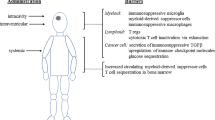
Abstract
Cell-based therapies are an emerging biopharmaceutical paradigm under investigation for the treatment of a range of neurological disorders. Accumulating evidence is demonstrating that cell-based therapies might be effective, but the mechanism of action remains unclear. In this Review, we synthesize results from over 20 years of animal studies that illustrate how transdifferentiation, cell replacement and restoration of damaged tissues in the CNS are highly unlikely mechanisms. We consider the evidence for an alternative model that we refer to as the bioreactor hypothesis, in which exogenous cells migrate to peripheral organs and modulate and reprogramme host immune cells to generate an anti-inflammatory, regenerative environment. The results of clinical trials clearly demonstrate a role for immunomodulation in the effects of cell-based therapies. Greater understanding of these mechanisms could facilitate the optimization of cell-based therapies for a variety of neurological disorders.
Key points
-
Cell-based therapies have high potential as novel therapeutics for a range of neurological disorders.
-
Transdifferentiation, cell replacement and restoration of damaged tissue in the CNS are unlikely mechanisms for the majority of cell-based therapies under development for neurological disorders.
-
An alternative model is the bioreactor hypothesis, in which exogenous cells migrate to peripheral organs and modulate and reprogramme host immune cells to generate an anti-inflammatory, regenerative environment.
-
The spleen and lungs are immediate therapeutic targets of intravenously administered cell-based therapies for neurological disorders.
-
Bioreactor mechanisms are important even when cell-based therapies are administered to the brain parenchyma.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Freed, C. R. et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N. Engl. J. Med. 344, 710–719 (2001).
Backlund, E. O. et al. Transplantation of adrenal medullary tissue to striatum in parkinsonism. First clinical trials. J. Neurosurg. 62, 169–173 (1985).
Ma, Y. et al. Dopamine cell implantation in Parkinson’s disease: long-term clinical and (18)F-FDOPA PET outcomes. J. Nucl. Med. 51, 7–15 (2010).
Piao, J. et al. Preclinical efficacy and safety of a human embryonic stem cell-derived midbrain dopamine progenitor product, MSK-DA01. Cell Stem Cell 28, 217–229 e217 (2021).
No authors listed. First Parkinson’s patients dosed with dopaminergic neurons. Nat. Biotechnol. 39, 785 (2021).
Riecke, J. et al. A meta-analysis of mesenchymal stem cells in animal models of Parkinson’s disease. Stem Cell Dev. 24, 2082–2090 (2015).
Jackson, M. L., Srivastava, A. K. & Cox, C. S. Jr Preclinical progenitor cell therapy in traumatic brain injury: a meta-analysis. J. Surg. Res. 214, 38–48 (2017).
Wang, Z. et al. Effects of stem cell transplantation on cognitive decline in animal models of Alzheimer’s disease: a systematic review and meta-analysis. Sci. Rep. 5, 12134 (2015).
Muthu, S., Jeyaraman, M., Gulati, A. & Arora, A. Current evidence on mesenchymal stem cell therapy for traumatic spinal cord injury: systematic review and meta-analysis. Cytotherapy 23, 186–197 (2021).
Misra, V., Lal, A., El Khoury, R., Chen, P. R. & Savitz, S. I. Intra-arterial delivery of cell therapies for stroke. Stem Cell Dev. 21, 1007–1015 (2012).
Petrou, P. et al. Beneficial effects of autologous mesenchymal stem cell transplantation in active progressive multiple sclerosis. Brain 143, 3574–3588 (2020).
Ohtaki, H. et al. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc. Natl Acad. Sci. USA 105, 14638–14643 (2008). One of the first studies to show that intracerebral injection of MSCs modifies microglia-mediated neuroinflammation in a rodent model of a common neurological condition resulting from global cerebral ischaemia.
Zhang, Y. T. et al. Advances in intranasal application of stem cells in the treatment of central nervous system diseases. Stem Cell Res. Ther. 12, 210 (2021).
Mahmood, A., Lu, D. & Chopp, M. Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J. Neurotrauma 21, 33–39 (2004).
Mahmood, A., Lu, D. & Chopp, M. Marrow stromal cell transplantation after traumatic brain injury promotes cellular proliferation within the brain. Neurosurgery 55, 1185–1193 (2004).
Mahmood, A., Lu, D., Lu, M. & Chopp, M. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery 53, 697–702 (2003). discussion 702-693.
Lv, F. J., Tuan, R. S., Cheung, K. M. & Leung, V. Y. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cell 32, 1408–1419 (2014).
Wang, Y. et al. The plasticity of mesenchymal stem cells in regulating surface HLA-I. iScience 15, 66–78 (2019).
Machado Cde, V., Telles, P. D. & Nascimento, I. L. Immunological characteristics of mesenchymal stem cells. Rev. Bras. Hematol. Hemoter. 35, 62–67 (2013).
Oh, J. Y. et al. MHC class I enables MSCs to evade NK-cell-mediated cytotoxicity and exert immunosuppressive activity. Stem Cell 40, 870–882 (2022).
Bloom, D. D. et al. A reproducible immunopotency assay to measure mesenchymal stromal cell-mediated T-cell suppression. Cytotherapy 17, 140–151 (2015).
Fischer, U. M. et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cell Dev. 18, 683–692 (2009). A comprehensive set of studies demonstrating that intravenously administered cell therapies are entrapped in the lung and only small percentages can traverse to the arterial circulation.
Barker, R. A. & Widner, H. Immune problems in central nervous system cell therapy. NeuroRx 1, 472–481 (2004).
Hickey, W. F. Basic principles of immunological surveillance of the normal central nervous system. Glia 36, 118–124 (2001).
Widner, H. & Brundin, P. Immunological aspects of grafting in the mammalian central nervous system. A review and speculative synthesis. Brain Res. 472, 287–324 (1988).
Coyne, T. M., Marcus, A. J., Woodbury, D. & Black, I. B. Marrow stromal cells transplanted to the adult brain are rejected by an inflammatory response and transfer donor labels to host neurons and glia. Stem Cell 24, 2483–2492 (2006).
Coyne, T. M., Marcus, A. J., Reynolds, K., Black, I. B. & Woodbury, D. Disparate host response and donor survival after the transplantation of mesenchymal or neuroectodermal cells to the intact rodent brain. Transplantation 84, 1507–1516 (2007).
Hwang, J. W. et al. A comparison of immune responses exerted following syngeneic, allogeneic, and xenogeneic transplantation of mesenchymal stem cells into the mouse brain. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21093052 (2020).
Mezey, E., Chandross, K. J., Harta, G., Maki, R. A. & McKercher, S. R. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science 290, 1779–1782 (2000).
Brazelton, T. R., Rossi, F. M., Keshet, G. I. & Blau, H. M. From marrow to brain: expression of neuronal phenotypes in adult mice. Science 290, 1775–1779 (2000).
Woodbury, D., Schwarz, E. J., Prockop, D. J. & Black, I. B. Adult rat and human bone marrow stromal cells differentiate into neurons. J. Neurosci. Res. 61, 364–370 (2000).
Sanchez-Ramos, J. et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp. Neurol. 164, 247–256 (2000).
Harting, M. T. et al. Intravenous mesenchymal stem cell therapy for traumatic brain injury. J. Neurosurg. 110, 1189–1197 (2009).
Sinden, J. D., Hicks, C., Stroemer, P., Vishnubhatla, I. & Corteling, R. Human neural stem cell therapy for chronic ischemic stroke: charting progress from laboratory to patients. Stem Cell Dev. 26, 933–947 (2017).
Selden, N. R. et al. Central nervous system stem cell transplantation for children with neuronal ceroid lipofuscinosis. J. Neurosurg. Pediatr. 11, 643–652 (2013).
Andres, R. H. et al. Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain 134, 1777–1789 (2011).
Teng, Y. D. et al. Multimodal actions of neural stem cells in a mouse model of ALS: a meta-analysis. Sci. Transl. Med. 4, 165ra164 (2012).
Redmond, D. E. Jr et al. Behavioral improvement in a primate Parkinson’s model is associated with multiple homeostatic effects of human neural stem cells. Proc. Natl Acad. Sci. USA 104, 12175–12180 (2007).
Tadesse, T. et al. Analysis of graft survival in a trial of stem cell transplant in ALS. Ann. Clin. Transl. Neurol. 1, 900–908 (2014).
Jiang, P. et al. Human iPSC-derived immature astroglia promote oligodendrogenesis by increasing TIMP-1 secretion. Cell Rep. 15, 1303–1315 (2016).
Llorente, I. L. et al. Patient-derived glial enriched progenitors repair functional deficits due to white matter stroke and vascular dementia in rodents.Sci. Transl. Med. 13, eaaz6747 (2021).
Lyczek, A. et al. Transplanted human glial-restricted progenitors can rescue the survival of dysmyelinated mice independent of the production of mature, compact myelin. Exp. Neurol. 291, 74–86 (2017).
Walker, P. A. et al. Bone marrow-derived stromal cell therapy for traumatic brain injury is neuroprotective via stimulation of non-neurologic organ systems. Surgery 152, 790–793 (2012).
Spees, J. L., Olson, S. D., Whitney, M. J. & Prockop, D. J. Mitochondrial transfer between cells can rescue aerobic respiration. Proc. Natl Acad. Sci. USA 103, 1283–1288 (2006).
Tan, Y. L. et al. Mesenchymal stromal cell mitochondrial transfer as a cell rescue strategy in regenerative medicine: a review of evidence in preclinical models. Stem Cell Transl. Med. 11, 814–827 (2022).
Einstein, O. et al. Neural precursors attenuate autoimmune encephalomyelitis by peripheral immunosuppression. Ann. Neurol. 61, 209–218 (2007). This study demonstrated that NPCs suppress pro-inflammatory T cells in a model of MS.
Mays, R. W. & Savitz, S. I. Intravenous cellular therapies for acute ischemic stroke. Stroke 49, 1058–1065 (2018).
Zhou, K. et al. Regulatory T cells ameliorate intracerebral hemorrhage-induced inflammatory injury by modulating microglia/macrophage polarization through the IL-10/GSK3beta/PTEN axis. J. Cereb. Blood Flow. Metab. 37, 967–979 (2017).
Giovannelli, I., Heath, P., Shaw, P. J. & Kirby, J. The involvement of regulatory T cells in amyotrophic lateral sclerosis and their therapeutic potential. Amyotroph. Lateral Scler. Frontotemporal Degener. 21, 435–444 (2020).
Dombrowski, Y. et al. Regulatory T cells promote myelin regeneration in the central nervous system. Nat. Neurosci. 20, 674–680 (2017).
Machhi, J. et al. Harnessing regulatory T cell neuroprotective activities for treatment of neurodegenerative disorders. Mol. Neurodegener. 15, 32 (2020).
Yang, B. et al. Multipotent adult progenitor cells enhance recovery after stroke by modulating the immune response from the spleen. Stem Cell 35, 1290–1302 (2017).
Menge, T. et al. Mesenchymal stem cells regulate blood-brain barrier integrity through TIMP3 release after traumatic brain injury. Sci. Transl. Med. 4, 161ra150 (2012).
Vigo, T. et al. Mesenchymal stem cells instruct a beneficial phenotype in reactive astrocytes. Glia 69, 1204–1215 (2021).
Greiner, T. & Kipp, M. What guides peripheral immune cells into the central nervous system? Cells https://doi.org/10.3390/cells10082041 (2021).
Gao, J., Dennis, J. E., Muzic, R. F., Lundberg, M. & Caplan, A. I. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cell Tissues Organs 169, 12–20 (2001).
Acosta, S. A., Tajiri, N., Hoover, J., Kaneko, Y. & Borlongan, C. V. Intravenous bone marrow stem cell grafts preferentially migrate to spleen and abrogate chronic inflammation in stroke. Stroke 46, 2616–2627 (2015).
Park, B. N., Lim, T. S., Yoon, J. K. & An, Y. S. In vivo tracking of intravenously injected mesenchymal stem cells in an Alzheimer’s animal model. Cell Transpl. 27, 1203–1209 (2018).
Xu, K. et al. Human stem cells transplanted into the rat stroke brain migrate to the spleen via lymphatic and inflammation pathways. Haematologica 104, 1062–1073 (2019).
Lee, S. T. et al. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain 131, 616–629 (2008).
Schwarting, S. et al. Hematopoietic stem cells reduce postischemic inflammation and ameliorate ischemic brain injury. Stroke 39, 2867–2875 (2008).
Golden, J. E. et al. Human umbilical cord blood cells alter blood and spleen cell populations after stroke. Transl. Stroke Res. 3, 491–499 (2012).
Offner, H. et al. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J. Immunol. 176, 6523–6531 (2006).
Ajmo, C. T. Jr et al. The spleen contributes to stroke-induced neurodegeneration. J. Neurosci. Res. 86, 2227–2234 (2008). One of the first studies of splenic contraction and the role of the spleen in the inflammatory response after stroke.
Vendrame, M. et al. Infusion of human umbilical cord blood cells in a rat model of stroke dose-dependently rescues behavioral deficits and reduces infarct volume. Stroke 35, 2390–2395 (2004).
Walker, P. A. et al. Intravenous multipotent adult progenitor cell therapy for traumatic brain injury: preserving the blood brain barrier via an interaction with splenocytes. Exp. Neurol. 225, 341–352 (2010).
Highfill, S. L. et al. Multipotent adult progenitor cells can suppress graft-versus-host disease via prostaglandin E2 synthesis and only if localized to sites of allopriming. Blood 114, 693–701 (2009).
Nemeth, K. et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 15, 42–49 (2009). This study showed that intravenous delivery of MSCs reprogrammes macrophages in the lungs in a mouse model of sepsis.
Schmidt, A. et al. Human macrophages induce CD4+Foxp3+ regulatory T cells via binding and re-release of TGF-beta. Immunol. Cell Biol. 94, 747–762 (2016).
Lee, R. H. et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 5, 54–63 (2009).
Dyer, D. P. et al. TSG-6 inhibits neutrophil migration via direct interaction with the chemokine CXCL8. J. Immunol. 192, 2177–2185 (2014).
Lu, D., Xu, Y., Liu, Q. & Zhang, Q. Mesenchymal stem cell-macrophage crosstalk and maintenance of inflammatory microenvironment homeostasis. Front. Cell Dev. Biol. 9, 681171 (2021).
Galipeau, J. Macrophages at the nexus of mesenchymal stromal cell potency: the emerging role of chemokine cooperativity. Stem Cell 39, 1145–1154 (2021).
Luu, N. T. et al. Crosstalk between mesenchymal stem cells and endothelial cells leads to downregulation of cytokine-induced leukocyte recruitment. Stem Cell 31, 2690–2702 (2013).
Wang, H. et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421, 384–388 (2003).
Dash, P. K. et al. Activation of alpha 7 cholinergic nicotinic receptors reduce blood-brain barrier permeability following experimental traumatic brain injury. J. Neurosci. 36, 2809–2818 (2016).
Rosas-Ballina, M. et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334, 98–101 (2011).
Su, X., Matthay, M. A. & Malik, A. B. Requisite role of the cholinergic alpha7 nicotinic acetylcholine receptor pathway in suppressing Gram-negative sepsis-induced acute lung inflammatory injury. J. Immunol. 184, 401–410 (2010).
Capcha, J. M. C. et al. Wharton’s jelly-derived mesenchymal stem cells attenuate sepsis-induced organ injury partially via cholinergic anti-inflammatory pathway activation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 318, R135–R147 (2020).
Yi, T. G. et al. A novel immunomodulatory mechanism dependent on acetylcholine secreted by human bone marrow-derived mesenchymal stem cells. Int. J. Stem Cell 12, 315–330 (2019).
Satani, N. et al. Peripheral blood monocytes as a therapeutic target for marrow stromal cells in stroke patients. Front. Neurol. https://doi.org/10.3389/fneur.2022.958579 (2022).
Giri, J., Das, R., Nylen, E., Chinnadurai, R. & Galipeau, J. CCL2 and CXCL12 derived from mesenchymal stromal cells cooperatively polarize IL-10+ tissue macrophages to mitigate gut injury. Cell Rep. 30, 1923–1934 e1924 (2020).
Yang, F. et al. Bone marrow mesenchymal stem cells induce M2 microglia polarization through PDGF-AA/MANF signaling. World J. Stem Cell 12, 633–658 (2020).
Daadi, M. M. et al. Human neural stem cell grafts modify microglial response and enhance axonal sprouting in neonatal hypoxic-ischemic brain injury. Stroke 41, 516–523 (2010).
Hassani, Z. et al. Human neural progenitor cell engraftment increases neurogenesis and microglial recruitment in the brain of rats with stroke. PLoS ONE 7, e50444 (2012).
Muir, K. W. et al. Intracerebral implantation of human neural stem cells and motor recovery after stroke: multicentre prospective single-arm study (PISCES-2). J. Neurol. Neurosurg. Psychiatry 91, 396–401 (2020).
Kim, D. K. et al. Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc. Natl Acad. Sci. USA 113, 170–175 (2016).
Zhang, Y. et al. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J. Neurosurg. 122, 856–867 (2015).
Chen, J. & Chopp, M. Exosome therapy for stroke. Stroke 49, 1083–1090 (2018).
Zhang, Z. G., Buller, B. & Chopp, M. Exosomes - beyond stem cells for restorative therapy in stroke and neurological injury. Nat. Rev. Neurol. 15, 193–203 (2019).
Liu, X. et al. Bone marrow mesenchymal stem cell-derived exosomes attenuate cerebral ischemia-reperfusion injury-induced neuroinflammation and pyroptosis by modulating microglia M1/M2 phenotypes. Exp. Neurol. 341, 113700 (2021).
Go, V. et al. Extracellular vesicles from mesenchymal stem cells reduce microglial-mediated neuroinflammation after cortical injury in aged Rhesus monkeys. Geroscience 42, 1–17 (2020).
Ruppert, K. A. et al. Human mesenchymal stromal cell-derived extracellular vesicles modify microglial response and improve clinical outcomes in experimental spinal cord injury. Sci. Rep. 8, 480 (2018).
de Couto, G. et al. Exosomal microRNA transfer into macrophages mediates cellular postconditioning. Circulation 136, 200–214 (2017).
Hess, D. C. et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 16, 360–368 (2017).
Cox, C. S. Jr et al. Treatment of severe adult traumatic brain injury using bone marrow mononuclear cells. Stem Cell 35, 1065–1079 (2017).
Schiess, M. et al. Allogeneic bone marrow-derived mesenchymal stem cell safety in idiopathic parkinsons disease. Mov. Disord. 36, 1825–1834 (2021).
Author information
Authors and Affiliations
Contributions
S.I.S. researched data for the article. Both authors contributed to writing the article and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
S.I.S. has served in the following roles as an employee of UTHealth: site investigator in clinical trials sponsored by Athersys, KM Pharma, ReNeuron and SanBio, for which UTHealth has received payments on the basis of clinical trial contracts; investigator on clinical trials supported by the Cord Blood Registry Systems, the Department of Defense, Let’s Cure CP, NIH, and the TIRR Foundation; investigator in the Clinical and Translational Science Awards (CTSA) Program funded by the NIH; principal investigator or co-investigator on NIH-funded grants in basic science and clinical research; investigator for an imaging analysis centre for clinical trials sponsored by Athersys, ReNeuron and SanBio. He has also provided consulting services on behalf of UTHealth to Abbvie, ArunA, Deck Therapeutics, KM Pharma, Lumosa, Neurastasis, Neurexcell and ReNeuron. All compensation from such consulting arrangements has been paid to UTHealth. C.S.C. has royalty, equity and advisory board interests in Cellvation, which is developing cell therapies and an ex vivo mechanotransductive bioreactor for cell expansion. He also has sponsored research agreements with Athersys, Biostage and Generate Life Sciences, has served on the advisory board for Biostage and Generate Life Sciences, and is a consultant for Stream Biomedical.
Peer review
Peer review information
Nature Reviews Neurology thanks T. Ben-hur, C. Borlongan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Savitz, S.I., Cox, C.S. Cell-based therapies for neurological disorders — the bioreactor hypothesis. Nat Rev Neurol 19, 9–18 (2023). https://doi.org/10.1038/s41582-022-00736-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-022-00736-4