is required for onset and offset of critical period plasticity in visual cortex. Sci. Rep. 7, 12646 (2017).
Kochlamazashvili, G. et al. The extracellular matrix molecule hyaluronic acid regulates hippocampal synaptic plasticity by modulating postsynaptic L-type Ca(2+) channels. Neuron 67, 116–128 (2010).
Massey, P. V. et al. Learning-specific changes in long-term depression in adult perirhinal cortex. J. Neurosci. 28, 7548–7554 (2008).
Griffiths, S. et al. Expression of long-term depression underlies visual recognition memory. Neuron 58, 186–194 (2008).
Romberg, C. et al. Depletion of perineuronal nets enhances recognition memory and long-term depression in the perirhinal cortex. J. Neurosci. 33, 7057–7065 (2013). PNN removal causes prolongation of object recognition memory and enhanced LTD in the perirhinal cortex.
de Vivo, L. et al. Extracellular matrix inhibits structural and functional plasticity of dendritic spines in the adult visual cortex. Nat. Commun. 4, 1484 (2013).
Matsuzaki, M., Honkura, N., Ellis-Davies, G. C. & Kasai, H. Structural basis of long-term potentiation in single dendritic spines. Nature 429, 761–766 (2004).
Harvey, C. D. & Svoboda, K. Locally dynamic synaptic learning rules in pyramidal neuron dendrites. Nature 450, 1195–1200 (2007).
Carstens, K. E. & Dudek, S. M. Regulation of synaptic plasticity in hippocampal area CA2. Curr. Opin. Neurobiol. 54, 194–199 (2019).
Carstens, K. E., Phillips, M. L., Pozzo-Miller, L., Weinberg, R. J. & Dudek, S. M. Perineuronal nets suppress plasticity of excitatory synapses on CA2 pyramidal neurons. J. Neurosci. 36, 6312–6320 (2016). PNN disruption enables synaptic potentiation of CA2 pyramidal neurons. Early-life enrichment modifies the development of PNNs on these neurons.
Hayani, H., Song, I. & Dityatev, A. Increased excitability and reduced excitatory synaptic input into fast-spiking CA2 interneurons after enzymatic attenuation of extracellular matrix. Front. Cell Neurosci. 12, 149 (2018).
Carulli, D. et al. The composition of perineuronal nets in the adult rat cerebellum and the cellular origin of their components. J. Comp. Neurol. 494, 559–577 (2006). This paper describes the composition and structure of PNNs in the deep cerebellar nucleus.
Edamatsu, M. et al. Hapln4/Bral2 is a selective regulator for formation and transmission of GABAergic synapses between Purkinje and deep cerebellar nuclei neurons. J. Neurochem. 147, 748–763 (2018).
Hirono, M. et al. Perineuronal nets in the deep cerebellar nuclei regulate GABAergic transmission and delay eyeblink conditioning. J. Neurosci. 38, 6130–6144 (2018).
Bukalo, O., Schachner, M. & Dityatev, A. Modification of extracellular matrix by enzymatic removal of chondroitin sulfate and by lack of tenascin-R differentially affects several forms of synaptic plasticity in the hippocampus. Neuroscience 104, 359–369 (2001). Chondroitinase digestion or deficiency of the PNN component tenascin-R affects hippocampal plasticity.
Hensch, T. K. Critical period regulation. Annu. Rev. Neurosci. 27, 549–579 (2004).
Southwell, D. G., Froemke, R. C., Alvarez-Buylla, A., Stryker, M. P. & Gandhi, S. P. Cortical plasticity induced by inhibitory neuron transplantation. Science 327, 1145–1148 (2010).
Gundelfinger, E. D., Frischknecht, R., Choquet, D. & Heine, M. Converting juvenile into adult plasticity: a role for the brain’s extracellular matrix. Eur. J. Neurosci. 31, 2156–2165 (2010).
Gianfranceschi, L. et al. Visual cortex is rescued from the effects of dark rearing by overexpression of BDNF. Proc. Natl Acad. Sci. USA 100, 12486–12491 (2003).
Cynader, M. & Mitchell, D. E. Prolonged sensitivity to monocular deprivation in dark-reared cats. J. Neurophysiol. 43, 1026–1040 (1980).
Galtrey, C. M., Asher, R. A., Nothias, F. & Fawcett, J. W. Promoting plasticity in the spinal cord with chondroitinase improves functional recoveryafter peripheral nerve repair. Brain 130, 926–939 (2007).
Kwok, J. C., Yang, S. & Fawcett, J. W. Neural ECM in regeneration and rehabilitation. Prog. Brain Res. 214, 179–192 (2014).
Kadomatsu, K. & Sakamoto, K. Sulfated glycans in network rewiring and plasticity after neuronal injuries. Neurosci. Res. 78, 50–54 (2014).
Takesian, A. E. & Hensch, T. K. Balancing plasticity/stability across brain development. Prog. Brain Res. 207, 3–34 (2013).
Nabel, E. M. & Morishita, H. Regulating critical period plasticity: insight from the visual system to fear circuitry for therapeutic interventions. Front. Psychiatry 4, 146 (2013).
Morawski, M., Bruckner, G., Arendt, T. & Matthews, R. T. Aggrecan: Beyond cartilage and into the brain. Int. J. Biochem. Cell Biol. 44, 690–693 (2012).
Apostolova, I., Irintchev, A. & Schachner, M. Tenascin-R restricts posttraumatic remodeling of motoneuron innervation and functional recovery after spinal cord injury in adult mice. J. Neurosci. 26, 7849–7859 (2006).
Loers, G., Chen, S., Grumet, M. & Schachner, M. Signal transduction pathways implicated in neural recognition molecule L1 triggered neuroprotection and neuritogenesis. J. Neurochem. 92, 1463–1476 (2005).
Happel, M. F. et al. Enhanced cognitive flexibility in reversal learning induced by removal of the extracellular matrix in auditory cortex. Proc. Natl Acad. Sci. USA 111, 2800–2805 (2014).
Gogolla, N., Caroni, P., Luthi, A. & Herry, C. Perineuronal nets protect fear memories from erasure. Science 325, 1258–1261 (2009). Digestion of PNNs in the amygdala enables juvenile-pattern unlearning of fear memory, implicating PNNs in memory stability.
Thompson, E. H. et al. Removal of perineuronal nets disrupts recall of a remote fear memory. Proc. Natl Acad. Sci. USA 115, 607–612 (2018).
Xue, Y. X. et al. Depletion of perineuronal nets in the amygdala to enhance the erasure of drug memories. J. Neurosci. 34, 6647–6658 (2014).
Slaker, M. et al. Removal of perineuronal nets in the medial prefrontal cortex impairs the acquisition and reconsolidation of a cocaine-induced conditioned place preference memory. J. Neurosci. 35, 4190–4202 (2015). PNN digestion affects cocaine-induced drug memory, suggesting that drug memories might be more modifiable after PNN modulation.
Morellini, F. et al. Improved reversal learning and working memory and enhanced reactivity to novelty in mice with enhanced GABAergic innervation in the dentate gyrus. Cereb. Cortex 20, 2712–2727 (2010).
Karunakaran, S. et al. PV plasticity sustained through D1/5 dopamine signaling required for long-term memory consolidation. Nat. Neurosci. 19, 454–464 (2016).
Caroni, P. Regulation of Parvalbumin Basket cell plasticity in rule learning. Biochem. Biophys. Res. Commun. 460, 100–103 (2015).
Sur, M., Nagakura, I., Chen, N. & Sugihara, H. Mechanisms of plasticity in the developing and adult visual cortex. Prog. Brain Res. 207, 243–254 (2013).
Faini, G. et al. Perineuronal nets control visual input via thalamic recruitment of cortical PV interneurons. eLife 7, e41520 (2018).
Rao-Ruiz, P., Yu, J., Kushner, S. A. & Josselyn, S. A. Neuronal competition: microcircuit mechanisms define the sparsity of the engram. Curr. Opin. Neurobiol. 54, 163–170 (2019).
Tsien, R. Y. Very long-term memories may be stored in the pattern of holes in the perineuronal net. Proc. Natl Acad. Sci. USA 110, 12456–12461 (2013).
Bradbury, E. J. et al. Chondroitinase ABC promotes axon regeneration and functional recovery following spinal cord injury. Nature 416, 636–640 (2002).
Takeda-Uchimura, Y. et al. Requirement of keratan sulfate proteoglycan phosphacan with a specific sulfation pattern for critical period plasticity in the visual cortex. Exp. Neurol. 274, 145–155 (2015).
Imagama, S. et al. Keratan sulfate restricts neural plasticity after spinal cord injury. J. Neurosci. 31, 17091–17102 (2011).
Soleman, S., Filippov, M. A., Dityatev, A. & Fawcett, J. W. Targeting the neural extracellular matrix in neurological disorders. Neuroscience 253, 194–213 (2013).
Bartus, K., James, N. D., Bosch, K. D. & Bradbury, E. J. Chondroitin sulphate proteoglycans: key modulators of spinal cord and brain plasticity. Exp. Neurol. 235, 5–17 (2012).
Frischknecht, R. & Gundelfinger, E. D. The brain’s extracellular matrix and its role in synaptic plasticity. Adv. Exp. Med. Biol. 970, 153–171 (2012).
Garcia-Alias, G. & Fawcett, J. W. Training and anti-CSPG combination therapy for spinal cord injury. Exp. Neurol. 235, 26–32 (2011).
Fitch, M. T. & Silver, J. CNS injury, glial scars, and inflammation: inhibitory extracellular matrices and regeneration failure. Exp. Neurol. 209, 294–301 (2008).
Suttkus, A., Morawski, M. & Arendt, T. Protective properties of neural extracellular matrix. Mol. Neurobiol. 53, 73–82 (2014).
Cabungcal, J. H. et al. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc. Natl Acad. Sci. USA 110, 9130–9135 (2013). PNNs have a neuroprotective action, allowing PNN-bearing neurons to survive in the presence of a global increase in oxidative stress.
Testa, D., Prochiantz, A. & Di Nardo, A. A. Perineuronal nets in brain physiology and disease. Semin. Cell Dev. Biol. 89, 125–135 (2019).
Yang, S. et al. Antibody recognizing 4-sulfated chondroitin sulfate proteoglycans restores memory in tauopathy-induced neurodegeneration. Neurobiol. Aging 59, 197–209 (2017). An antibody that blocks 4-sulfated CSPGs enables recovery of memory in an Alzheimer disease model. This finding supports the concept that plasticity depends on the balance of inhibitory 4-sulfated and permissive 6-sulfated glycans.
Yang, S. et al. Perineuronal net digestion with chondroitinase restores memory in mice with tau pathology. Exp. Neurol. 265, 48–58 (2015).
Morawski, M. et al. Involvement of perineuronal and perisynaptic extracellular matrix in Alzheimer’s disease neuropathology. Brain Pathol. 22, 547–561 (2012).
Baig, S., Wilcock, G. K. & Love, S. Loss of perineuronal net N-acetylgalactosamine in Alzheimer’s disease. Acta Neuropathol. 110, 393–401 (2005).
Morawski, M., Bruckner, G., Jager, C., Seeger, G. & Arendt, T. Neurons associated with aggrecan-based perineuronal nets are protected against tau pathology in subcortical regions in Alzheimer’s disease. Neuroscience 169, 1347–1363 (2010).
Bruckner, G. et al. Cortical areas abundant in extracellular matrix chondroitin sulphate proteoglycans are less affected by cytoskeletal changes in Alzheimer’s disease. Neuroscience 92, 791–805 (1999).
Suttkus, A., Holzer, M., Morawski, M. & Arendt, T. The neuronal extracellular matrix restricts distribution and internalization of aggregated Tau-protein. Neuroscience 313, 225–235 (2016).
Suttkus, A. et al. Aggrecan, link protein and tenascin-R are essential components of the perineuronal net to protect neurons against iron-induced oxidative stress. Cell Death Dis. 5, e1119 (2014). PNNs protect neurons against oxidative stress from iron. This protection is dependent on their content of aggrecan, link protein and tenascin-R.
Allen, B. et al. Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J. Neurosci. 22, 9340–9351 (2002).
Vegh, M. J. et al. Reducing hippocampal extracellular matrix reverses early memory deficits in a mouse model of Alzheimer’s disease. Acta Neuropathol. Commun. 2, 76 (2014).
Wilczynski, G. M. et al. Important role of matrix metalloproteinase 9 in epileptogenesis. J. Cell Biol. 180, 1021–1035 (2008).
Forostyak, S. et al. Intrathecal delivery of mesenchymal stromal cells protects the structure of altered perineuronal nets in SOD1 rats and amends the course of ALS. Stem Cells 32, 3163–3172 (2014).
Lewis, D. A. Inhibitory neurons in human cortical circuits: substrate for cognitive dysfunction in schizophrenia. Curr. Opin. Neurobiol. 26, 22–26 (2014).
Steullet, P. et al. Oxidative stress-driven parvalbumin interneuron impairment as a common mechanism in models of schizophrenia. Mol. Psychiatry 22, 936–943 (2017).
Mauney, S. A. et al. Developmental pattern of perineuronal nets in the human prefrontal cortex and their deficit in schizophrenia. Biol. Psychiatry 74, 427–435 (2013). In unaffected individuals, the density of PNNs in the prefrontal cortex increases during pre-pubertal and early adolescence. In patients with schizophrenia, there is a 70% reduction in PNN number in the prefrontal cortex.
Lewis, D. A., Curley, A. A., Glausier, J. R. & Volk, D. W. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 35, 57–67 (2012).
Berretta, S. Extracellular matrix abnormalities in schizophrenia. Neuropharmacology 62, 1584–1597 (2012).
Steullet, P. et al. The thalamic reticular nucleus in schizophrenia and bipolar disorder: role of parvalbumin-expressing neuron networks and oxidative stress. Mol. Psychiatry 23, 2057–2065 (2018).
Pantazopoulos, H., Woo, T. U., Lim, M. P., Lange, N. & Berretta, S. Extracellular matrix-glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia. Arch. Gen. Psychiatry 67, 155–166 (2010).
Enwright, J. F. et al. Reduced labeling of parvalbumin neurons and perineuronal nets in the dorsolateral prefrontal cortex of subjects with schizophrenia. Neuropsychopharmacology 41, 2206–2214 (2016).
Arion, D., Horvath, S., Lewis, D. A. & Mirnics, K. Infragranular gene expression disturbances in the prefrontal cortex in schizophrenia: signature of altered neural development? Neurobiol. Dis. 37, 738–746 (2010).
Pantazopoulos, H. et al. Aggrecan and chondroitin-6-sulfate abnormalities in schizophrenia and bipolar disorder: a postmortem study on the amygdala. Transl Psychiatry 5, e496 (2015).
Gogolla, N., Takesian, A. E., Feng, G., Fagiolini, M. & Hensch, T. K. Sensory integration in mouse insular cortex reflects GABA circuit maturation. Neuron 83, 894–905 (2014).
Gandal, M. J. et al. Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science 362, eaat8127 (2018).
Do, K. Q., Cuenod, M. & Hensch, T. K. Targeting oxidative stress and aberrant critical period plasticity in the developmental trajectory to schizophrenia. Schizophr. Bull. 41, 835–846 (2015).
Takei, Y., Kikkawa, Y. S., Atapour, N., Hensch, T. K. & Hirokawa, N. Defects in synaptic plasticity, reduced NMDA-receptor transport, and instability of postsynaptic density proteins in mice lacking microtubule-associated protein 1A. J. Neurosci. 35, 15539–15554 (2015).
Cabungcal, J. H., Steullet, P., Kraftsik, R., Cuenod, M. & Do, K. Q. Early-life insults impair parvalbumin interneurons via oxidative stress: reversal by N-acetylcysteine. Biol. Psychiatry 73, 574–582 (2013).
Morishita, H., Cabungcal, J. H., Chen, Y., Do, K. Q. & Hensch, T. K. Prolonged period of cortical plasticity upon redox dysregulation in fast-spiking interneurons. Biol. Psychiatry 78, 396–402 (2015).
Riga, D. et al. Hippocampal extracellular matrix alterations contribute to cognitive impairment associated with a chronic depressive-like state in rats. Sci. Transl Med. 9, eaai8753 (2017).
Miyata, S., Nadanaka, S., Igarashi, M. & Kitagawa, H. Structural variation of chondroitin sulfate chains contributes to the molecular heterogeneity of perineuronal nets. Front. Integr. Neurosci. 12, 3 (2018).
Zimmermann, D. R. & Dours-Zimmermann, M. T. Extracellular matrix of the central nervous system: from neglect to challenge. Histochem. Cell Biol. 130, 635–653 (2008).
Yamaguchi, Y. Lecticans: organizers of the brain extracellular matrix. Cell. Mol. Life Sci. 57, 276–289 (2000).
Hagihara, K. et al. Immunohistochemical evidence for the brevican-tenascin-R interaction: colocalization in perineuronal nets suggests a physiological role for the interaction in the adult rat brain. J. Comp. Neurol. 410, 256–264 (1999).
Aspberg, A. et al. The C-type lectin domains of lecticans, a family of aggregating chondroitin sulfate proteoglycans, bind tenascin-R by protein-protein interactions independent of carbohydrate moiety. Proc. Natl Acad. Sci. USA 94, 10116–10121 (1997).
Bekku, Y. et al. Molecular cloning of Bral2, a novel brain-specific link protein, and immunohistochemical colocalization with brevican in perineuronal nets. Mol. Cell. Neurosci. 24, 148–159 (2003).
Miyata, S. & Kitagawa, H. Formation and remodeling of the brain extracellular matrix in neural plasticity: Roles of chondroitin sulfate and hyaluronan. Biochim. Biophys. Acta 1861, 2420–2434 (2017).
Bekku, Y., Rauch, U., Ninomiya, Y. & Oohashi, T. Brevican distinctively assembles extracellular components at the large diameter nodes of Ranvier in the CNS. J. Neurochem. 108, 1266–1276 (2009).
Dours-Zimmermann, M. T. et al. Versican V2 assembles the extracellular matrix surrounding the nodes of ranvier in the CNS. J. Neurosci. 29, 7731–7742 (2009).
Carulli, D., Kwok, J. C. & Pizzorusso, T. Perineuronal nets and CNS plasticity and repair. Neural Plast. 2016, 4327082 (2016).
Kwok, J. C., Dick, G., Wang, D. & Fawcett, J. W. Extracellular matrix and perineuronal nets in CNS repair. Dev. Neurobiol. 71, 1073–1089 (2011).
Bruckner, G., Szeoke, S., Pavlica, S., Grosche, J. & Kacza, J. Axon initial segment ensheathed by extracellular matrix in perineuronal nets. Neuroscience 138, 365–375 (2006).
John, N. et al. Brevican-containing perineuronal nets of extracellular matrix in dissociated hippocampal primary cultures. Mol. Cell. Neurosci. 31, 774–784 (2006).
Hedstrom, K. L. et al. Neurofascin assembles a specialized extracellular matrix at the axon initial segment. J. Cell Biol. 178, 875–886 (2007).
Bekku, Y. & Oohashi, T. Neurocan contributes to the molecular heterogeneity of the perinodal ECM. Arch. Histol. Cytol. 73, 95–102 (2010).
Bekku, Y. et al. Bral1: its role in diffusion barrier formation and conduction velocity in the CNS. J. Neurosci. 30, 3113–3123 (2010). The link protein HAPLN2 (also known as BRAL1) participates in the formation of the perinodal ECM. In its absence, the perinodal ECM does not form normally and axon conduction velocity is decreased.
Susuki, K. et al. Three mechanisms assemble central nervous system nodes of Ranvier. Neuron 78, 469–482 (2013).
Elliott, L. T. et al. Genome-wide association studies of brain imaging phenotypes in UK Biobank. Nature 562, 210–216 (2018).
Eshed, Y., Feinberg, K., Carey, D. J. & Peles, E. Secreted gliomedin is a perinodal matrix component of peripheral nerves. J. Cell Biol. 177, 551–562 (2007).
Bandtlow, C. E. & Zimmermann, D. R. Proteoglycans in the developing brain: new conceptual insights for old proteins. Physiol. Rev. 80, 1267–1290 (2000).
Hunter, G. K., Wong, K. S. & Kim, J. J. Binding of calcium to glycosaminoglycans: an equilibrium dialysis study. Arch. Biochem. Biophys. 260, 161–167 (1988).
Lopreore, C. L. et al. Computational modeling of three-dimensional electrodiffusion in biological systems: application to the node of Ranvier. Biophys. J. 95, 2624–2635 (2008).
Takada, W., Fukushima, M., Pothacharoen, P., Kongtawelert, P. & Sugahara, K. A sulfated glycosaminoglycan array for molecular interactions between glycosaminoglycans and growth factors or anti-glycosaminoglycan antibodies. Anal. Biochem. 435, 123–130 (2013).
Kitagawa, H., Tsutsumi, K., Tone, Y. & Sugahara, K. Developmental regulation of the sulfation profile of chondroitin sulfate chains in the chicken embryo brain. J. Biol. Chem. 272, 31377–31381 (1997).
Foscarin, S., Raha-Chowdhury, R., Fawcett, J. W. & Kwok, J. C. F. Brain ageing changes proteoglycan sulfation, rendering perineuronal nets more inhibitory. Aging 9, 1607–1622 (2017).
Wang, H. et al. Chondroitin-4-sulfation negatively regulates axonal guidance and growth. J. Cell Sci. 121, 3083–3091 (2008).
Lin, R., Rosahl, T. W., Whiting, P. J., Fawcett, J. W. & Kwok, J. C. 6-Sulphated chondroitins have a positive influence on axonal regeneration. PLOS ONE 6, e21499 (2011).
Sahu, S., Li, R., Loers, G. & Schachner, M. Knockdown of chondroitin-4-sulfotransferase-1, but not of dermatan-4-sulfotransferase-1, accelerates regeneration of zebrafish after spinal cord injury. FASEB J. 33, 2252–2262 (2019).
Caroni, P. Inhibitory microcircuit modules in hippocampal learning. Curr. Opin. Neurobiol. 35, 66–73 (2015).
Caroni, P., Chowdhury, A. & Lahr, M. Synapse rearrangements upon learning: from divergent-sparse connectivity to dedicated sub-circuits. Trends Neurosci. 37, 604–614 (2014).
Letzkus, J. J., Wolff, S. B. & Luthi, A. Disinhibition, a circuit mechanism for associative learning and memory. Neuron 88, 264–276 (2015).
Donato, F., Chowdhury, A., Lahr, M. & Caroni, P. Early- and late-born parvalbumin basket cell subpopulations exhibiting distinct regulation and roles in learning. Neuron 85, 770–786 (2015).
Acknowledgements
The authors’ work is supported by the UK Medical Research Council; the Christopher and Dana Reeve Foundation; the International Foundation for Research in Paraplegia; the EU European Research Area Networks (ERA-NET) AxonRepair project; the European Research Council; the Czech Centre of Reconstructive Neuroscience; the Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT; grant number 26110713); and the Mizutani Foundation for Glycoscience.
Reviewer information
Nature Reviews Neuroscience thanks H. Kitagawa and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Extracellular matrix
-
(ECM). A diffuse extracellular matrix that surrounds the cells of the brain and is specialized around some neurons into a much more compacted structure, the perineuronal nets.
- Nodes of Ranvier
-
The gaps between myelinating glia at which the naked axonal membrane fires action potentials.
- Chondroitin sulfate proteoglycan
-
(CSPG). A molecule that consists of a protein core with a varying number of chondroitin sulfate chains attached through a covalent link to serine.
- Glycosaminoglycan
-
(GAG). One of the repeating disaccharides that form the carbohydrate chains of heparin sulfate and chondroitin sulfate proteoglycans and hyaluronan.
- Critical periods
-
Periods of enhanced plasticity at the end of neural development during which the final pattern of CNS connectivity is refined. They are followed by critical period closure when plasticity declines to the adult level.
- Sulfation
-
The addition of a sulfo group to a molecule. Chondroitin sulfate glycosaminoglycan chains are sulfated at the 4, 6, 2–6 and 4–6 positions. Sulfation motifs can give charge structures that define specific binding sites.
- Ocular dominance plasticity
-
A phenomenon in which, when one mammalian eye is disadvantaged during the critical period by eye closure or an equivalent intervention, the projections from that eye in the brain lose in the competition for space to projections from the other eye, which leads to increased innervation from the non-deprived eye.
- Long-term potentiation
-
(LTP). An increase in the postsynaptic potential caused by an input that occurs when this and another input to the same neuron are active simultaneously.
- Long-term depression
-
(LTD). A decline in the postsynaptic potential caused by an input following prolonged stimulation.
- Oxidative stress
-
Active metabolic events that can lead to the release of various free radicals and other oxidant molecules, usually from mitochondria.
Rights and permissions
About this article
Cite this article
Fawcett, J.W., Oohashi, T. & Pizzorusso, T. The roles of perineuronal nets and the perinodal extracellular matrix in neuronal function. Nat Rev Neurosci 20, 451–465 (2019). https://doi.org/10.1038/s41583-019-0196-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41583-019-0196-3
This article is cited by
-
Collagen in the central nervous system: contributions to neurodegeneration and promise as a therapeutic target
Molecular Neurodegeneration (2024)
-
Intersectional strategy to study cortical inhibitory parvalbumin-expressing interneurons
Scientific Reports (2024)
-
Coexpression network analysis of the adult brain sheds light on the pathogenic mechanism of DDR1 in schizophrenia and bipolar disorder
Translational Psychiatry (2024)
-
Erythropoietin restrains the inhibitory potential of interneurons in the mouse hippocampus
Molecular Psychiatry (2024)
-
Choice impulsivity after repeated social stress is associated with increased perineuronal nets in the medial prefrontal cortex
Scientific Reports (2024)