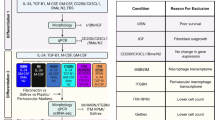
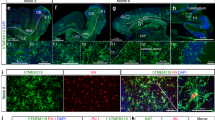
Abstract
It is becoming increasingly apparent that microglia, the immune cells of the CNS, and their peripheral counterparts, macrophages, have a major role in normal physiology and pathology. Recent technological advances in the production of particular cell types from induced pluripotent stem cells have led to an interest in applying this methodology to the production of microglia. Here, we discuss recent advances in this area and describe how they will aid our future understanding of microglia.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Wolf, S. A. Boddeke, H. W. & Kettenmann, H. Microglia in physiology and disease. Annu. Rev. Physiol. 10, 619–643 (2017).
Amor, S. et al. Inflammation in neurodegenerative diseases – an update. Immunology 142, 151–166 (2014).
Brazelton, T. R. Rossi, F. M. Keshet, G. I. & Blau, H. M. From marrow to brain: expression of neuronal phenotypes in adult mice. Science 290, 1775–1779 (2000).
Waisman, A. Ginhoux, F. Greter, M. Bruttger, J. Homeostasis of microglia in the adult brain: review of novel microglia depletion systems. Trends Immunol. 36, 625–636 (2015).
Guerreiro, R. et al. TREM2 variants in Alzheimer’s disease. N. Engl. J. Med. 368, 117–127 (2013).
Karch, C. M. & Goate, A. M. Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biol. Psychiatry 77, 43–51 (2015).
Villegas-Llerena, C. et al. Microglial genes regulating neuroinflammation in the progression of Alzheimer’s disease. Curr. Opin. Neurobiol. 36, 74–81 (2016).
Gonsette, R. E. Neurodegeneration in multiple sclerosis: the role of oxidative stress and excitotoxicity. J. Neurol. Sci. 274, 48–53 (2008).
Tansey, M. G. & Goldberg, M. S. Neuroinflammation in Parkinson’s disease; its role in neuronal death and implications for therapeutic intervention. Neurobiol. Dis. 37, 510–518 (2010).
Crotti, A. & Glass, C. K. The choreography of neuroinflammation in Huntington’s disease. Trends Immunol. 36, 364–373 (2015).
Morello, G. Spampinato, A. G. Cavallaro, S. Neuroinflammation and ALS: transcriptomic insights into molecular disease mechanisms and therapeutic targets. Mediators Inflamm. https://doi.org/10.1155/2017/7070469 (2017).
Sha nks, N. Greek, R. Greek, J. Are animal models predictive for humans? Philos. Ethics Humanit. Med. 4, 2 (2009).
Keene, C. D. et al. Neuropathological assessment and validation of mouse models for Alzheimer’s disease: applying NIA-AA guidelines. Pathobiol. Aging Age Relat. Dis. 6, 32397 (2016).
Swami, V., Furnham, A. & Christopher, A. N. Free the animals? Investigating attitudes toward animal testing in Britain and the United States. Scand. J. Psychol. 49, 269–276 (2008).
Ohgidani, M. et al. Direct induction of ramified microglia-like cells from human monocytes: dynamic microglial dysfunction in Nasu-Hakola disease. Sci. Rep. 4, 4957 (2014).
Ryan, K. J. et al. A human microglia-like cellular model for assessing the effects of neurodegenerative disease gene variants. Sci. Trans. Med. 20, 421 (2017).
Sturgeon, C. M. et al. Wnt signaling controls the specification of definitive and primitive hematopoiesis from human pluripotent stem cells. Nat. Biotechnol. 32, 554–561 (2014).
Arber, C. Lovejoy, C. Wray, S. Stem cell models of Alzheimer’s disease: progress and challenges. Alzheimers Res. Ther. 9, 42–59 (2017).
Ginhoux, F. et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845 (2010).
Kierdorf, K. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 16, 273–280 (2013).
Yona, S. et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38, 79–91 (2013).
Davies, L. C. & Taylor, P. R. Tissue-resident macrophages: then and now. Immunology 144, 541–548 (2015).
Butovsky, O. et al. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat. Neurosci. 17, 131–143 (2014).
Hashimoto, D. et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38, 792–804 (2013).
Hoeffel, G. et al. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity 42, 665–678 (2015).
Buchrieser, J. James, W. Moore, M. D. Human induced pluripotent stem cell-derived macrophages share ontogeny with MYB-independent tissue-resident macrophages. Stem Cell Rep. 8, 334–345 (2017).
Muffat, J. et al. Efficient derivation of microglia-like cells from human pluripotent stem cells. Nat. Med. 22, 1358–1367 (2016).
Douvaras, P. et al. Directed Differentiation of Human Pluripotent Stem Cells to Microglia. Stem Cell Rep. 8, 1516–1524 (2017).
Pandya, H. et al. Differentiation of human and murine induced pluripotent stem cells to microglia-like cells. Nat. Neurosci. 20, 753–759 (2017).
Abud, E. et al. iPSC-derived human microglia-like cells to study neurological diseases. Neuron 94, 278–293 (2017).
Haenseler, W. et al. A highly efficient human pluripotent stem cell microglia model displays a neuronal-co-culture-specific expression profile and inflammatory response. Stem Cell Rep. 8, 1727–1742 (2017).
Takata, K. et al. Induced-pluripotent-stem-cell-derived primitive macrophages provide a platform for modeling tissue-resident macrophage differentiation and function. Immunity 47, 183–198 (2017).
Karlsson, K. R. Homogeneous monocytes and macrophages from human embryonic stem cells following coculture-free differentiation in M-CSF and IL-3. Exp. Hematol. 36, 1167–1175 (2008).
Lachmann, N. et al. Large-scale haematopoietic differentiation of human induced pluripotent stem cells provides granulocytes or macrophages for cell replacement therapies. Stem Cell Rep. 4, 282–296 (2015).
Murray, P. J. et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20 (2014).
Moore, C. S. et al. P2Y12 expression and function in alternatively activated human microglia. Neurol. Neuroimmunol. Neuroinflamm. 2, e80 (2015).
Pocock, J. M. & Kettenmann, H. Neurotransmitter receptors on microglia. Trends Neurosci. 30, 527–535 (2007).
Domercq, M. Vázquez-Villoldo, N. Matute, C. Neurotransmitter signaling in the pathophysiology of microglia. Front. Cell Neurosci. 7, 49 (2013).
Greter, M. Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity 37, 1050–1060 (2012).
Gosselin, D. An environment-dependent transcriptional network specifies human microglia identity. Science 356, eaal3222 (2017).
Stevens, B. et al. The classical complement cascade mediates CNS synapse elimination. Cell 131, 1164–1178 (2007).
Sousa, C. Biber, K. & Michelucci, A. Cellular and molecular characterization of microglia: a unique immune cell population. Front. Immunol. 8, 198 (2017).
Bennett, M. L. et al. New tools for studying microglia in the mouse and human CNS. Proc. Natl Acad. Sci. USA 113, E1738–E1746 (2016).
Hickman, S. E. et al. The microglial sensome revealed by direct RNA sequencing. Nat. Neurosci. 16, 1896–1905 (2013).
Perry, V. H. Cunningham, C. Holmes, C. Systemic infections and inflammation affect chronic neurodegeneration. Nat. Rev. Immunol. 7, 161–167 (2007).
Morris, J. K. et al. Is Alzheimer’s disease a systemic disease? Biochim. Biophys. Acta. 1842, 1340–1349 (2014).
Yamasaki, R. et al. Differential roles of microglia and monocytes in the inflamed central nervous system. J. Exp. Med. 211, 1533–1549 (2014).
Hooper, C. et al. Differential effects of albumin on microglia and macrophages: implications for neurodegeneration following blood-brain barrier damage. J. Neurochem. 109, 694–705 (2009).
Nimmerjahn, A. Kirchhoff, F. & Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318 (2005).
Vogel, D. Y. et al. Human macrophage polarization in vitro: maturation and activation methods compared. Immunobiology 219, 695–703 (2014).
Suzuki, H. et al. Glycolytic pathway affects differentiation of human monocytes to regulatory macrophages. Immunol. Lett. 176, 18–27 (2016).
Bershteyn, M. et al. Human iPSC-derived cerebral organoids model cellular features of lissencephaly and reveal prolonged mitosis of outer radial glia. Cell Stem Cell 20, 435–449 (2017).
Mucci, A. et al. Murine iPSC-derived macrophages as a tool for disease modelling of hereditary pulmonary alveolar proteinosis due to Csf2rb deficiency. Stem Cell Rep. 7, 292–305 (2016).
Biber, K. Möller, T. Boddeke, E. Prinz, M. Central nervous system myeloid cells as drug targets: current status and translational challenges. Nat. Rev. Drug Discov. 15, 110–124 (2016).
Chen, S. K. et al. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell 141, 775–785 (2010).
Biju, K. C. et al. Bone-marrow-derived microglia-based neurturin delivery protects against dopaminergic neurodegeneration in a mouse model of Parkinson’s disease. Neurosci. Lett. 535, 24–29 (2013).
Conde, J. R. & Streit, W. J. Microglia in the aging brain. J. Neuropathol. Exp. Neurol. 65, 199–203 (2006).
Angelov, D. N. et al. Temporospatial relationships between macroglia and microglia during in vitro differentiation of murine stem cells. Dev. Neurosci 20, 42–51 (1998).
Tsuchiya, T. et al. Characterization of microglia induced from mouse embryonic stem cells and their migration into the brain parenchyma. J. Neuroimmunol. 160, 210–218 (2005).
Napoli, I. Kierdorf, K. Neumann, H. Microglial precursors derived from mouse embryonic stem cells. Glia 57, 1660–1671 (2009).
Beutner, C. Roy, K. Linnartz, B. Napoli, I. Neumann, H. Generation of microglial cells from mouse embryonic stem cells. Nat. Protoc. 5, 1481–1494 (2010).
van Wilgenburg, B. Browne, C. Vowles, J. Cowley, S. A. Efficient, long term production of monocyte-derived macrophages from human pluripotent stem cells under partly-defined and fully-defined conditions. PLoS ONE 8, e71098 (2013).
Author information
Authors and Affiliations
Contributions
J.M.P. made a substantial contribution to discussions of the content of the article. J.M.P. and T.M.P. researched data for the article, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pocock, J.M., Piers, T.M. Modelling microglial function with induced pluripotent stem cells: an update. Nat Rev Neurosci 19, 445–452 (2018). https://doi.org/10.1038/s41583-018-0030-3
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41583-018-0030-3
This article is cited by
-
Alzheimer’s disease-related transcriptional sex differences in myeloid cells
Journal of Neuroinflammation (2022)
-
Synthetic amyloid beta does not induce a robust transcriptional response in innate immune cell culture systems
Journal of Neuroinflammation (2022)
-
Human iPSC co-culture model to investigate the interaction between microglia and motor neurons
Scientific Reports (2022)
-
Functional microglia derived from human pluripotent stem cells empower retinal organs
Science China Life Sciences (2022)
-
The heterogeneity of microglial activation and its epigenetic and non-coding RNA regulations in the immunopathogenesis of neurodegenerative diseases
Cellular and Molecular Life Sciences (2022)