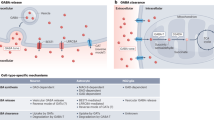
Abstract
Cellular mechanisms that regulate the interplay of synaptic excitation and inhibition are thought to be central to the functional stability of healthy neuronal circuits. A growing body of literature demonstrates the capacity for inhibitory GABAergic synapses to exhibit long-term plasticity in response to changes in neuronal activity. Here, we review this expanding field of research, focusing on the diversity of mechanisms that link glutamatergic signalling, postsynaptic action potentials and inhibitory synaptic strength. Several lines of evidence indicate that multiple, parallel forms of plasticity serve to regulate activity at both the input and output domains of individual neurons. Overall, these varied phenomena serve to promote both stability and flexibility over the life of the organism.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Lisman, J. Glutamatergic synapses are structurally and biochemically complex because of multiple plasticity processes: long-term potentiation, long-term depression, short-term potentiation and scaling. Philos. Trans. R. Soc. Lond. B Biol. Sci. 372, 20160260 (2017).
Malenka, R. C. & Bear, M. F. LTP and LTD: an embarrassment of riches. Neuron 44, 5–21 (2004).
Castillo, P. E., Chiu, C. Q. & Carroll, R. C. Long-term plasticity at inhibitory synapses. Curr. Opin. Neurobiol. 21, 328–338 (2011).
Kullmann, D. M., Moreau, A. W., Bakiri, Y. & Nicholson, E. Plasticity of inhibition. Neuron 75, 951–962 (2012).
Griffen, T. C. & Maffei, A. GABAergic synapses: their plasticity and role in sensory cortex. Front. Cell Neurosci. 8, 91 (2014).
Isaacson, J. S. & Scanziani, M. How inhibition shapes cortical activity. Neuron 72, 231–243 (2011).
Gogolla, N. et al. Common circuit defect of excitatory-inhibitory balance in mouse models of autism. J. Neurodev. Disord. 1, 172–181 (2009).
Lewis, D. A. & Hashimoto, T. Deciphering the disease process of schizophrenia: the contribution of cortical GABA neurons. Int. Rev. Neurobiol. 78, 109–131 (2007).
Higley, M. J. & Contreras, D. Balanced excitation and inhibition determine spike timing during frequency adaptation. J. Neurosci. 26, 448–457 (2006).
Wehr, M. & Zador, A. M. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature 426, 442–446 (2003).
Okun, M. & Lampl, I. Instantaneous correlation of excitation and inhibition during ongoing and sensory-evoked activities. Nat. Neurosci. 11, 535–537 (2008).
Wilent, W. B. & Contreras, D. Dynamics of excitation and inhibition underlying stimulus selectivity in rat somatosensory cortex. Nat. Neurosci. 8, 1364–1370 (2005).
Turrigiano, G. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annu. Rev. Neurosci. 34, 89–103 (2011).
Xue, M., Atallah, B. V. & Scanziani, M. Equalizing excitation-inhibition ratios across visual cortical neurons. Nature 511, 596–600 (2014).This study demonstrates that in vivo manipulation of neuronal output selectively modifies perisomatic inhibition in the neocortex.
Chen, J. L. et al. Clustered dynamics of inhibitory synapses and dendritic spines in the adult neocortex. Neuron 74, 361–373 (2012).This is one of two studies to first demonstrate the in vivo structural dynamics of dendritic GABAergic synapses on cortical PNs.
Chiu, C. Q. et al. Compartmentalization of GABAergic inhibition by dendritic spines. Science 340, 759–762 (2013).This work provides the first evidence that GABAergic inhibition can modulate electrical and biochemical signalling in highly localized dendritic compartments.
Kubota, Y., Hatada, S., Kondo, S., Karube, F. & Kawaguchi, Y. Neocortical inhibitory terminals innervate dendritic spines targeted by thalamocortical afferents. J. Neurosci. 27, 1139–1150 (2007).
Ascoli, G. A. et al. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat. Rev. Neurosci. 9, 557–568 (2008).
Rudy, B., Fishell, G., Lee, S. & Hjerling-Leffler, J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev. Neurobiol. 71, 45–61 (2011).
Cardin, J. A. et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459, 663–667 (2009).
Pouille, F. & Scanziani, M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science 293, 1159–1163 (2001).
Cardin, J. A. Snapshots of the brain in action: local circuit operations through the lens of gamma oscillations. J. Neurosci. 36, 10496–10504 (2016).
Sohal, V. S., Zhang, F., Yizhar, O. & Deisseroth, K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459, 698–702 (2009).
Pelkey, K. A. et al. Hippocampal GABAergic inhibitory interneurons. Physiol. Rev. 97, 1619–1747 (2017).
Chevaleyre, V., Takahashi, K. A. & Castillo, P. E. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu. Rev. Neurosci. 29, 37–76 (2006).
Lee, S. H., Foldy, C. & Soltesz, I. Distinct endocannabinoid control of GABA release at perisomatic and dendritic synapses in the hippocampus. J. Neurosci. 30, 7993–8000 (2010).
Glickfeld, L. L., Atallah, B. V. & Scanziani, M. Complementary modulation of somatic inhibition by opioids and cannabinoids. J. Neurosci. 28, 1824–1832 (2008).
Pouzat, C. & Hestrin, S. Developmental regulation of basket/stellate cell→Purkinje cell synapses in the cerebellum. J. Neurosci. 17, 9104–9112 (1997).
He, Q. et al. Interneuron- and GABAA receptor-specific inhibitory synaptic plasticity in cerebellar Purkinje cells. Nat. Commun. 6, 7364 (2015).This study demonstrates that cerebellar GABAergic plasticity is dependent on the identity of the presynaptic interneuron.
Higley, M. J. Localized GABAergic inhibition of dendritic Ca2+ signalling. Nat. Rev. Neurosci. 15, 567–572 (2014).
Muller, C. & Remy, S. Dendritic inhibition mediated by O-LM and bistratified interneurons in the hippocampus. Front. Synaptic Neurosci. 6, 23 (2014).
Straub, C. et al. Principles of synaptic organization of GABAergic interneurons in the striatum. Neuron 92, 84–92 (2016).
Mullner, F. E., Wierenga, C. J. & Bonhoeffer, T. Precision of inhibition: dendritic inhibition by individual GABAergic synapses on hippocampal pyramidal cells is confined in space and time. Neuron 87, 576–589 (2015).
Hayama, T. et al. GABA promotes the competitive selection of dendritic spines by controlling local Ca2+ signaling. Nat. Neurosci. 16, 1409–1416 (2013).
Murayama, M. et al. Dendritic encoding of sensory stimuli controlled by deep cortical interneurons. Nature 457, 1137–1141 (2009).
Veit, J., Hakim, R., Jadi, M. P., Sejnowski, T. J. & Adesnik, H. Cortical gamma band synchronization through somatostatin interneurons. Nat. Neurosci. 20, 951–959 (2017).
Olah, S. et al. Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature 461, 1278–1281 (2009).
Overstreet-Wadiche, L. & McBain, C. J. Neurogliaform cells in cortical circuits. Nat. Rev. Neurosci. 16, 458–468 (2015).
Tamas, G., Lorincz, A., Simon, A. & Szabadics, J. Identified sources and targets of slow inhibition in the neocortex. Science 299, 1902–1905 (2003).
Pfeffer, C. K., Xue, M., He, M., Huang, Z. J. & Scanziani, M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat. Neurosci. 16, 1068–1076 (2013).
Chiu, C. Q. et al. Input-specific NMDAR-dependent potentiation of dendritic GABAergic inhibition. Neuron 97, 368–377 (2018).This study demonstrates that dendritic GABAergic synapses formed by somatostatin-expressing interneurons in the neocortex are selectively potentiated in response to NMDAR signalling.
Zhou, X., Rickmann, M., Hafner, G. & Staiger, J. F. Subcellular targeting of VIP boutons in mouse barrel cortex is layer-dependent and not restricted to interneurons. Cereb. Cortex 27, 5353–5368 (2017).
Farrant, M. & Nusser, Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat. Rev. Neurosci. 6, 215–229 (2005).
Vithlani, M. & Moss, S. J. The role of GABAAR phosphorylation in the construction of inhibitory synapses and the efficacy of neuronal inhibition. Biochem. Soc. Trans. 37, 1355–1358 (2009).
Fritschy, J. M., Harvey, R. J. & Schwarz, G. Gephyrin: where do we stand, where do we go? Trends Neurosci. 31, 257–264 (2008).
Tretter, V. et al. Gephyrin, the enigmatic organizer at GABAergic synapses. Front. Cell Neurosci. 6, 23 (2012).
Tyagarajan, S. K. & Fritschy, J. M. Gephyrin: a master regulator of neuronal function? Nat. Rev. Neurosci. 15, 141–156 (2014).
Essrich, C., Lorez, M., Benson, J. A., Fritschy, J. M. & Luscher, B. Postsynaptic clustering of major GABAA receptor subtypes requires the γ2 subunit and gephyrin. Nat. Neurosci. 1, 563–571 (1998).
Kneussel, M. et al. Loss of postsynaptic GABAA receptor clustering in gephyrin-deficient mice. J. Neurosci. 19, 9289–9297 (1999).
Levi, S., Logan, S. M., Tovar, K. R. & Craig, A. M. Gephyrin is critical for glycine receptor clustering but not for the formation of functional GABAergic synapses in hippocampal neurons. J. Neurosci. 24, 207–217 (2004).
O’Sullivan, G. A. et al. Forebrain-specific loss of synaptic GABAA receptors results in altered neuronal excitability and synaptic plasticity in mice. Mol. Cell. Neurosci. 72, 101–113 (2016).
Fritschy, J. M., Panzanelli, P. & Tyagarajan, S. K. Molecular and functional heterogeneity of GABAergic synapses. Cell. Mol. Life Sci. 69, 2485–2499 (2012).
Panzanelli, P., Fruh, S. & Fritschy, J. M. Differential role of GABAA receptors and neuroligin 2 for perisomatic GABAergic synapse formation in the hippocampus. Brain Struct. Funct. 222, 4149–4161 (2017).
Saiepour, L. et al. Complex role of collybistin and gephyrin in GABAA receptor clustering. J. Biol. Chem. 285, 29623–29631 (2010).
Hines, R. M. et al. Developmental seizures and mortality result from reducing GABAA receptor alpha2-subunit interaction with collybistin. Nat. Commun. 9, 3130 (2018).
Tyagarajan, S. K., Ghosh, H., Harvey, K. & Fritschy, J. M. Collybistin splice variants differentially interact with gephyrin and Cdc42 to regulate gephyrin clustering at GABAergic synapses. J. Cell Sci. 124, 2786–2796 (2011).
Xiang, S. et al. The crystal structure of Cdc42 in complex with collybistin II, a gephyrin-interacting guanine nucleotide exchange factor. J. Mol. Biol. 359, 35–46 (2006).
Papadopoulos, T. et al. Impaired GABAergic transmission and altered hippocampal synaptic plasticity in collybistin-deficient mice. EMBO J. 26, 3888–3899 (2007).
Varoqueaux, F., Jamain, S. & Brose, N. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur. J. Cell Biol. 83, 449–456 (2004).
Poulopoulos, A. et al. Neuroligin 2 drives postsynaptic assembly at perisomatic inhibitory synapses through gephyrin and collybistin. Neuron 63, 628–642 (2009).This is one of the first studies to suggest molecular heterogeneity across perisomatic and dendritic GABAergic synapses.
Fritschy, J. M. & Tyagarajan, S. K. GABAergic synaptogenesis: a case for cooperation. Neuron 96, 709–711 (2017).
Li, J. et al. Molecular dissection of neuroligin 2 and Slitrk3 reveals an essential framework for GABAergic synapse development. Neuron 96, 808–826 (2017).
Woo, J. et al. The adhesion protein IgSF9b is coupled to neuroligin 2 via S-SCAM to promote inhibitory synapse development. J. Cell Biol. 201, 929–944 (2013).
Knuesel, I. et al. Short communication: altered synaptic clustering of GABAA receptors in mice lacking dystrophin (mdx mice). Eur. J. Neurosci. 11, 4457–4462 (1999).
Panzanelli, P. et al. Distinct mechanisms regulate GABAA receptor and gephyrin clustering at perisomatic and axo-axonic synapses on CA1 pyramidal cells. J. Physiol. 589, 4959–4980 (2011).
Fruh, S. et al. Neuronal dystroglycan is necessary for formation and maintenance of functional CCK-positive basket cell terminals on pyramidal cells. J. Neurosci. 36, 10296–10313 (2016).
Sumita, K. et al. Synaptic scaffolding molecule (S-SCAM) membrane-associated guanylate kinase with inverted organization (MAGI)-2 is associated with cell adhesion molecules at inhibitory synapses in rat hippocampal neurons. J. Neurochem. 100, 154–166 (2007).
Davenport, E. C. et al. An essential role for the tetraspanin LHFPL4 in the cell-type-specific targeting and clustering of synaptic GABAA receptors. Cell Rep. 21, 70–83 (2017).
Yamasaki, T., Hoyos-Ramirez, E., Martenson, J. S., Morimoto-Tomita, M. & Tomita, S. GARLH family proteins stabilize GABAA receptors at synapses. Neuron 93, 1138–1152 (2017).This is the first study to identify GARLH4/LHFPL4 as an auxiliary subunit for the GABA A R that, along with NL2, modulates inhibitory synaptic clustering.
Turrigiano, G. G. Homeostatic plasticity in neuronal networks: the more things change, the more they stay the same. Trends Neurosci. 22, 221–227 (1999).
Hartman, K. N., Pal, S. K., Burrone, J. & Murthy, V. N. Activity-dependent regulation of inhibitory synaptic transmission in hippocampal neurons. Nat. Neurosci. 9, 642–649 (2006).
Peng, Y. R. et al. Postsynaptic spiking homeostatically induces cell-autonomous regulation of inhibitory inputs via retrograde signaling. J. Neurosci. 30, 16220–16231 (2010).
Ibata, K., Sun, Q. & Turrigiano, G. G. Rapid synaptic scaling induced by changes in postsynaptic firing. Neuron 57, 819–826 (2008).
Rannals, M. D. & Kapur, J. Homeostatic strengthening of inhibitory synapses is mediated by the accumulation of GABAA receptors. J. Neurosci. 31, 17701–17712 (2011).
Swanwick, C. C., Murthy, N. R. & Kapur, J. Activity-dependent scaling of GABAergic synapse strength is regulated by brain-derived neurotrophic factor. Mol. Cell Neurosci. 31, 481–492 (2006).
Lourenco, J. et al. Non-associative potentiation of perisomatic inhibition alters the temporal coding of neocortical layer 5 pyramidal neurons. PLOS Biol. 12, e1001903 (2014).This study provides a novel mechanism linking postsynaptic spiking with the plasticity of perisomatic inhibition.
Vruwink, M., Schmidt, H. H., Weinberg, R. J. & Burette, A. Substance P and nitric oxide signaling in cerebral cortex: anatomical evidence for reciprocal signaling between two classes of interneurons. J. Comp. Neurol. 441, 288–301 (2001).
Jiao, Y. et al. A key mechanism underlying sensory experience-dependent maturation of neocortical GABAergic circuits in vivo. Proc. Natl Acad. Sci. USA 108, 12131–12136 (2011).
Sun, Q. Q. et al. Functional and structural specific roles of activity-driven BDNF within circuits formed by single spiny stellate neurons of the barrel cortex. Front. Cell Neurosci. 8, 372 (2014).
Kuczewski, N. et al. Spontaneous glutamatergic activity induces a BDNF-dependent potentiation of GABAergic synapses in the newborn rat hippocampus. J. Physiol. 586, 5119–5128 (2008).
Inagaki, T. et al. Brain-derived neurotrophic factor-mediated retrograde signaling required for the induction of long-term potentiation at inhibitory synapses of visual cortical pyramidal neurons. Neurosci. Res. 61, 192–200 (2008).
Diana, M. A. & Marty, A. Endocannabinoid-mediated short-term synaptic plasticity: depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE). Br. J. Pharmacol. 142, 9–19 (2004).
Kreitzer, A. C. & Regehr, W. G. Cerebellar depolarization-induced suppression of inhibition is mediated by endogenous cannabinoids. J. Neurosci. 21, RC174 (2001).
Klausberger, T. et al. Complementary roles of cholecystokinin- and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations. J. Neurosci. 25, 9782–9793 (2005).
Daw, M. I., Tricoire, L., Erdelyi, F., Szabo, G. & McBain, C. J. Asynchronous transmitter release from cholecystokinin-containing inhibitory interneurons is widespread and target-cell independent. J. Neurosci. 29, 11112–11122 (2009).
Trettel, J., Fortin, D. A. & Levine, E. S. Endocannabinoid signalling selectively targets perisomatic inhibitory inputs to pyramidal neurones in juvenile mouse neocortex. J. Physiol. 556, 95–107 (2004).
Garkun, Y. & Maffei, A. Cannabinoid-dependent potentiation of inhibition at eye opening in mouse V1. Front. Cell Neurosci. 8, 46 (2014).
Kurotani, T., Yamada, K., Yoshimura, Y., Crair, M. C. & Komatsu, Y. State-dependent bidirectional modification of somatic inhibition in neocortical pyramidal cells. Neuron 57, 905–916 (2008).
Kurotani, T., Yoshimura, Y. & Komatsu, Y. Postsynaptic firing produces long-term depression at inhibitory synapses of rat visual cortex. Neurosci. Lett. 337, 1–4 (2003).
Kano, M., Rexhausen, U., Dreessen, J. & Konnerth, A. Synaptic excitation produces a long-lasting rebound potentiation of inhibitory synaptic signals in cerebellar Purkinje cells. Nature 356, 601–604 (1992).
Holmgren, C. D. & Zilberter, Y. Coincident spiking activity induces long-term changes in inhibition of neocortical pyramidal cells. J. Neurosci. 21, 8270–8277 (2001).
Aizenman, C. D., Manis, P. B. & Linden, D. J. Polarity of long-term synaptic gain change is related to postsynaptic spike firing at a cerebellar inhibitory synapse. Neuron 21, 827–835 (1998).
Koester, H. J. & Sakmann, B. Calcium dynamics in single spines during coincident pre- and postsynaptic activity depend on relative timing of back-propagating action potentials and subthreshold excitatory postsynaptic potentials. Proc. Natl Acad. Sci. USA 95, 9596–9601 (1998).
Maffei, A., Nataraj, K., Nelson, S. B. & Turrigiano, G. G. Potentiation of cortical inhibition by visual deprivation. Nature 443, 81–84 (2006).This is one of the earliest reports of inhibitory synaptic plasticity in vivo.
Sivakumaran, S., Mohajerani, M. H. & Cherubini, E. At immature mossy-fiber-CA3 synapses, correlated presynaptic and postsynaptic activity persistently enhances GABA release and network excitability via BDNF and cAMP-dependent PKA. J. Neurosci. 29, 2637–2647 (2009).
Woodin, M. A., Ganguly, K. & Poo, M. M. Coincident pre- and postsynaptic activity modifies GABAergic synapses by postsynaptic changes in Cl− transporter activity. Neuron 39, 807–820 (2003).
Chevaleyre, V. & Castillo, P. E. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron 38, 461–472 (2003).This is one of the first studies to demonstrate a mechanism for long-term presynaptic plasticity at GABAergic synapses.
Chevaleyre, V., Heifets, B. D., Kaeser, P. S., Sudhof, T. C. & Castillo, P. E. Endocannabinoid-mediated long-term plasticity requires cAMP/PKA signaling and RIM1α. Neuron 54, 801–812 (2007).
Heifets, B. D., Chevaleyre, V. & Castillo, P. E. Interneuron activity controls endocannabinoid-mediated presynaptic plasticity through calcineurin. Proc. Natl Acad. Sci. USA 105, 10250–10255 (2008).
Battaglia, S. et al. Activity-dependent inhibitory synapse scaling is determined by gephyrin phosphorylation and subsequent regulation of GABAA receptor diffusion. eNeuro https://doi.org/10.1523/ENEURO.0203-17.2017 (2018).
Flores, C. E. et al. Activity-dependent inhibitory synapse remodeling through gephyrin phosphorylation. Proc. Natl Acad. Sci. USA 112, E65–E72 (2015).
Petrini, E. M. et al. Synaptic recruitment of gephyrin regulates surface GABAA receptor dynamics for the expression of inhibitory LTP. Nat. Commun. 5, 3921 (2014).This is the first study demonstrating that the potentiation of GABAergic synapses is associated with decreased lateral mobility of synaptic GABA A Rs.
Pennacchietti, F. et al. Nanoscale molecular reorganization of the inhibitory postsynaptic density is a determinant of GABAergic synaptic potentiation. J. Neurosci. 37, 1747–1756 (2017).
Marsden, K. C., Beattie, J. B., Friedenthal, J. & Carroll, R. C. NMDA receptor activation potentiates inhibitory transmission through GABA receptor-associated protein-dependent exocytosis of GABAA receptors. J. Neurosci. 27, 14326–14337 (2007).This is one of the first studies to show that glutamatergic signalling can produce long-term potentiation of GABAergic synapses.
Muir, J. et al. NMDA receptors regulate GABAA receptor lateral mobility and clustering at inhibitory synapses through serine 327 on the γ2 subunit. Proc. Natl Acad. Sci. USA 107, 16679–16684 (2010).
Houston, C. M., He, Q. & Smart, T. G. CaMKII phosphorylation of the GABAA receptor: receptor subtype- and synapse-specific modulation. J. Physiol. 587, 2115–2125 (2009).
Houston, C. M., Hosie, A. M. & Smart, T. G. Distinct regulation of β2 and β3 subunit-containing cerebellar synaptic GABAA receptors by calcium/calmodulin-dependent protein kinase II. J. Neurosci. 28, 7574–7584 (2008).
Houston, C. M., Lee, H. H., Hosie, A. M., Moss, S. J. & Smart, T. G. Identification of the sites for CaMK-II-dependent phosphorylation of GABAA receptors. J. Biol. Chem. 282, 17855–17865 (2007).
McDonald, B. J. & Moss, S. J. Conserved phosphorylation of the intracellular domains of GABAA receptor β2 and β3 subunits by cAMP-dependent protein kinase, cGMP-dependent protein kinase protein kinase C and Ca2+/calmodulin type II-dependent protein kinase. Neuropharmacology 36, 1377–1385 (1997).
Bannai, H. et al. Activity-dependent tuning of inhibitory neurotransmission based on GABAAR diffusion dynamics. Neuron 62, 670–682 (2009).
Niwa, F. et al. Gephyrin-independent GABAAR mobility and clustering during plasticity. PLOS ONE 7, e36148 (2012).
Marsden, K. C., Shemesh, A., Bayer, K. U. & Carroll, R. C. Selective translocation of Ca2+/calmodulin protein kinase IIalpha (CaMKIIα) to inhibitory synapses. Proc. Natl Acad. Sci. USA 107, 20559–20564 (2010).
Beattie, E. C. et al. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat. Neurosci. 3, 1291–1300 (2000).
Bannai, H. et al. Bidirectional control of synaptic GABAAR clustering by glutamate and calcium. Cell Rep. 13, 2768–2780 (2015).
Choquet, D. & Triller, A. The dynamic synapse. Neuron 80, 691–703 (2013).
Petrini, E. M. & Barberis, A. Diffusion dynamics of synaptic molecules during inhibitory postsynaptic plasticity. Front. Cell Neurosci. 8, 300 (2014).
de Luca, E. et al. Inter-synaptic lateral diffusion of GABAA receptors shapes inhibitory synaptic currents. Neuron 95, 63–69 (2017).This study demonstrates that the lateral mobility of GABA A Rs can functionally link adjacent inhibitory synapses.
Petrini, E. M. et al. Influence of GABAAR monoliganded states on GABAergic responses. J. Neurosci. 31, 1752–1761 (2011).
Jiang, L., Kang, D. & Kang, J. Potentiation of tonic GABAergic inhibition by activation of postsynaptic kainate receptors. Neuroscience 298, 448–454 (2015).
Gu, X., Zhou, L. & Lu, W. An NMDA receptor-dependent mechanism underlies inhibitory synapse development. Cell Rep. 14, 471–478 (2016).
Jaenisch, N. et al. Reduced tonic inhibition after stroke promotes motor performance and epileptic seizures. Sci. Rep. 6, 26173 (2016).
Golovko, T. et al. Control of inhibition by the direct action of cannabinoids on GABAA receptors. Cereb. Cortex 25, 2440–2455 (2015).
Sigel, E. et al. The major central endocannabinoid directly acts at GABAA receptors. Proc. Natl Acad. Sci. USA 108, 18150–18155 (2011).
Gasulla, J. & Calvo, D. J. Enhancement of tonic and phasic GABAergic currents following nitric oxide synthase inhibition in hippocampal CA1 pyramidal neurons. Neurosci. Lett. 590, 29–34 (2015).
Dominguez, S., Fernandez de Sevilla, D. & Buno, W. Muscarinic long-term enhancement of tonic and phasic GABAA inhibition in rat CA1 pyramidal neurons. Front. Cell Neurosci. 10, 244 (2016).
Bettler, B., Kaupmann, K., Mosbacher, J. & Gassmann, M. Molecular structure and physiological functions of GABAB receptors. Physiol. Rev. 84, 835–867 (2004).
Couve, A., Moss, S. J. & Pangalos, M. N. GABAB receptors: a new paradigm in G protein signaling. Mol. Cell. Neurosci. 16, 296–312 (2000).
Terunuma, M., Pangalos, M. N. & Moss, S. J. Functional modulation of GABAB receptors by protein kinases and receptor trafficking. Adv. Pharmacol. 58, 113–122 (2010).
Lecca, S., Trusel, M. & Mameli, M. Footshock-induced plasticity of GABAB signalling in the lateral habenula requires dopamine and glucocorticoid receptors. Synapse 71, e21948 (2017).
Chandler, K. E. et al. Plasticity of GABAB receptor-mediated heterosynaptic interactions at mossy fibers after status epilepticus. J. Neurosci. 23, 11382–11391 (2003).
Gross, G. G. et al. Recombinant probes for visualizing endogenous synaptic proteins in living neurons. Neuron 78, 971–985 (2013).
Kannan, M., Gross, G. G., Arnold, D. B. & Higley, M. J. Visual deprivation during the critical period enhances layer 2/3 GABAergic inhibition in mouse V1. J. Neurosci. 36, 5914–5919 (2016).
van Versendaal, D. et al. Elimination of inhibitory synapses is a major component of adult ocular dominance plasticity. Neuron 74, 374–383 (2012).This is one of two studies to first demonstrate the in vivo structural dynamics of dendritic GABAergic synapses on cortical PNs.
Villa, K. L. et al. Inhibitory synapses are repeatedly assembled and removed at persistent sites in vivo. Neuron 90, 662–664 (2016).
Uezu, A. et al. Identification of an elaborate complex mediating postsynaptic inhibition. Science 353, 1123–1129 (2016).
Tamas, G., Buhl, E. H. & Somogyi, P. Fast IPSPs elicited via multiple synaptic release sites by different types of GABAergic neurone in the cat visual cortex. J. Physiol. 500, 715–738 (1997).
Somogyi, P., Tamas, G., Lujan, R. & Buhl, E. H. Salient features of synaptic organisation in the cerebral cortex. Brain Res. Brain Res. Rev. 26, 113–135 (1998).
Lopez-Bendito, G. et al. Distribution of metabotropic GABA receptor subunits GABAB1a/b and GABAB2 in the rat hippocampus during prenatal and postnatal development. Hippocampus 14, 836–848 (2004).
Sabaliauskas, N., Shen, H., Homanics, G. E., Smith, S. S. & Aoki, C. Knockout of the gamma-aminobutyric acid receptor subunit alpha4 reduces functional delta-containing extrasynaptic receptors in hippocampal pyramidal cells at the onset of puberty. Brain Res. 1450, 11–23 (2012).
Serwanski, D. R. et al. Synaptic and nonsynaptic localization of GABAA receptors containing the α5 subunit in the rat brain. J. Comp. Neurol. 499, 458–470 (2006).
Wang, Y. et al. Anatomical, physiological and molecular properties of Martinotti cells in the somatosensory cortex of the juvenile rat. J. Physiol. 561, 65–90 (2004).
Kanemoto, Y. et al. Spatial distributions of GABA receptors and local inhibition of Ca2+ transients studied with GABA uncaging in the dendrites of CA1 pyramidal neurons. PLOS ONE 6, e22652 (2011).
Gidon, A. & Segev, I. Principles governing the operation of synaptic inhibition in dendrites. Neuron 75, 330–341 (2012).
Patrizi, A. et al. Synapse formation and clustering of neuroligin-2 in the absence of GABAA receptors. Proc. Natl Acad. Sci. USA 105, 13151–13156 (2008).
Tyagarajan, S. K. et al. Extracellular signal-regulated kinase and glycogen synthase kinase 3β regulate gephyrin postsynaptic aggregation and GABAergic synaptic function in a calpain-dependent mechanism. J. Biol. Chem. 288, 9634–9647 (2013).
Tyagarajan, S. K. et al. Regulation of GABAergic synapse formation and plasticity by GSK3β-dependent phosphorylation of gephyrin. Proc. Natl Acad. Sci. USA 108, 379–384 (2011).This is one of the first studies demonstrating that gephyrin phosphorylation modulates synaptic clustering.
Kuhse, J. et al. Phosphorylation of gephyrin in hippocampal neurons by cyclin-dependent kinase CDK5 at Ser-270 is dependent on collybistin. J. Biol. Chem. 287, 30952–30966 (2012).
Costa, J. T. et al. Gephyrin cleavage in in vitro brain ischemia decreases GABAA receptor clustering and contributes to neuronal death. Mol. Neurobiol. 53, 3513–3527 (2016).
Dejanovic, B. & Schwarz, G. Neuronal nitric oxide synthase-dependent S-nitrosylation of gephyrin regulates gephyrin clustering at GABAergic synapses. J. Neurosci. 34, 7763–7768 (2014).
Dejanovic, B. et al. Palmitoylation of gephyrin controls receptor clustering and plasticity of GABAergic synapses. PLOS Biol. 12, e1001908 (2014).
Ghosh, H. et al. Several posttranslational modifications act in concert to regulate gephyrin scaffolding and GABAergic transmission. Nat. Commun. 7, 13365 (2016).
Specht, C. G. et al. Quantitative nanoscopy of inhibitory synapses: counting gephyrin molecules and receptor binding sites. Neuron 79, 308–321 (2013).
Acknowledgements
The authors thank members of the Higley laboratory, J. Cardin, E. Petrini and T. Ravasenga, for critical reading and fruitful discussions during the preparation of this manuscript.
Reviewer information
Nature Reviews Neuroscience thanks B. Rudy and A. Sebastião for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all aspects of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Organotypic cultures
-
Cell culture systems prepared from slices of neonatal or early postnatal brain, in which the general synaptic and circuit architecture is preserved.
- Tonic inhibition
-
An inhibitory signal that is thought to utilize extrasynaptic receptors (not directly apposed to presynaptic release sites), may be decoupled from presynaptic spiking and occurs over long (minutes or more) periods.
- Phasic inhibition
-
An inhibitory signal that relies on postsynaptic receptors closely apposed to presynaptic release sites, is typically coupled to presynaptic action potentials and is brief (tens to hundreds of milliseconds) in duration.
Rights and permissions
About this article
Cite this article
Chiu, C.Q., Barberis, A. & Higley, M.J. Preserving the balance: diverse forms of long-term GABAergic synaptic plasticity. Nat Rev Neurosci 20, 272–281 (2019). https://doi.org/10.1038/s41583-019-0141-5
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41583-019-0141-5
This article is cited by
-
The Coordinating Role of the Actin Cytoskeleton in Short-Term Neural Network Plasticity Involving Excitatory and Inhibitory Synapses
Neuroscience and Behavioral Physiology (2024)
-
Heterosynaptic plasticity-induced modulation of synapses
The Journal of Physiological Sciences (2023)
-
GABAergic synapses onto SST and PV interneurons in the CA1 hippocampal region show cell-specific and integrin-dependent plasticity
Scientific Reports (2023)
-
The plasticitome of cortical interneurons
Nature Reviews Neuroscience (2023)
-
Synthesis and Antinociceptive Activity of GABA and Pyroglutamic Acid Short Peptides
Pharmaceutical Chemistry Journal (2022)