Abstract
Post-traumatic stress disorder (PTSD) is a prevalent, debilitating and sometimes deadly consequence of exposure to severe psychological trauma. Although effective treatments exist for some individuals, they are limited. New approaches to intervention, treatment and prevention are therefore much needed. In the past few years, the field has rapidly developed a greater understanding of the dysfunctional brain circuits underlying PTSD, a shift in understanding that has been made possible by technological revolutions that have allowed the observation and perturbation of the macrocircuits and microcircuits thought to underlie PTSD-related symptoms. These advances have allowed us to gain a more translational knowledge of PTSD, have provided further insights into the mechanisms of risk and resilience and offer promising avenues for therapeutic discovery.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Morris, D. J. The Evil Hours: A Biography of Post-Traumatic Stress Disorder. (Houghton Mifflin Harcourt, 2015).
van der Kolk, B. Interview: what is PTSD really? Surprises, twists of history, and the politics of diagnosis and treatment. Interview by Lisa M Najavits. J. Clin. Psychol. 69, 516–522 (2013).
American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-3). (APA Publishing, 1980).
Logue, M. W. et al. The Psychiatric Genomics Consortium Posttraumatic Stress Disorder Workgroup: posttraumatic stress disorder enters the age of large-scale genomic collaboration. Neuropsychopharmacology 40, 2287–2297 (2015).
Insel, T. et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry 167, 748–751 (2010).
Rauch, S. L., Shin, L. M. & Phelps, E. A. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research—past, present, and future. Biol. Psychiatry 60, 376–382 (2006).
Tovote, P., Fadok, J. P. & Luthi, A. Neuronal circuits for fear and anxiety. Nat. Rev. Neurosci. 16, 317–331 (2015).This review offers an excellent overview of current literature on fear circuitry.
Janak, P. H. & Tye, K. M. From circuits to behaviour in the amygdala. Nature 517, 284–292 (2015).
Hu, H. Reward and aversion. Annu. Rev. Neurosci. 39, 297–324 (2016).
Namburi, P., Al-Hasani, R., Calhoon, G. G., Bruchas, M. R. & Tye, K. M. Architectural representation of valence in the limbic system. Neuropsychopharmacology 41, 1697–1715 (2016).This review takes a comprehensive, forward-looking view of the basic neuroscience of valence representation in the brain.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5). (APA Publishing, 2013).
Yehuda, R. et al. Post-traumatic stress disorder. Nat. Rev. Dis. Primers 1, 15057 (2015).
Shalev, A., Liberzon, I. & Marmar, C. Post-traumatic stress disorder. N. Engl. J. Med. 376, 2459–2469 (2017).
Kessler, R. C. Posttraumatic stress disorder: the burden to the individual and to society. J. Clin. Psychiatry 61, (Suppl. 5), 4–12; discussion 13–14 (2000).
Holbrook, T. L., Hoyt, D. B., Stein, M. B. & Sieber, W. J. Gender differences in long-term posttraumatic stress disorder outcomes after major trauma: women are at higher risk of adverse outcomes than men. J. Trauma 53, 882–888 (2002).
Ehring, T. & Quack, D. Emotion regulation difficulties in trauma survivors: the role of trauma type and PTSD symptom severity. Behav. Ther. 41, 587–598 (2010).
Kelley, L. P., Weathers, F. W., McDevitt-Murphy, M. E., Eakin, D. E. & Flood, A. M. A comparison of PTSD symptom patterns in three types of civilian trauma. J. Trauma Stress 22, 227–235 (2009).
Wolf, E. J. et al. A latent class analysis of dissociation and posttraumatic stress disorder: evidence for a dissociative subtype. Arch. Gen. Psychiatry 69, 698–705 (2012). This paper offers pivotal psychometric evidence in favour of a dissociative subtype of PTSD with critical clinical implications.
Cuthbert, B. N. & Insel, T. R. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 11, 126 (2013).
Lanius, R. A. et al. Emotion modulation in PTSD: clinical and neurobiological evidence for a dissociative subtype. Am. J. Psychiatry 167, 640–647 (2010). This paper provides important biological evidence in favour of a dissociative subtype of PTSD.
Bremner, J. D. et al. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol. Psychiatry 45, 806–816 (1999).
Bremner, J. D. et al. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am. J. Psychiatry 156, 1787–1795 (1999).
Lanius, R. A. et al. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. Am. J. Psychiatry 158, 1920–1922 (2001).
Liberzon, I. et al. Brain activation in PTSD in response to trauma-related stimuli. Biol. Psychiatry 45, 817–826 (1999).
Osuch, E. A. et al. Regional cerebral blood flow correlated with flashback intensity in patients with posttraumatic stress disorder. Biol. Psychiatry 50, 246–253 (2001).
Pissiota, A. et al. Neurofunctional correlates of posttraumatic stress disorder: a PET symptom provocation study. Eur. Arch. Psychiatry Clin. Neurosci. 252, 68–75 (2002).
Rauch, S. L. et al. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script driven imagery. Arch. Gen. Psychiatry 53, 380–387 (1996). This study is one of the first to demonstrate increased limbic activation in a symptom provocation study paradigm for individuals with PTSD.
Shin, L. M. et al. Regional cerebral blood flow during scriptdriven imagery in childhood sexual abuse-related PTSD: a PET investigation. Am. J. Psychiatry 156, 575–584 (1999).
Shin, L. M. et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch. Gen. Psychiatry 61, 168–176 (2004).
Zubieta, J.-K. et al. Medial frontal cortex involvement in PTSD symptoms: a SPECT study. J. Psychiatr. Res. 33, 259–264 (1999).
Phelps, E. A., D. M., Nearing, K. I. & LeDoux, J. E. Extinction learning in humans: role of the amygdala and vmPFC. Neuron 43, 897–905 (2004).
Hopper, J. W., Frewen, P. A., van der Kolk, B. A. & Lanius, R. A. Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. J. Trauma Stress 20, 713–725 (2007).
Craig, A. D. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 3, 655–666 (2002).
Phan, K. L., Britton, J. C., Taylor, S. F., Fig, L. M. & Liberzon, I. Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Arch. Gen. Psychiatry 63, 184–192 (2006).
Milad, M. R. et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol. Psychiatry 66, 1075–1082 (2009).
Milad, M. R. & Quirk, G. J. Fear extinction as a model for translational neuroscience: ten years of progress. Annu. Rev. Psychol. 63, 129–151 (2012).
Rescorla, R. A. & Heth, C. D. Reinstatement of fear to an extinguished conditioned stimulus. J. Exp. Psychol. Anim. Behav. Process 1, 88–96 (1975).
Kroes, M. C., Schiller, D., LeDoux, J. E. & Phelps, E. A. Translational approaches targeting reconsolidation. Curr. Top. Behav. Neurosci. 28, 197–230 (2016).
Garfinkel, S. N. et al. Impaired contextual modulation of memories in PTSD: an fMRI and psychophysiological study of extinction retention and fear renewal. J. Neurosci. 34, 13435–13443 (2014).
Norrholm, S. D. et al. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol. Psychiatry 69, 556–563 (2011).
Wicking, M. et al. Deficient fear extinction memory in posttraumatic stress disorder. Neurobiol. Learn. Mem. 136, 116–126 (2016).
Rauch, S. L. et al. Selectively reduced regional cortical volumes in post-traumatic stress disorder. Neuroreport 14, 913–916 (2003).
Stevens, J. S. et al. Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J. Psychiatr. Res. 47, 1469–1478 (2013).
Orr, S. P. et al. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J. Abnorm Psychol. 109, 290–298 (2000).
Kaczkurkin, A. N. et al. Neural substrates of overgeneralized conditioned fear in PTSD. Am. J. Psychiatry 174, 125–134 (2017).
Thome, J. et al. Generalisation of fear in PTSD related to prolonged childhood maltreatment: an experimental study. Psychol. Med. 28, 1–12 (2017).
Morey, R. A. et al. Fear learning circuitry is biased toward generalization of fear associations in posttraumatic stress disorder. Transl Psychiatry 5, e700 (2015).
Vogt, B. A. & Paxinos, G. Cytoarchitecture of mouse and rat cingulate cortex with human homologies. Brain Struct. Funct. 219, 185–192 (2014).
Heilbronner, S. R., Rodriguez-Romaguera, J., Quirk, G. J., Groenewegen, H. J. & Haber, S. N. Circuit-based corticostriatal homologies between rat and primate. Biol. Psychiatry 80, 509–521 (2016).
Sierra-Mercado, D., Padilla-Coreano, N. & Quirk, G. J. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology 36, 529–538 (2011).
Gourley, S. L. & Taylor, J. R. Going and stopping: dichotomies in behavioral control by the prefrontal cortex. Nat. Neurosci. 19, 656–664 (2016).
Do-Monte, F. H., Quinones-Laracuente, K. & Quirk, G. J. A temporal shift in the circuits mediating retrieval of fear memory. Nature 519, 460–463 (2015).
Courtin, J. et al. Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature 505, 92–96 (2014).
Do-Monte, F. H., Manzano-Nieves, G., Quinones-Laracuente, K., Ramos-Medina, L. & Quirk, G. J. Revisiting the role of infralimbic cortex in fear extinction with optogenetics. J. Neurosci. 35, 3607–3615 (2015).
Santini, E., Ge, H., Ren, K., Pena de Ortiz, S. & Quirk, G. J. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J. Neurosci. 24, 5704–5710 (2004).
Hong, J. & Kim, D. Freezing response-independent facilitation of fear extinction memory in the prefrontal cortex. Sci. Rep. 7, 5363 (2017).
Stein, M. B. & Paulus, M. P. Imbalance of approach and avoidance: the yin and yang of anxiety disorders. Biol. Psychiatry 66, 1072–1074 (2009).This review contains a concise theoretical discussion suggesting that PTSD is characterized by an imbalance of approach-avoidance systems.
Sripada, R. K., Garfinkel, S. N. & Liberzon, I. Avoidant symptoms in PTSD predict fear circuit activation during multimodal fear extinction. Front. Hum. Neurosci. 7, 672 (2013).
LeDoux, J. E., Moscarello, J., Sears, R. & Campese, V. The birth, death and resurrection of avoidance: a reconceptualization of a troubled paradigm. Mol. Psychiatry 22, 24–36 (2017).
Maier, S. F. Behavioral control blunts reactions to contemporaneous and future adverse events: medial prefrontal cortex plasticity and a corticostriatal network. Neurobiol. Stress 1, 12–22 (2015).
Boeke, E. A., Moscarello, J. M., LeDoux, J. E., Phelps, E. A. & Hartley, C. A. Active avoidance: neural mechanisms and attenuation of pavlovian conditioned responding. J. Neurosci. 37, 4808–4818 (2017).
Galatzer-Levy, I. R. et al. Heterogeneity in signaled active avoidance learning: substantive and methodological relevance of diversity in instrumental defensive responses to threat cues. Front. Syst. Neurosci. 8, 179 (2014).
Choi, J. S., Cain, C. K. & LeDoux, J. E. The role of amygdala nuclei in the expression of auditory signaled two-way active avoidance in rats. Learn. Mem. 17, 139–147 (2010).
Lazaro-Munoz, G., LeDoux, J. E. & Cain, C. K. Sidman instrumental avoidance initially depends on lateral and basal amygdala and is constrained by central amygdala-mediated Pavlovian processes. Biol. Psychiatry 67, 1120–1127 (2010).
Ramirez, F., Moscarello, J. M., LeDoux, J. E. & Sears, R. M. Active avoidance requires a serial basal amygdala to nucleus accumbens shell circuit. J. Neurosci. 35, 3470–3477 (2015).
Moscarello, J. M. & LeDoux, J. E. Active avoidance learning requires prefrontal suppression of amygdala-mediated defensive reactions. J. Neurosci. 33, 3815–3823 (2013).
Fadok, J. P. et al. A competitive inhibitory circuit for selection of active and passive fear responses. Nature 542, 96–100 (2017).This paper begins to elucidate the divergent neurocircuitries for active and passive fear responses.
Pellman, B. A. & Kim, J. J. What can ethobehavioral studies tell us about the brain’s fear system? Trends Neurosci. 39, 420–431 (2016).
Paré, D. & Quirk, G. J. When scientific paradigms lead to tunnel vision: lessons from the study of fear. Sci. Learn. 2, 6 (2017).
Choi, J.-S. & Kim, J. J. Amygdala regulates risk of predation in rats foraging in a dynamic fear environment. Proc. Natl Acad. Sci. USA 107, 21773–21777 (2010).
Amir, A., Lee, S. C., Headley, D. B., Herzallah, M. M. & Pare, D. Amygdala signaling during foraging in a hazardous environment. J. Neurosci. 35, 12994–13005 (2015).
Davis, M. & Whalen, P. J. The amygdala: vigilance and emotion. Mol. Psychiatry 6, 13–34 (2001).
Squire, L. R. & Zola-Morgan, S. The medial temporal lobe memory system. Science 253, 1380–1386 (1991).
Elzinga, B. M. & Bremner, J. D. Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD)? J. Affect Disord. 70, 1–17 (2002).This excellent review synthesizes the memory-related symptoms in PTSD into a neurobiological model.
Pitman, R. K. et al. Biological studies of post-traumatic stress disorder. Nat. Rev. Neurosci. 13, 769–787 (2012).
Logue, M. W. et al. Smaller hippocampal volume in posttraumatic stress disorder: a multisite ENIGMA-PGC study: subcortical volumetry results from Posttraumatic Stress Disorder Consortia. Biol. Psychiatry 83, 244–253 (2018).
Kremen, W. S., Koenen, K. C., Afari, N. & Lyons, M. J. Twin studies of posttraumatic stress disorder: differentiating vulnerability factors from sequelae. Neuropharmacology 62, 647–653 (2012).
Bremner, J. D. et al. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am. J. Psychiatry 160, 924–932 (2003).
Polak, A. R., Witteveen, A. B., Reitsma, J. B. & Olff, M. The role of executive function in posttraumatic stress disorder: a systematic review. J. Affect Disord. 141, 11–21 (2012).
Aupperle, R. L., Melrose, A. J., Stein, M. B. & Paulus, M. P. Executive function and PTSD: disengaging from trauma. Neuropharmacology 62, 686–694 (2012).
Bryant, R. A. et al. Neural networks of information processing in posttraumatic stress disorder: a functional magnetic resonance imaging study. Biol. Psychiatry 58, 111–118 (2005).
Falconer, E. et al. The neural networks of inhibitory control in posttraumatic stress disorder. J. Psychiatry Neurosci. 33, 413–422 (2008).
Moores, K. A. et al. Abnormal recruitment of working memory updating networks during maintenance of trauma-neutral information in post-traumatic stress disorder. Psychiatry Res. 163, 156–170 (2008).
Clausen, A. N. et al. PTSD and cognitive symptoms relate to inhibition-related prefrontal activation and functional connectivity. Depress. Anxiety 34, 427–436 (2017).
McEwen, B. S., Nasca, C. & Gray, J. D. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology 41, 3–23 (2016).
Gould, E. & Tanapat, P. Stress and hippocampal neurogenesis. Biol. Psychiatry 46, 1472–1479 (1999).
Gronli, J. et al. Chronic mild stress inhibits BDNF protein expression and CREB activation in the dentate gyrus but not in the hippocampus proper. Pharmacol. Biochem. Behav. 85, 842–849 (2006).
Segal, M., Richter-Levin, G. & Maggio, N. Stress-induced dynamic routing of hippocampal connectivity: a hypothesis. Hippocampus 20, 1332–1338 (2010).
Gray, J. D. et al. Translational profiling of stress-induced neuroplasticity in the CA3 pyramidal neurons of BDNF Val66Met mice. Mol. Psychiatry 23, 904–913 (2016).
Tomar, A., Polygalov, D., Chattarji, S. & McHugh, T. J. The dynamic impact of repeated stress on the hippocampal spatial map. Hippocampus 25, 38–50 (2015).
Shansky, R. M., Rubinow, K., Brennan, A. & Arnsten, A. F. The effects of sex and hormonal status on restraint-stress-induced working memory impairment. Behav. Brain Funct. 2, 8 (2006).
Benchenane, K. et al. Coherent theta oscillations and reorganization of spike timing in the hippocampal- prefrontal network upon learning. Neuron 66, 921–936 (2010).
Spellman, T. et al. Hippocampal-prefrontal input supports spatial encoding in working memory. Nature 522, 309–314 (2015).
Barrett, L. F. Are emotions natural kinds? Perspect. Psychol. Sci. 1, 28–58 (2006).
Russell, J. A. Core affect and the psychological construction of emotion. Psychol. Rev. 110, 145–172 (2003).
Kober, H. et al. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage 42, 998–1031 (2008).
Wilson-Mendenhall, C. D., Barrett, L. F. & Barsalou, L. W. Neural evidence that human emotions share core affective properties. Psychol. Sci. 24, 947–956 (2013).
Ochsner, K. N., G. J. The cognitive control of emotion. Trends Cogn. Sci. 9, 242–249 (2005).
New, A. S. et al. A functional magnetic resonance imaging study of deliberate emotion regulation in resilience and posttraumatic stress disorder. Biol. Psychiatry 66, 656–664 (2009).
Lanius, R. A. et al. Recall of emotional states in posttraumatic stress disorder: an fMRI investigation. Biol. Psychiatry 53, 204–210 (2003).
Goldin, P. R., M. K., Ramel, W. & Gross, J. J. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol. Psychiatry 63, 577–586 (2008).
Keding, T. J. & Herringa, R. J. Paradoxical prefrontal-amygdala recruitment to angry and happy expressions in pediatric posttraumatic stress disorder. Neuropsychopharmacology 41, 2903–2912 (2016).
Fonzo, G. A., Huemer, J. & Etkin, A. History of childhood maltreatment augments dorsolateral prefrontal processing of emotional valence in PTSD. J. Psychiatr. Res. 74, 45–54 (2016).
Stuber, G. D. et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475, 377–380 (2011).
Kim, J., Zhang, X., Muralidhar, S., LeBlanc, S. A. & Tonegawa, S. Basolateral to central amygdala neural circuits for appetitive behaviors. Neuron 93, 1464–1479.e5 (2017).
Airan, R. D., Thompson, K. R., Fenno, L. E., Bernstein, H. & Deisseroth, K. Temporally precise in vivo control of intracellular signalling. Nature 458, 1025–1029 (2009).
Tsai, H. C. et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 324, 1080–1084 (2009).
Redondo, R. L. et al. Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nature 513, 426–430 (2014).
Reynolds, S. M. & Berridge, K. C. Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens. Nat. Neurosci. 11, 423–425 (2008).
Der-Avakian, A. & Markou, A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 35, 68–77 (2012).
Treadway, M. T. & Zald, D. H. Parsing anhedonia: translational models of reward-processing deficits in psychopathology. Curr. Dir. Psychol. Sci. 22, 244–249 (2013).
Nawijn, L. et al. Reward functioning in PTSD: a systematic review exploring the mechanisms underlying anhedonia. Neurosci. Biobehav Rev. 51, 189–204 (2015).This paper is a meta-analytic study of reward functioning in PTSD that finds evidence for both decreased reward anticipation and approach and reduced hedonic responses.
Liu, X., Hairston, J., Schrier, M. & Fan, J. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci. Biobehav Rev. 35, 1219–1236 (2011).
Elman, I. et al. Functional neuroimaging of reward circuitry responsivity to monetary gains and losses in posttraumatic stress disorder. Biol. Psychiatry 66, 1083–1090 (2009).
Sailer, U. et al. Altered reward processing in the nucleus accumbens and mesial prefrontal cortex of patients with posttraumatic stress disorder. Neuropsychologia 46, 2836–2844 (2008).
Litz, B. Emotional numbing in combat-related post-traumatic stress disorder: a critical review and reformulation. Clin. Psychol. Rev. 12, 417–432 (1992).
Frewen, P. A. et al. Emotional numbing in posttraumatic stress disorder: a functional magnetic resonance imaging study. J. Clin. Psychiatry 73, 431–436 (2012).
Chaudhury, D. et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493, 532–536 (2013).
Tye, K. M. et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 493, 537–541 (2013).
Covington, H. E. 3rd et al. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J. Neurosci. 30, 16082–16090 (2010).
Ferenczi, E. A. et al. Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science 351, aac9698 (2016).
Liberzon, I. & Sripada, C. S. The functional neuroanatomy of PTSD: a critical review. Prog. Brain Res. 167, 151–169 (2008).
Shin, L. M. & Liberzon, I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 35, 169–191 (2010).
Sripada, R. K. et al. Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom. Med. 74, 904–911 (2012).
Terburg, D. et al. Hypervigilance for fear after basolateral amygdala damage in humans. Transl Psychiatry 2, e115 (2012).
Tye, K. M. et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471, 358–362 (2011).
Jasnow, A. M. et al. Thy1-expressing neurons in the basolateral amygdala may mediate fear inhibition. J. Neurosci. 33, 10396–10404 (2013).
McCullough, K. M. et al. Molecular characterization of Thy1 expressing fear-inhibiting neurons within the basolateral amygdala. Nat. Commun. 7, 13149 (2016).
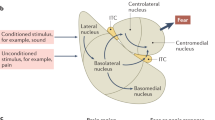
Ciocchi, S. et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 468, 277–282 (2010).This study draws attention to the existence of opposing microcircuits within the extended amygdala.
Li, H. et al. Experience-dependent modification of a central amygdala fear circuit. Nat. Neurosci. 16, 332–339 (2013).
Somerville, L. H., Whalen, P. J. & Kelley, W. M. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biol. Psychiatry 68, 416–424 (2010).
Avery, S. N., Clauss, J. A. & Blackford, J. U. The Human BNST: functional role in anxiety and addiction. Neuropsychopharmacology 41, 126–141 (2016).
Lebow, M. A. & Chen, A. Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol. Psychiatry 21, 450–463 (2016).
Kim, S. Y. et al. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature 496, 219–223 (2013).This paper begins the dissection of microcircuits within the BNST.
Rau, V., DeCola, J. P. & Fanselow, M. S. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci. Biobehav Rev. 29, 1207–1223 (2005).
Lebow, M. et al. Susceptibility to PTSD-like behavior is mediated by corticotropin-releasing factor receptor type 2 levels in the bed nucleus of the stria terminalis. J. Neurosci. 32, 6906–6916 (2012).
Blair, R. J. Psychopathy, frustration, and reactive aggression: the role of ventromedial prefrontal cortex. Br. J. Psychol. 101, 383–399 (2010).
Haden, S. C. & Scarpa, A. The noradrenergic system and its involvement in aggressive behaviors. Aggression Violent Behav. 12, 1–15 (2007).
Haller, J., Makara, G. & Kruk, M. Catecholaminergic involvement in the control of aggression: hormones, the peripheral sympathetic, and central noradrenergic systems. Neurosci. Biobehav Rev. 22, 85–97 (1997).
Gilam, G., Lin, T., Fruchter, E. & Hendler, T. Neural indicators of interpersonal anger as cause and consequence of combat training stress symptoms. Psychol. Med. 47, 1561–1572 (2017).
Davidson, R. J., Putnam, K. M. & Larson, C. L. Dysfunction in the neural circuitry of emotion regulation—a possible prelude to violence. Science 289, 591–594 (2000).
Dileo, J. F., Brewer, W. J., Hopwood, M., Anderson, V. & Creamer, M. Olfactory identification dysfunction, aggression and impulsivity in war veterans with post-traumatic stress disorder. Psychol. Med. 38, 523–531 (2008).
Siever, L. J. Neurobiology of aggression and violence. Am. J. Psychiatry 165, 429–442 (2008).
Gilam, G. et al. Neural substrates underlying the tendency to accept anger-infused ultimatum offers during dynamic social interactions. Neuroimage 120, 400–411 (2015).
Denson, T. F., Pedersen, W. C., Ronquillo, J. & Nandy, A. S. The angry brain: neural correlates of anger, angry rumination, and aggressive personality. J. Cogn. Neurosci. 21, 734–744 (2009).
Pietrini, P., Guazzelli, M., Basso, G., Jaffe, K. & Grafman, J. Neural correlates of imaginal aggressive behavior assessed by positron emission tomography in healthy subjects. Am. J. Psychiatry 157, 1772–1781 (2000).
Best, M., Williams, J. M. & Coccaro, E. F. Evidence for a dysfunctional prefrontal circuit in patients with an impulsive aggressive disorder. Proc. Natl Acad. Sci. USA 99, 8448–8453 (2002).
Blair, R. J. The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain Cogn. 55, 198–208 (2004).
Falkner, A. L., Dollar, P., Perona, P., Anderson, D. J. & Lin, D. Decoding ventromedial hypothalamic neural activity during male mouse aggression. J. Neurosci. 34, 5971–5984 (2014).
Lee, H. et al. Scalable control of mounting and attack by Esr1+ neurons in the ventromedial hypothalamus. Nature 509, 627–632 (2014).
Lin, D. et al. Functional identification of an aggression locus in the mouse hypothalamus. Nature 470, 221–226 (2011).
Han, W. et al. Integrated control of predatory hunting by the central nucleus of the amygdala. Cell 168, 311–324.e318 (2017).
Hong, W., Kim, D. W. & Anderson, D. J. Antagonistic control of social versus repetitive self-grooming behaviors by separable amygdala neuronal subsets. Cell 158, 1348–1361 (2014).
Gerfen, C. R. & Surmeier, D. J. Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci. 34, 441–466 (2011).
Lee, J. H. et al. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature 465, 788–792 (2010).
Bernal-Casas, D., Lee, H. J., Weitz, A. J. & Lee, J. H. Studying brain circuit function with dynamic causal modeling for optogenetic fMRI. Neuron 93, 522–532.e5 (2017).
Reznikov, R. & Hamani, C. Posttraumatic stress disorder: perspectives for the use of deep brain stimulation. Neuromodulation 20, 7–14 (2017).
Boggio, P. S. et al. Noninvasive brain stimulation with high-frequency and low-intensity repetitive transcranial magnetic stimulation treatment for posttraumatic stress disorder. J. Clin. Psychiatry 71, 992–999 (2010).
Cohen, H. et al. Repetitive transcranial magnetic stimulation of the right dorsolateral prefrontal cortex in posttraumatic stress disorder: a double-blind, placebo-controlled study. Am. J. Psychiatry 161, 515–524 (2004).
Watts, B. V., Landon, B., Groft, A. & Young-Xu, Y. A sham controlled study of repetitive transcranial magnetic stimulation for posttraumatic stress disorder. Brain Stimul 5, 38–43 (2012).
Taghva, A. et al. Magnetic resonance therapy improves clinical phenotype and EEG alpha power in posttraumatic stress disorder. Trauma Mon. 20, e27360 (2015).
Fonzo, G. A. et al. PTSD psychotherapy outcome predicted by brain activation during emotional reactivity and regulation. Am. J. Psychiatry 174, 1163–1174 (2017).
Rajasethupathy, P., Ferenczi, E. & Deisseroth, K. Targeting neural circuits. Cell 165, 524–534 (2016).
Admon, R., Milad, M. R. & Hendler, T. A causal model of post-traumatic stress disorder: disentangling predisposed from acquired neural abnormalities. Trends Cogn. Sci. 17, 337–347 (2013).
Shin, L. M. et al. Exaggerated activation of dorsal anterior cingulate cortex during cognitive interference: a monozygotic twin study of posttraumatic stress disorder. Am. J. Psychiatry 168, 979–985 (2011).
Heiman, M., Kulicke, R., Fenster, R. J., Greengard, P. & Heintz, N. Cell type-specific mRNA purification by translating ribosome affinity purification (TRAP). Nat. Protoc. 9, 1282–1291 (2014).
Macosko, E. Z. et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214 (2015).
Kessler, R. C., Sonnega, A., Bromet, E., Hughes, M. & Nelson, C. B. Posttraumatic stress disorder in the National Comorbidity Survey. Arch. Gen. Psychiatry 52, 1048–1060 (1995).
Afifi, T. O., Asmundson, G. J., Taylor, S. & Jang, K. L. The role of genes and environment on trauma exposure and posttraumatic stress disorder symptoms: a review of twin studies. Clin. Psychol. Rev. 30, 101–112 (2010).
Lappalainen, T. & Greally, J. M. Associating cellular epigenetic models with human phenotypes. Nat. Rev. Genet. 18, 441–451 (2017).
Daskalakis, N. P., Rijal, C. M., King, C., Huckins, L. M. & Ressler, K. J. Recent genetics and epigenetics approaches to PTSD. Curr. Psychiatry Rep. 20, 30 (2018).
Cornelis, M. C., Nugent, N. R., Amstadter, A. B. & Koenen, K. C. Genetics of post-traumatic stress disorder: review and recommendations for genome-wide association studies. Curr. Psychiatry Rep. 12, 313–326 (2010).
Tabery, J. Biometric and developmental gene-environment interactions: looking back, moving forward. Dev. Psychopathol 19, 961–976 (2007).
Fani, N. et al. FKBP5 genotype and structural integrity of the posterior cingulum. Neuropsychopharmacology 39, 1206–1213 (2014).
Lind, M. J. et al. Association of posttraumatic stress disorder with rs2267735 in the ADCYAP1R1 gene: a meta-analysis. J. Trauma Stress 30, 389–398 (2017).
Ressler, K. J. et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature 470, 492–497 (2011).
Almli, L. M. et al. A genome-wide identified risk variant for PTSD is a methylation quantitative trait locus and confers decreased cortical activation to fearful faces. Am. J. Med. Genet. B Neuropsychiatr. Genet. 168B, 327–336 (2015).
Duncan, L. E. et al. Largest GWAS of PTSD (N = 20 070) yields genetic overlap with schizophrenia and sex differences in heritability. Mol. Psychiatry 23, 666–673 (2017).
Guffanti, G. et al. Genome-wide association study implicates a novel RNA gene, the lincRNA AC068718.1, as a risk factor for post-traumatic stress disorder in women. Psychoneuroendocrinology 38, 3029–3038 (2013).
Kilaru, V. et al. Genome-wide gene-based analysis suggests an association between Neuroligin 1 (NLGN1) and post-traumatic stress disorder. Transl Psychiatry 6, e820 (2016).
Stein, M. B. et al. Genome-wide association studies of posttraumatic stress disorder in 2 cohorts of US Army soldiers. JAMA Psychiatry 73, 695–704 (2016).
Ashley-Koch, A. E. et al. Genome-wide association study of posttraumatic stress disorder in a cohort of Iraq-Afghanistan era veterans. J. Affect Disord. 184, 225–234 (2015).
Nievergelt, C. M. et al. Genomic predictors of combat stress vulnerability and resilience in U. S. Marines: a genome-wide association study across multiple ancestries implicates PRTFDC1 as a potential PTSD gene. Psychoneuroendocrinology 51, 459–471 (2015).
Liberzon, I. et al. Interaction of the ADRB2 gene polymorphism with childhood trauma in predicting adult symptoms of posttraumatic stress disorder. JAMA Psychiatry 71, 1174–1182 (2014).
Almli, L. M. et al. Follow-up and extension of a prior genome-wide association study of posttraumatic stress disorder: gene x environment associations and structural magnetic resonance imaging in a highly traumatized African-American civilian population. Biol. Psychiatry 76, e3–4 (2014).
Xie, P. et al. Genome-wide association study identifies new susceptibility loci for posttraumatic stress disorder. Biol. Psychiatry 74, 656–663 (2013).
Logue, M. W. et al. A genome-wide association study of post-traumatic stress disorder identifies the retinoid-related orphan receptor alpha (RORA) gene as a significant risk locus. Mol. Psychiatry 18, 937–942 (2013).
Sudhof, T. C. Neuroligins and neurexins link synaptic function to cognitive disease. Nature 455, 903–911 (2008).
Paul, C. et al. Signaling through cGMP-dependent protein kinase I in the amygdala is critical for auditory-cued fear memory and long-term potentiation. J. Neurosci. 28, 14202–14212 (2008).
Dell, P. F. The multidimensional inventory of dissociation (MID): a comprehensive measure of pathological dissociation. J. Trauma Dissociation 7, 77–106 (2006).
Holmes, E. A. et al. Are there two qualitatively distinct forms of dissociation? A review and some clinical implications. Clin. Psychol. Rev. 25, 1–23 (2005).
Lanius, R. A., Bluhm, R., Lanius, U. & Pain, C. A review of neuroimaging studies in PTSD: heterogeneity of response to symptom provocation. J. Psychiatr. Res. 40, 709–729 (2006).
Lanius, R. A., Hopper, J. W. & Menon, R. S. Individual differences in a husband and wife who developed PTSD after a motor vehicle accident: a functional MRI case study. Am. J. Psychiatry 160, 667–669 (2003).
Felmingham, K. et al. Dissociative responses to conscious and non-conscious fear impact underlying brain function in post-traumatic stress disorder. Psychol. med. 38, 1771–1780 (2008).
Lanius, R. A. et al. Brain activation during script-driven imagery induced dissociative responses in PTSD: a functional magnetic resonance imaging investigation. Biol. Psychiatry 52, 305–311 (2002).
Lanius, R. A. et al. Functional connectivity of dissociative responses in posttraumatic stress disorder: a functional magnetic resonance imaging investigation. Biol. Psychiatry 57, 873–884 (2005).
Pitman, R. K., Orr, S. P., Forgue, D. F., de Jong, J. B. & Claiborn, J. M. Psychophysiologic assessment of posttraumatic stress disorder imagery in Vietnam combat veterans. Arch. Gen. Psychiatry 44, 970–975 (1987).
Orr, S. P., Metzger, L. J. & Kaloupek, D. G. Psychophysiological Assessment of PTSD. Assessing psychological trauma and PTSD, 289 (2004).
Etkin, A., Egner, T. & Kalisch, R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends sci 15, 85–93 (2011).
Robinson, O. J. et al. Towards a mechanistic understanding of pathological anxiety: the dorsal medial prefrontal-amygdala ‘aversive amplification’circuit in unmedicated generalized and social anxiety disorders. Lancet. Psychiatry 1, 294 (2014).
Shin, L. M. in Neurobiology of PTSD (eds Shiromani, P. J., Keane, T. M. & LeDoux, J. E.) (Humana Press, 2009).
Nicholson, A. A. et al. The dissociative subtype of posttraumatic stress disorder: unique resting-state functional connectivity of basolateral and centromedial amygdala complexes. Neuropsychopharmacology 40, 2317–2326 (2015).
Nicholson, A. A. et al. Unique insula subregion resting-state functional connectivity with amygdala complexes in posttraumatic stress disorder and its dissociative subtype. Psychiatry Res. 250, 61–72 (2016).
Harricharan, S. et al. fMRI functional connectivity of the periaqueductal gray in PTSD and its dissociative subtype. Brain Behav. 6, e00579. (2016).
Dalenberg, C. J. et al. Evaluation of the evidence for the trauma and fantasy models of dissociation. Psychol. Bull. 138, 550–588 (2012).
Reinders, A. A. T. S. et al. One brain, two selves. Neuroimage 20, 2119–2125 (2003).
Reinders, A. S. et al. Psychobiological characteristics of dissociative identity disorder: a symptom provocation study. Biol. Psychiatry 60, 730–740 (2006).
Schiller, D. & Delgado, M. R. Overlapping neural systems mediating extinction, reversal and regulation of fear. Trends Cogn sci 14, 268–276 (2010).
Ebner-Priemer, U. W. et al. Emotional learning during dissociative states in borderline personality disorder. J. Psychiatry Neurosci. 34, 214–222 (2009).
Bae, H., Kim, D. & Park, Y. C. Dissociation predicts treatment response in eye-movement desensitization and reprocessing for posttraumatic stress disorder. J. Trauma Dissoci. 17, 112–130 (2016).
Kleindienst, N. et al. State dissociation moderates response to dialectical behavior therapy for posttraumatic stress disorder in women with and without borderline personality disorder. Eur. J. Psychotraumatol 7, 30375 (2016).
Kleindienst, N. et al. Dissociation predicts poor response to dialectial behavioral therapy in female patients with borderline personality disorder. J. Pers Disord. 25, 432–447 (2011).
Price, M., Kearns, M., Houry, D. & Rothbaum, B. O. Emergency department predictors of posttraumatic stress reduction for trauma-exposed individuals with and without an early intervention. J. Consult Clin. Psychol. 82, 336–341 (2014).
Wolf, E. J., Lunney, C. A. & Schnurr, P. P. The influence of the dissociative subtype of posttraumatic stress disorder on treatment efficacy in female veterans and active duty service members. J. Consult Clin. Psychol. 84, 95–100 (2016).
Cloitre, M., Petkova, E., Wang, J. & Lu Lassell, F. An examination of the influence of a sequential treatment on the course and impact of dissociation among women with PTSD related to childhood abuse. Depress. Anxiety 29, 709–717 (2012).
Resick, P. A., Suvak, M. K., Johnides, B. D., Mitchell, K. S. & Iverson, K. M. The impact of dissociation on PTSD treatment with cognitive processing therapy. Depress. Anxiety 29, 718–730 (2012).
Hagenaars, M. A., van Minnen, A. & Hoogduin, K. A. The impact of dissociation and depression on the efficacy of prolonged exposure treatment for PTSD. Behav. Res. Ther. 48, 19–27 (2010).
Halvorsen, J. O., Stenmark, H., Neuner, F. & Nordahl, H. M. Does dissociation moderate treatment outcomes of narrative exposure therapy for PTSD? A secondary analysis from a randomized controlled clinical trial. Behav. Res. Ther. 57, 21–28 (2014).
Jaycox, L. H., Foa, E. B. & Morral, A. R. Influence of emotional engagement and habituation on exposure therapy for PTSD. J. Consult Clin. Psychol. 66, 185–192 (1998).
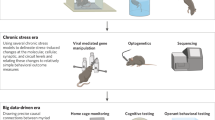
Nestler, E. J. & Hyman, S. E. Animal models of neuropsychiatric disorders. Nat. Neurosci. 13, 1161–1169 (2010).
Hendriksen, H., Olivier, B. & Oosting, R. S. From non-pharmacological treatments for post-traumatic stress disorder to novel therapeutic targets. Eur. J. Pharmacol. 732, 139–158 (2014).
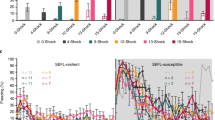
Sillivan, S. E. et al. Susceptibility and resilience to posttraumatic stress disorder-like behaviors in inbred mice. Biol. Psychiatry 82, 924–933 (2017).
Cohen, H. & Zohar, J. An animal model of posttraumatic stress disorder: the use of cut-off behavioral criteria. Ann. NY Acad. Sci. 1032, 167–178 (2004).
Acknowledgements
The work was supported by US National Institutes of Health (NIH) grants R01MH108665, R01MH094757 and R21MH112956 to K.J.R., NIH fellowship grant F32MH109274 to L.A.M.L. and the Frazier Foundation Grant for Mood and Anxiety Research to K.J.R. K.J.R. has received research funding from the US National Institute of Mental Health, the Howard Hughes Medical Institute, the National Alliance for Research on Schizophrenia & Depression and the Burroughs Wellcome Foundation.
Author information
Authors and Affiliations
Contributions
R.J.F., L.A.M.L., K.J.R. and J.S. researched data for the article, made substantial contributions to discussions of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
K.J.R. is on the scientific advisory boards for Resilience Therapeutics, the Sheppard Pratt–Lieber Research Institute, the Laureate Institute for Brain Research, the Army Study to Assess Risk and Resilience in Servicemembers (STARRS) project, the University of California–San Diego VA Center of Excellence for Stress and Mental Health (CESAMH) and the Anxiety and Depression Association of America; provides fee-for-service consultation for Biogen and Resilience Therapeutics; and holds patents for the use of d-cycloserine and psychotherapy, targeting the pituitary adenylate cyclase-activating polypeptide (PACAP) type 1 receptor for extinction, targeting tachykinin 2 for prevention of fear and targeting angiotensin to improve extinction of fear. R.J.F., L.A.M.L. and J.S. declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Genome-wide association studies
-
(GWAS). Studies in which statistical associations between genetic variants and a disease or trait of interest are identified by genotyping individuals with disease and healthy controls for a set of single-nucleotide polymorphisms that capture variation across the entire genome.
- Optogenetics
-
The use of genetically encoded light-activated proteins (for example, ion channels) to control the functional parameters (for example, membrane potential) of targeted neuronal populations.
- Chemogenetics
-
The use of exogenous macromolecules to manipulate activity of genetically encoded receptors with no endogenous ligands (that is, designer receptors exclusively activated by designer drugs).
- Fibre photometry
-
Technology that utilizes an optical fibre for monitoring of activity of neuronal ensembles through genetically encoded activity indicators.
- Valence
-
The appetitive or aversive nature of a stimulus.
- Gene by environmental risk
-
The interaction between a genotype and environmental variation.
- Symptom provocation studies
-
Studies designed to elicit PTSD symptoms by exposing participants to their own trauma narratives.
- Fear generalization
-
Describes a situation in which conditioned fear responses are elicited in response to stimuli related to the conditioned stimulus.
- Blood-oxygen-level-dependent (BOLD) signalling
-
An index of brain activation based on detecting changes in blood oxygenation with fMRI.
- Memory fragmentation
-
Trauma memory retrieval that is experienced as only portions of various sensory and emotional representations and that lacks an integrative personal narrative.
- Executive function
-
A set of top-down cognitive control processes including inhibition (resisting habits, temptations or distractions), working memory (mentally holding and using information) and cognitive flexibility (adjusting to change).
- Long-term potentiation
-
A long-lasting (hours or days) increase in the response of neurons to stimulation of their afferents following a brief patterned stimulus (for example, a 100 Hz stimulus).
- Salience detection
-
The detection of information relevant to basic biological drives and psychological needs (for example, potential threats).
- Default-mode network
-
A large-scale brain network that is more active when individuals are not directing attention to the external environment.
Rights and permissions
About this article
Cite this article
Fenster, R.J., Lebois, L.A.M., Ressler, K. et al. Brain circuit dysfunction in post-traumatic stress disorder: from mouse to man. Nat Rev Neurosci 19, 535–551 (2018). https://doi.org/10.1038/s41583-018-0039-7
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41583-018-0039-7
This article is cited by
-
Closing the loop in psychiatric deep brain stimulation: physiology, psychometrics, and plasticity
Neuropsychopharmacology (2024)
-
Direct paraventricular thalamus-basolateral amygdala circuit modulates neuropathic pain and emotional anxiety
Neuropsychopharmacology (2024)
-
Prefrontal control of superior colliculus modulates innate escape behavior following adversity
Nature Communications (2024)
-
Neurocognitive effects of stress: a metaparadigm perspective
Molecular Psychiatry (2023)
-
Effects of acute stress and depression on functional connectivity between prefrontal cortex and the amygdala
Molecular Psychiatry (2023)