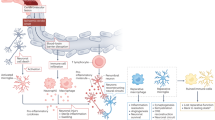
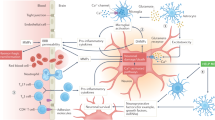
Abstract
Ischaemic stroke is a leading cause of disability and death for which no acute treatments exist beyond recanalization. The development of novel therapies has been repeatedly hindered by translational failures that have changed the way we think about tissue damage after stroke. What was initially a neuron-centric view has been replaced with the concept of the neurovascular unit (NVU), which encompasses neuronal, glial and vascular compartments, and the biphasic nature of neural–glial–vascular signalling. However, it is now clear that the brain is not the private niche it was traditionally thought to be and that the NVU interacts bidirectionally with systemic biology, such as systemic metabolism, the peripheral immune system and the gut microbiota. Furthermore, these interactions are profoundly modified by internal and external factors, such as ageing, temperature and day–night cycles. In this Review, we propose an extension of the concept of the NVU to include its dynamic interactions with systemic biology. We anticipate that this integrated view will lead to the identification of novel mechanisms of stroke pathophysiology, potentially explain previous translational failures, and improve stroke care by identifying new biomarkers of and treatment targets in stroke.
Key points
-
According to the prevailing concept, the neurovascular unit (NVU) comprises multiple cell types from the neuronal, glial and vascular compartments but is functionally confined to cell-to-cell signalling within the CNS.
-
Novel pathways for brain–body communication, such as the glymphatic system and skull microchannels, have been identified, indicating that the brain is not the private compartment it was thought to be.
-
The NVU interacts with systemic biological processes and systems, including systemic metabolism, the peripheral immune system and the gut microbiota.
-
Factors such as age, temperature and the circadian clock not only affect the NVU but also profoundly modify the interactions between the NVU and systemic biology.
-
A conceptual extension of the NVU to include its interactions with systemic biology could provide a more accurate depiction of the biology after stroke.
-
This extended NVU framework might help to explain previous translational failures and improve stroke care by identifying novel biomarkers of and treatment targets in stroke.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Lo, E. H., Dalkara, T. & Moskowitz, M. A. Mechanisms, challenges and opportunities in stroke. Nat. Rev. Neurosci. 4, 399–415 (2003).
Lo, E. H., Broderick, J. P. & Moskowitz, M. A. tPA and proteolysis in the neurovascular unit. Stroke 35, 354–356 (2004).
Hayakawa, K. et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535, 551–555 (2016).
Yemisci, M. et al. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat. Med. 15, 1031–1037 (2009).
Horng, S. et al. Astrocytic tight junctions control inflammatory CNS lesion pathogenesis. J. Clin. Invest. 127, 3136–3151 (2017).
Asahi, M. et al. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J. Neurosci. 21, 7724–7732 (2001).
Lee, S. R. et al. Involvement of matrix metalloproteinase in neuroblast cell migration from the subventricular zone after stroke. J. Neurosci. 26, 3491–3495 (2006).
Zhao, B. Q. et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat. Med. 12, 441–445 (2006).
Hu, X. et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 43, 3063–3070 (2012).
Xing, C., Li, W., Deng, W., Ning, M. & Lo, E. H. A potential gliovascular mechanism for microglial activation: differential phenotypic switching of microglia by endothelium versus astrocytes. J. Neuroinflamm. 15, 143 (2018).
Lo, E. H. A new penumbra: transitioning from injury into repair after stroke. Nat. Med. 14, 497–500 (2008).
Moskowitz, M. A., Lo, E. H. & Iadecola, C. The science of stroke: mechanisms in search of treatments. Neuron 67, 181–198 (2010).
Ishikawa, H. et al. Ischemic stroke brain sends indirect cell death signals to the heart. Stroke 44, 3175–3182 (2013).
Sposato, L. A. et al. Post-stroke cardiovascular complications and neurogenic cardiac injury: JACC state-of-the-art review. JACC 76, 2768–2785 (2020).
Liesz, A. et al. DAMP signaling is a key pathway inducing immune modulation after brain injury. J. Neurosci. 35, 583–598 (2015).
Brooks, T. A. et al. Biphasic cytoarchitecture and functional changes in the BBB induced by chronic inflammatory pain. Brain Res. 1120, 172–182 (2006).
Carloni, S. et al. Identification of a choroid plexus vascular barrier closing during intestinal inflammation. Science 374, 439–448 (2021).
Choi, C. S. et al. Cytotoxic tau released from lung microvascular endothelial cells upon infection with Pseudomonas aeruginosa promotes neuronal tauopathy. J. Biol. Chem. 298, 101482 (2021).
MahmoudianDehkordi, S. et al. Altered bile acid profile associates with cognitive impairment in Alzheimer’s disease-an emerging role for gut microbiome. Alzheimers Dement. 15, 76–92 (2019).
Kumar, S., Selim, M. H. & Caplan, L. R. Medical complications after stroke. Lancet Neurol. 9, 105–118 (2010).
Malik, R. et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat. Genet. 50, 524–537 (2018).
Traylor, M. et al. Genetic basis of lacunar stroke: a pooled analysis of individual patient data and genome-wide association studies. Lancet Neurol. 20, 351–361 (2021).
Dichgans, M., Pulit, S. L. & Rosand, J. Stroke genetics: discovery, biology, and clinical applications. Lancet Neurol. 18, 587–599 (2019).
Yang, A. C. et al. Physiological blood-brain transport is impaired with age by a shift in transcytosis. Nature 583, 425–430 (2020). In this study, extensive labelling and proteome studies provided compelling evidence for the extent of physiological blood–brain transport and its change with age.
Xing, C. et al. in The Vasculome: From Many, One (ed. Galis, Z. S.) 427–438 (Elsevier, 2022).
Krueger, M. et al. Blood-brain barrier breakdown involves four distinct stages of vascular damage in various models of experimental focal cerebral ischemia. J. Cereb. Blood Flow Metab. 35, 292–303 (2015).
Jickling, G. C. et al. Hemorrhagic transformation after ischemic stroke in animals and humans. J. Cereb. Blood Flow Metab. 34, 185–199 (2014).
Gershon, M. D. & Margolis, K. G. The gut, its microbiome, and the brain: connections and communications. J. Clin. Invest. 131, e143768 (2021).
Iliff, J. J. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 4, 147ra111 (2012).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015).
Rasmussen, M. K., Mestre, H. & Nedergaard, M. The glymphatic pathway in neurological disorders. Lancet Neurol. 17, 1016–1024 (2018).
Herisson, F. et al. Direct vascular channels connect skull bone marrow and the brain surface enabling myeloid cell migration. Nat. Neurosci. 21, 1209–1217 (2018).
Partridge, L., Deelen, J. & Slagboom, P. E. Facing up to the global challenges of ageing. Nature 561, 45–56 (2018).
Belsky, D. W. et al. Quantification of biological aging in young adults. Proc. Natl Acad. Sci. USA 112, E4104–E4110 (2015).
Chahal, H. S. & Drake, W. M. The endocrine system and ageing. J. Pathol. 211, 173–180 (2007).
Lakatta, E. G. & Levy, D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part II: the aging heart in health: links to heart disease. Circulation 107, 346–354 (2003).
Hou, Y. et al. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 15, 565–581 (2019).
Campisi, J. et al. From discoveries in ageing research to therapeutics for healthy ageing. Nature 571, 183–192 (2019).
Mattson, M. P. & Magnus, T. Ageing and neuronal vulnerability. Nat. Rev. Neurosci. 7, 278–294 (2006).
Ma, J., Ma, Y., Shuaib, A. & Winship, I. R. Impaired collateral flow in pial arterioles of aged rats during ischemic stroke. Transl. Stroke Res. 11, 243–253 (2020).
Ungvari, Z., Tarantini, S., Donato, A. J., Galvan, V. & Csiszar, A. Mechanisms of vascular aging. Circ. Res. 123, 849–867 (2018).
Kress, B. T. et al. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 76, 845–861 (2014).
Ma, Q., Ineichen, B. V., Detmar, M. & Proulx, S. T. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat. Commun. 8, 1434 (2017).
Banks, W. A., Reed, M. J., Logsdon, A. F., Rhea, E. M. & Erickson, M. A. Healthy aging and the blood-brain barrier. Nat. Aging 1, 243–254 (2021).
Segarra, M., Aburto, M. R. & Acker-Palmer, A. Blood-brain barrier dynamics to maintain brain homeostasis. Trends Neurosci. 44, 393–405 (2021).
Wollenweber, F. A. et al. Functional outcome following stroke thrombectomy in clinical practice. Stroke 50, 2500–2506 (2019).
GBD 2016 Stroke Collaborators. Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 18, 439–458 (2019).
Boehme, A. K., Esenwa, C. & Elkind, M. S. V. Stroke risk factors, genetics, and prevention. Circ. Res. 120, 472–495 (2017).
Ainslie, P. N. et al. Early morning impairment in cerebral autoregulation and cerebrovascular CO2 reactivity in healthy humans: relation to endothelial function. Exp. Physiol. 92, 769–777 (2007).
Schaeffer, S. & Iadecola, C. Revisiting the neurovascular unit. Nat. Neurosci. 24, 1198–1209 (2021).
Brown, L. S. et al. Pericytes and neurovascular function in the healthy and diseased brain. Front. Cell. Neurosci. 13, 282 (2019).
Santisteban, M. M. et al. Endothelium-macrophage crosstalk mediates blood-brain barrier dysfunction in hypertension. Hypertension 76, 795–807 (2020).
Ungvari, Z. et al. Hypertension-induced cognitive impairment: from pathophysiology to public health. Nat. Rev. Nephrol. 17, 639–654 (2021).
Leonardi-Bee, J., Bath, P. M., Phillips, S. J., Sandercock, P. A. & Group, I. S. T. C. Blood pressure and clinical outcomes in the International Stroke Trial. Stroke 33, 1315–1320 (2002).
Hood, S. & Amir, S. The aging clock: circadian rhythms and later life. J. Clin. Invest. 127, 437–446 (2017).
Amorim, J. A. et al. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat. Rev. Endocrinol. 18, 243–258 (2022).
Montecino-Rodriguez, E., Berent-Maoz, B. & Dorshkind, K. Causes, consequences, and reversal of immune system aging. J. Clin. Invest. 123, 958–965 (2013).
Nikolich-Zugich, J. The twilight of immunity: emerging concepts in aging of the immune system. Nat. Immunol. 19, 10–19 (2018).
Yatsunenko, T. et al. Human gut microbiome viewed across age and geography. Nature 486, 222–227 (2012).
Ritzel, R. M. et al. Aging alters the immunological response to ischemic stroke. Acta Neuropathol. 136, 89–110 (2018).
Spychala, M. S. et al. Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann. Neurol. 84, 23–36 (2018).
Marton, A. et al. Organ protection by SGLT2 inhibitors: role of metabolic energy and water conservation. Nat. Rev. Nephrol. 17, 65–77 (2021).
Hyder, F., Rothman, D. L. & Bennett, M. R. Cortical energy demands of signaling and nonsignaling components in brain are conserved across mammalian species and activity levels. Proc. Natl Acad. Sci. USA 110, 3549–3554 (2013).
Belanger, M., Allaman, I. & Magistretti, P. J. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 14, 724–738 (2011).
Zhao, H. et al. Akt contributes to neuroprotection by hypothermia against cerebral ischemia in rats. J. Neurosci. 25, 9794–9806 (2005).
Yenari, M. A. & Han, H. S. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat. Rev. Neurosci. 13, 267–278 (2012).
Hamann, G. F. et al. Mild to moderate hypothermia prevents microvascular basal lamina antigen loss in experimental focal cerebral ischemia. Stroke 35, 764–769 (2004).
Baumann, E., Preston, E., Slinn, J. & Stanimirovic, D. Post-ischemic hypothermia attenuates loss of the vascular basement membrane proteins, agrin and SPARC, and the blood-brain barrier disruption after global cerebral ischemia. Brain Res. 1269, 185–197 (2009).
Duz, B., Oztas, E., Erginay, T., Erdogan, E. & Gonul, E. The effect of moderate hypothermia in acute ischemic stroke on pericyte migration: an ultrastructural study. Cryobiology 55, 279–284 (2007).
Huang, Z. G., Xue, D., Preston, E., Karbalai, H. & Buchan, A. M. Biphasic opening of the blood-brain barrier following transient focal ischemia: effects of hypothermia. Can. J. Neurol. Sci. 26, 298–304 (1999).
van der Worp, H. B., Sena, E. S., Donnan, G. A., Howells, D. W. & Macleod, M. R. Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta-analysis. Brain J. Neurol. 130, 3063–3074 (2007).
van der Worp, H. B. et al. Therapeutic hypothermia for acute ischaemic stroke. Results of a European multicentre, randomised, phase III clinical trial. Eur. Stroke J. 4, 254–262 (2019).
Obermeyer, Z., Samra, J. K. & Mullainathan, S. Individual differences in normal body temperature: longitudinal big data analysis of patient records. BMJ 359, j5468 (2017).
Reith, J. et al. Body temperature in acute stroke: relation to stroke severity, infarct size, mortality, and outcome. Lancet 347, 422–425 (1996).
Tagin, M. A., Woolcott, C. G., Vincer, M. J., Whyte, R. K. & Stinson, D. A. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Arch. Pediatr. Adolesc. Med. 166, 558–566 (2012).
Engelman, R. et al. The Society of Thoracic Surgeons, The Society of Cardiovascular Anesthesiologists, and The American Society of ExtraCorporeal Technology: clinical practice guidelines for cardiopulmonary bypass–temperature management during cardiopulmonary bypass. Ann. Thorac. Surg. 100, 748–757 (2015).
Nolan, J. P. et al. Therapeutic hypothermia after cardiac arrest: an advisory statement by the Advanced Life Support Task Force of the International Liaison Committee on Resuscitation. Circulation 108, 118–121 (2003).
Terpstra, A. H. Differences between humans and mice in efficacy of the body fat lowering effect of conjugated linoleic acid: role of metabolic rate. J. Nutr. 131, 2067–2068 (2001).
Baracos, V. E., Whitmore, W. T. & Gale, R. The metabolic cost of fever. Can. J. Physiol. Pharmacol. 65, 1248–1254 (1987).
Lyden, P. Selective cerebral cooling for acute ischemic stroke. J. Cereb. Blood Flow Metab. 40, 1365–1367 (2020).
Fuller, P. M., Gooley, J. J. & Saper, C. B. Neurobiology of the sleep-wake cycle: sleep architecture, circadian regulation, and regulatory feedback. J. Biol. Rhythms 21, 482–493 (2006).
Dyar, K. A. et al. Atlas of circadian metabolism reveals system-wide coordination and communication between clocks. Cell 174, 1571–1585.e11 (2018).
Geiger, S. S., Fagundes, C. T. & Siegel, R. M. Chrono-immunology: progress and challenges in understanding links between the circadian and immune systems. Immunology 146, 349–358 (2015).
Roenneberg, T. & Merrow, M. The circadian clock and human health. Curr. Biol. 26, R432–R443 (2016). This review links circadian clocks to human health and introduces key terms of circadian biology.
Smolensky, M. H., Hermida, R. C. & Portaluppi, F. Circadian mechanisms of 24-hour blood pressure regulation and patterning. Sleep. Med. Rev. 33, 4–16 (2017).
Scheer, F. A. & Shea, S. A. Human circadian system causes a morning peak in prothrombotic plasminogen activator inhibitor-1 (PAI-1) independent of the sleep/wake cycle. Blood 123, 590–593 (2014).
Takahashi, J. S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 18, 164–179 (2017).
Zhang, R., Lahens, N. F., Ballance, H. I., Hughes, M. E. & Hogenesch, J. B. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc. Natl Acad. Sci. USA 111, 16219–16224 (2014).
Rabinovich-Nikitin, I., Lieberman, B., Martino, T. A. & Kirshenbaum, L. A. Circadian-regulated cell death in cardiovascular diseases. Circulation 139, 965–980 (2019).
Musiek, E. S. et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J. Clin. Invest. 123, 5389–5400 (2013).
Kondratova, A. A. & Kondratov, R. V. The circadian clock and pathology of the ageing brain. Nat. Rev. Neurosci. 13, 325–335 (2012).
Schmitt, K. et al. Circadian control of DRP1 activity regulates mitochondrial dynamics and bioenergetics. Cell Metab. 27, 657–666.e5 (2018).
Cavey, M., Collins, B., Bertet, C. & Blau, J. Circadian rhythms in neuronal activity propagate through output circuits. Nat. Neurosci. 19, 587–595 (2016).
Chi-Castaneda, D. & Ortega, A. Circadian regulation of glutamate transporters. Front. Endocrinol. 9, 340 (2018).
Lang, N. et al. Circadian modulation of GABA-mediated cortical inhibition. Cereb. Cortex 21, 2299–2306 (2011).
Womac, A. D., Burkeen, J. F., Neuendorff, N., Earnest, D. J. & Zoran, M. J. Circadian rhythms of extracellular ATP accumulation in suprachiasmatic nucleus cells and cultured astrocytes. Eur. J. Neurosci. 30, 869–876 (2009).
Marpegan, L. et al. Circadian regulation of ATP release in astrocytes. J. Neurosci. 31, 8342–8350 (2011).
Brancaccio, M., Patton, A. P., Chesham, J. E., Maywood, E. S. & Hastings, M. H. Astrocytes control circadian timekeeping in the suprachiasmatic nucleus via glutamatergic signaling. Neuron 93, 1420–1435.e5 (2017).
Brancaccio, M. et al. Cell-autonomous clock of astrocytes drives circadian behavior in mammals. Science 363, 187–192 (2019).
Bellesi, M. et al. Effects of sleep and wake on oligodendrocytes and their precursors. J. Neurosci. 33, 14288–14300 (2013).
Durgan, D. J., Crossland, R. F. & Bryan, R. M. Jr. The rat cerebral vasculature exhibits time-of-day-dependent oscillations in circadian clock genes and vascular function that are attenuated following obstructive sleep apnea. J. Cereb. Blood Flow Metab. 37, 2806–2819 (2017).
Anea, C. B. et al. Increased superoxide and endothelial NO synthase uncoupling in blood vessels of Bmal1-knockout mice. Circ. Res. 111, 1157–1165 (2012).
Zhang, S. L., Yue, Z., Arnold, D. M., Artiushin, G. & Sehgal, A. A circadian clock in the blood-brain barrier regulates xenobiotic efflux. Cell 173, 130–139.e10 (2018).
Nakazato, R. et al. Disruption of Bmal1 impairs blood-brain barrier integrity via pericyte dysfunction. J. Neurosci. 37, 10052–10062 (2017).
Pulido, R. S. et al. Neuronal activity regulates blood-brain barrier efflux transport through endothelial circadian genes. Neuron 108, 937–952.e7 (2020).
Xie, L. et al. Sleep drives metabolite clearance from the adult brain. Science 342, 373–377 (2013).
Nilsson, C. et al. Circadian variation in human cerebrospinal fluid production measured by magnetic resonance imaging. Am. J. Physiol. 262, R20–R24 (1992).
Hablitz, L. M. et al. Circadian control of brain glymphatic and lymphatic fluid flow. Nat. Commun. 11, 4411 (2020).
Lo, E. H. et al. Circadian biology and stroke. Stroke 52, 2180–2190 (2021).
Wiebking, N., Maronde, E. & Rami, A. Increased neuronal injury in clock gene Per-1 deficient-mice after cerebral ischemia. Curr. Neurovasc. Res. 10, 112–125 (2013).
Magnone, M. C. et al. The mammalian circadian clock gene per2 modulates cell death in response to oxidative stress. Front. Neurol. 5, 289 (2014).
Lananna, B. V. et al. Cell-autonomous regulation of astrocyte activation by the circadian clock protein BMAL1. Cell Rep. 25, 1–9.e5 (2018).
Mullenders, J., Fabius, A. W., Madiredjo, M., Bernards, R. & Beijersbergen, R. L. A large scale shRNA barcode screen identifies the circadian clock component ARNTL as putative regulator of the p53 tumor suppressor pathway. PLoS ONE 4, e4798 (2009).
Esposito, E. et al. Potential circadian effects on translational failure for neuroprotection. Nature 582, 395–398 (2020). This study systematically investigated whether the efficacy of neuroprotective strategies depends on zeitgeber time and offers pathophysiological explanations for the identified differences.
Beker, M. C. et al. Time-of-day dependent neuronal injury after ischemic stroke: implication of circadian clock transcriptional factor Bmal1 and survival kinase AKT. Mol. Neurobiol. 55, 2565–2576 (2018).
Reidler, P. et al. Circadian rhythm of ischaemic core progression in human stroke. J. Neurol. Neurosurg. Psychiatry, https://doi.org/10.1136/jnnp-2021-326072 (2021).
Ryu, W. S. et al. Association of ischemic stroke onset time with presenting severity, acute progression, and long-term outcome: a cohort study. PLoS Med. 19, e1003910 (2022).
Lorenzano, S. et al. Within-day and weekly variations of thrombolysis in acute ischemic stroke: results from Safe Implementation of Treatments in Stroke–International Stroke Thrombolysis Register. Stroke 45, 176–184 (2014).
Hajdu, S. D. et al. Association of time of day when endovascular therapy for stroke starts and functional outcome. Neurology 96, e1124–e1136 (2021).
Hirota, T. et al. High-throughput chemical screen identifies a novel potent modulator of cellular circadian rhythms and reveals CKIα as a clock regulatory kinase. PLoS Biol. 8, e1000559 (2010).
Preiser, J. C., Ichai, C., Orban, J. C. & Groeneveld, A. B. Metabolic response to the stress of critical illness. Br. J. Anaesth. 113, 945–954 (2014).
Porter, C. et al. The metabolic stress response to burn trauma: current understanding and therapies. Lancet 388, 1417–1426 (2016).
Skafida, A. et al. In-hospital dynamics of glucose, blood pressure and temperature predict outcome in patients with acute ischaemic stroke. Eur. Stroke J. 3, 174–184 (2018).
Oesch, L., Tatlisumak, T., Arnold, M. & Sarikaya, H. Obesity paradox in stroke–myth or reality? A systematic review. PLoS ONE 12, e0171334 (2017).
Jonsson, A. C., Lindgren, I., Norrving, B. & Lindgren, A. Weight loss after stroke: a population-based study from the Lund Stroke Register. Stroke 39, 918–923 (2008).
Kim, Y. et al. Prognostic importance of weight change on short-term functional outcome in acute ischemic stroke. Int. J. Stroke 10, 62–68 (2015).
Uyttenboogaart, M. et al. Moderate hyperglycaemia is associated with favourable outcome in acute lacunar stroke. Brain 130, 1626–1630 (2007).
Capes, S. E., Hunt, D., Malmberg, K., Pathak, P. & Gerstein, H. C. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke 32, 2426–2432 (2001).
Davalos, A. et al. Effect of malnutrition after acute stroke on clinical outcome. Stroke 27, 1028–1032 (1996).
Chalela, J. A., Haymore, J., Schellinger, P. D., Kang, D. W. & Warach, S. Acute stroke patients are being underfed: a nitrogen balance study. Neurocrit. Care 1, 331–334 (2004).
Springer, J. et al. Catabolic signaling and muscle wasting after acute ischemic stroke in mice: indication for a stroke-specific sarcopenia. Stroke 45, 3675–3683 (2014).
Luitse, M. J., Biessels, G. J., Rutten, G. E. & Kappelle, L. J. Diabetes, hyperglycaemia, and acute ischaemic stroke. Lancet Neurol. 11, 261–271 (2012). An excellent review that disentangles the effects of diabetes and hyperglycaemia on stroke outcome.
Soty, M., Gautier-Stein, A., Rajas, F. & Mithieux, G. Gut-brain glucose signaling in energy homeostasis. Cell Metab. 25, 1231–1242 (2017).
Magistretti, P. J. & Allaman, I. A cellular perspective on brain energy metabolism and functional imaging. Neuron 86, 883–901 (2015).
Jais, A. et al. Myeloid-cell-derived VEGF maintains brain glucose uptake and limits cognitive impairment in obesity. Cell 165, 882–895 (2016).
Herrero-Mendez, A. et al. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat. Cell Biol. 11, 747–752 (2009).
Yip, J., Geng, X., Shen, J. & Ding, Y. Cerebral gluconeogenesis and diseases. Front. Pharmacol. 7, 521 (2016).
Poe, G. R. et al. Locus coeruleus: a new look at the blue spot. Nat. Rev. Neurosci. 21, 644–659 (2020).
Gibson, C. L., Murphy, A. N. & Murphy, S. P. Stroke outcome in the ketogenic state–a systematic review of the animal data. J. Neurochem. 123, 52–57 (2012).
Puchalska, P. & Crawford, P. A. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 25, 262–284 (2017).
Shimazu, T. et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 339, 211–214 (2013).
Kimura, I. et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc. Natl Acad. Sci. USA 108, 8030–8035 (2011).
Rahman, M. et al. The beta-hydroxybutyrate receptor HCA2 activates a neuroprotective subset of macrophages. Nat. Commun. 5, 3944 (2014).
Youm, Y. H. et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 21, 263–269 (2015).
Saxton, R. A. & Sabatini, D. M. mTOR signaling in growth, metabolism, and disease. Cell 169, 361–371 (2017).
Chong, Z. Z., Yao, Q. & Li, H. H. The rationale of targeting mammalian target of rapamycin for ischemic stroke. Cell Signal 25, 1598–1607 (2013).
DeYoung, M. P., Horak, P., Sofer, A., Sgroi, D. & Ellisen, L. W. Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev. 22, 239–251 (2008).
Papadakis, M. et al. Tsc1 (hamartin) confers neuroprotection against ischemia by inducing autophagy. Nat. Med. 19, 351–357 (2013).
Hadley, G. et al. Rapamycin in ischemic stroke: old drug, new tricks? J. Cereb. Blood Flow Metab. 39, 20–35 (2019).
Wang, C. et al. Targeting the mTOR signaling network for Alzheimer’s disease therapy. Mol. Neurobiol. 49, 120–135 (2014).
Sharkey, J. & Butcher, S. P. Immunophilins mediate the neuroprotective effects of FK506 in focal cerebral ischaemia. Nature 371, 336–339 (1994).
Chi, O. Z. et al. Effects of rapamycin on cerebral oxygen supply and consumption during reperfusion after cerebral ischemia. Neuroscience 316, 321–327 (2016).
Chauhan, A., Sharma, U., Jagannathan, N. R., Reeta, K. H. & Gupta, Y. K. Rapamycin protects against middle cerebral artery occlusion induced focal cerebral ischemia in rats. Behav. Brain Res. 225, 603–609 (2011).
Beard, D. J. et al. The effect of rapamycin treatment on cerebral ischemia: a systematic review and meta-analysis of animal model studies. Int. J. Stroke 14, 137–145 (2019).
Goossens, C. et al. Adipose tissue protects against sepsis-induced muscle weakness in mice: from lipolysis to ketones. Crit. Care 23, 236 (2019).
Lumeng, C. N., Bodzin, J. L. & Saltiel, A. R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 (2007).
Pepping, J. K., Freeman, L. R., Gupta, S., Keller, J. N. & Bruce-Keller, A. J. NOX2 deficiency attenuates markers of adiposopathy and brain injury induced by high-fat diet. Am. J. Physiol. Endocrinol. Metab. 304, E392–E404 (2013).
Takechi, R., Pallebage-Gamarallage, M. M., Lam, V., Giles, C. & Mamo, J. C. Nutraceutical agents with anti-inflammatory properties prevent dietary saturated-fat induced disturbances in blood-brain barrier function in wild-type mice. J. Neuroinflamm. 10, 73 (2013).
Haley, M. J. et al. Acute high-fat feeding leads to disruptions in glucose homeostasis and worsens stroke outcome. J. Cereb. Blood Flow Metab. 39, 1026–1037 (2019).
Wu, M. H. et al. Obesity exacerbates rat cerebral ischemic injury through enhancing ischemic adiponectin-containing neuronal apoptosis. Mol. Neurobiol. 53, 3702–3713 (2016).
Peterson, T. C. et al. Obesity drives delayed infarct expansion, inflammation, and distinct gene networks in a mouse stroke model. Transl. Stroke Res. 12, 331–346 (2021).
Guo, D. H. et al. Beige adipocytes mediate the neuroprotective and anti-inflammatory effects of subcutaneous fat in obese mice. Nat. Commun. 12, 4623 (2021).
Liu, Z. et al. Adiposity and outcome after ischemic stroke: obesity paradox for mortality and obesity parabola for favorable functional outcomes. Stroke 52, 144–151 (2021).
McCay, C. M., Maynard, L. A., Sperling, G. & Barnes, L. L. Retarded growth, life span, ultimate body size and age changes in the albino rat after feeding diets restricted in calories. Nutr. Rev. 18, 1–13 (1939).
Mattison, J. A. et al. Caloric restriction improves health and survival of rhesus monkeys. Nat. Commun. 8, 14063 (2017).
Gabande-Rodriguez, E., Gomez de Las Heras, M. M. & Mittelbrunn, M. Control of inflammation by calorie restriction mimetics: on the crossroad of autophagy and mitochondria. Cells 9, 82 (2019).
Duregon, E., Pomatto-Watson, L., Bernier, M., Price, N. L. & de Cabo, R. Intermittent fasting: from calories to time restriction. Geroscience 43, 1083–1092 (2021).
Mattson, M. P., Longo, V. D. & Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 39, 46–58 (2017).
FOOD Trial Collaboration. Poor nutritional status on admission predicts poor outcomes after stroke: observational data from the FOOD trial. Stroke 34, 1450–1456 (2003).
Scherbakov, N., Dirnagl, U. & Doehner, W. Body weight after stroke: lessons from the obesity paradox. Stroke 42, 3646–3650 (2011).
Lourbopoulos, A. et al. Inadequate food and water intake determine mortality following stroke in mice. J. Cereb. Blood Flow Metab. 37, 2084–2097 (2017).
Dennis, M. S., Lewis, S. C., Warlow, C. & Collaboration, F. T. Routine oral nutritional supplementation for stroke patients in hospital (FOOD): a multicentre randomised controlled trial. Lancet 365, 755–763 (2005).
Faraco, G. et al. Dietary salt promotes cognitive impairment through tau phosphorylation. Nature 574, 686–690 (2019). An outstanding example of how a dietary component can be mechanistically linked to outcomes at the NVU.
Shi, K. et al. Global brain inflammation in stroke. Lancet Neurol. 18, 1058–1066 (2019).
Iadecola, C. & Anrather, J. The immunology of stroke: from mechanisms to translation. Nat. Med. 17, 796–808 (2011).
Zhang, S. R., Phan, T. G. & Sobey, C. G. Targeting the immune system for ischemic stroke. Trends Pharmacol. Sci. 42, 96–105 (2021).
Iadecola, C., Buckwalter, M. S. & Anrather, J. Immune responses to stroke: mechanisms, modulation, and therapeutic potential. J. Clin. Invest. 130, 2777–2788 (2020).
Carson, M. J., Doose, J. M., Melchior, B., Schmid, C. D. & Ploix, C. C. CNS immune privilege: hiding in plain sight. Immunol. Rev. 213, 48–65 (2006).
Croese, T., Castellani, G. & Schwartz, M. Immune cell compartmentalization for brain surveillance and protection. Nat. Immunol. 22, 1083–1092 (2021).
Kierdorf, K., Masuda, T., Jordao, M. J. C. & Prinz, M. Macrophages at CNS interfaces: ontogeny and function in health and disease. Nat. Rev. Neurosci. 20, 547–562 (2019).
Wilson, E. H., Weninger, W. & Hunter, C. A. Trafficking of immune cells in the central nervous system. J. Clin. Invest. 120, 1368–1379 (2010).
Mastorakos, P. & McGavern, D. The anatomy and immunology of vasculature in the central nervous system. Sci. Immunol. 4, eaav0492 (2019).
Rustenhoven, J. et al. Functional characterization of the dural sinuses as a neuroimmune interface. Cell 184, 1000–1016.e27 (2021). One of the key studies in which novel neuroimmune interfaces were identified.
Li, Q. & Barres, B. A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 18, 225–242 (2018).
Mogensen, F. L., Delle, C. & Nedergaard, M. The Glymphatic System (En)during Inflammation. Int. J. Mol. Sci. 22, 7491 (2021).
Borlongan, C. V. Bone marrow stem cell mobilization in stroke: a ‘bonehead’ may be good after all! Leukemia 25, 1674–1686 (2011).
Denes, A. et al. Experimental stroke-induced changes in the bone marrow reveal complex regulation of leukocyte responses. J. Cereb. Blood Flow. Metab. 31, 1036–1050 (2011).
Pulous, F. E. et al. Cerebrospinal fluid can exit into the skull bone marrow and instruct cranial hematopoiesis in mice with bacterial meningitis. Nat Neurosci. 25, 567–576 (2022).
Cugurra, A. et al. Skull and vertebral bone marrow are myeloid cell reservoirs for the meninges and CNS parenchyma. Science 373, eabf7844 (2021).
Brioschi, S. et al. Heterogeneity of meningeal B cells reveals a lymphopoietic niche at the CNS borders. Science 373, eabf9277 (2021).
Kolabas, Z. I. et al. Multi-omics and 3D-imaging reveal bone heterogeneity and unique calvaria cells in neuroinflammation. bioRxiv https://doi.org/10.1101/2021.12.24.473988 (2021).
Lucas, D., Battista, M., Shi, P. A., Isola, L. & Frenette, P. S. Mobilized hematopoietic stem cell yield depends on species-specific circadian timing. Cell Stem Cell 3, 364–366 (2008).
Mendez-Ferrer, S., Lucas, D., Battista, M. & Frenette, P. S. Haematopoietic stem cell release is regulated by circadian oscillations. Nature 452, 442–447 (2008).
Natoli, G. & Ostuni, R. Adaptation and memory in immune responses. Nat. Immunol. 20, 783–792 (2019).
Ng, L. G., Ostuni, R. & Hidalgo, A. Heterogeneity of neutrophils. Nat. Rev. Immunol. 19, 255–265 (2019).
Casanova-Acebes, M. et al. Neutrophils instruct homeostatic and pathological states in naive tissues. J. Exp. Med. 215, 2778–2795 (2018).
Ballesteros, I. et al. Co-option of neutrophil fates by tissue environments. Cell 183, 1282–1297.e18 (2020).
Cuartero, M. I. et al. N2 neutrophils, novel players in brain inflammation after stroke: modulation by the PPARγ agonist rosiglitazone. Stroke 44, 3498–3508 (2013).
Garcia-Culebras, A. et al. Role of TLR4 (Toll-like receptor 4) in N1/N2 neutrophil programming after stroke. Stroke 50, 2922–2932 (2019).
Cai, W. et al. Functional dynamics of neutrophils after ischemic stroke. Transl. Stroke Res. 11, 108–121 (2020).
Sas, A. R. et al. A new neutrophil subset promotes CNS neuron survival and axon regeneration. Nat. Immunol. 21, 1496–1505 (2020).
Roy-O’Reilly, M. A. et al. Aging exacerbates neutrophil pathogenicity in ischemic stroke. Aging 12, 436–461 (2020).
Camilleri, M. Gastrointestinal motility disorders in neurologic disease. J. Clin. Invest. 131, e143771 (2021).
Benakis, C. et al. The microbiome-gut-brain axis in acute and chronic brain diseases. Curr. Opin. Neurobiol. 61, 1–9 (2020).
Gilbert, J. A. et al. Current understanding of the human microbiome. Nat. Med. 24, 392–400 (2018).
Rooks, M. G. & Garrett, W. S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 16, 341–352 (2016).
Claesson, M. J. et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 488, 178–184 (2012).
Wastyk, H. C. et al. Gut-microbiota-targeted diets modulate human immune status. Cell 184, 4137–4153.e14 (2021).
Bishehsari, F., Voigt, R. M. & Keshavarzian, A. Circadian rhythms and the gut microbiota: from the metabolic syndrome to cancer. Nat. Rev. Endocrinol. 16, 731–739 (2020).
Klunemann, M. et al. Bioaccumulation of therapeutic drugs by human gut bacteria. Nature 597, 533–538 (2021).
Mazmanian, S. K., Liu, C. H., Tzianabos, A. O. & Kasper, D. L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122, 107–118 (2005).
Blander, J. M., Longman, R. S., Iliev, I. D., Sonnenberg, G. F. & Artis, D. Regulation of inflammation by microbiota interactions with the host. Nat. Immunol. 18, 851–860 (2017).
Benakis, C. et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat. Med. 22, 516–523 (2016).
Agirman, G. & Hsiao, E. Y. SnapShot: the microbiota-gut-brain axis. Cell 184, 2524–2524.e1 (2021).
Delgado Jimenez, R. & Benakis, C. The gut ecosystem: a critical player in stroke. Neuromolecular Med. 23, 236–241 (2021).
Schroeder, B. O. & Backhed, F. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 22, 1079–1089 (2016).
Sampson, T. R. & Mazmanian, S. K. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe 17, 565–576 (2015).
Tang, W. H. et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 368, 1575–1584 (2013).
Wang, Z. et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472, 57–63 (2011). This seminal study identified TMAO as a key microbiota-dependent metabolite that triggers cardiovascular disease.
Farzi, A., Frohlich, E. E. & Holzer, P. Gut microbiota and the neuroendocrine system. Neurotherapeutics 15, 5–22 (2018).
Singh, V. et al. Microbiota dysbiosis controls the neuroinflammatory response after stroke. J. Neurosci. 36, 7428–7440 (2016). Through a series of transplantation experiments, this study disentangled the bidirectional effects between gut microbiota and the brain after experimental stroke.
Houlden, A. et al. Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain Behav. Immun. 57, 10–20 (2016).
Stanley, D., Moore, R. J. & Wong, C. H. Y. An insight into intestinal mucosal microbiota disruption after stroke. Sci. Rep. 8, 568 (2018).
Duvallet, C., Gibbons, S. M., Gurry, T., Irizarry, R. A. & Alm, E. J. Meta-analysis of gut microbiome studies identifies disease-specific and shared responses. Nat. Commun. 8, 1784 (2017).
Singh, V. et al. The gut microbiome primes a cerebroprotective immune response after stroke. J. Cereb. Blood Flow Metab. 38, 1293–1298 (2018).
Stanley, D. et al. Translocation and dissemination of commensal bacteria in post-stroke infection. Nat. Med. 22, 1277–1284 (2016).
Erny, D. et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18, 965–977 (2015).
Erny, D. et al. Microbiota-derived acetate enables the metabolic fitness of the brain innate immune system during health and disease. Cell Metab. 33, 2260–2276.e7 (2021).
Lee, J. et al. Gut microbiota-derived short-chain fatty acids promote poststroke recovery in aged mice. Circ. Res. 127, 453–465 (2020).
Sadler, R. et al. Short-chain fatty acids improve poststroke recovery via immunological mechanisms. J. Neurosci. 40, 1162–1173 (2020).
Braniste, V. et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 6, 263ra158 (2014).
Marques, F. Z., Mackay, C. R. & Kaye, D. M. Beyond gut feelings: how the gut microbiota regulates blood pressure. Nat. Rev. Cardiol. 15, 20–32 (2018).
Chu, C. et al. The microbiota regulate neuronal function and fear extinction learning. Nature 574, 543–548 (2019).
Benakis, C. et al. Distinct commensal bacterial signature in the gut is associated with acute and long-term protection from ischemic stroke. Stroke 51, 1844–1854 (2020).
Walter, J., Armet, A. M., Finlay, B. B. & Shanahan, F. Establishing or exaggerating causality for the gut microbiome: lessons from human microbiota-associated rodents. Cell 180, 221–232 (2020).
Yamashiro, K. et al. Gut dysbiosis is associated with metabolism and systemic inflammation in patients with ischemic stroke. PLoS ONE 12, e0171521 (2017).
Zhu, W. et al. Gut microbes impact stroke severity via the trimethylamine N-oxide pathway. Cell Host Microbe 29, 1199–1208.e5 (2021). This study showed that the metabolites produced by the microbiota are important for stroke outcome and provided an insight into what personalized and targeted approaches for microbiota recolonization could look like.
Maier, L. et al. Unravelling the collateral damage of antibiotics on gut bacteria. Nature 599, 120–124 (2021).
Yang, A. C. et al. A human brain vascular atlas reveals diverse mediators of Alzheimer’s risk. Nature 603, 885–892 (2022).
Hugenholtz, F. & de Vos, W. M. Mouse models for human intestinal microbiota research: a critical evaluation. Cell. Mol. Life Sci. 75, 149–160 (2018).
Nguyen, T. L., Vieira-Silva, S., Liston, A. & Raes, J. How informative is the mouse for human gut microbiota research. Dis. Model. Mech. 8, 1–16 (2015).
Nagpal, R. et al. Comparative microbiome signatures and short-chain fatty acids in mouse, rat, non-human primate, and human feces. Front. Microbiol. 9, 2897 (2018).
Mestas, J. & Hughes, C. C. Of mice and not men: differences between mouse and human immunology. J. Immunol. 172, 2731–2738 (2004).
Healy, L. M., Yaqubi, M., Ludwin, S. & Antel, J. P. Species differences in immune-mediated CNS tissue injury and repair: a (neuro)inflammatory topic. Glia 68, 811–829 (2020).
Becker, K. J. Strain-related differences in the immune response: relevance to human stroke. Transl. Stroke Res. 7, 303–312 (2016).
Bosetti, F. et al. Translational stroke research: vision and opportunities. Stroke 48, 2632–2637 (2017).
Cho, S. & Yang, J. What do experimental models teach us about comorbidities in stroke. Stroke 49, 501–507 (2018).
Winter, C. et al. Chrono-pharmacological targeting of the CCL2-CCR2 axis ameliorates atherosclerosis. Cell Metab. 28, 175–182.e5 (2018). Serving as an example for future studies in the context of stroke, this work identified the diurnal rhythm of a driver of myeloid cell recruitment and used this to develop a chrono-pharmacological approach against atherosclerosis.
George, P. M. & Steinberg, G. K. Novel stroke therapeutics: unraveling stroke pathophysiology and its impact on clinical treatments. Neuron 87, 297–309 (2015).
Lyden, P. et al. Final results of the RHAPSODY trial: a multi-center, phase 2 trial using a continual reassessment method to determine the safety and tolerability of 3K3A-APC, a recombinant variant of human activated protein C, in combination with tissue plasminogen activator, mechanical thrombectomy or both in moderate to severe acute ischemic stroke. Ann. Neurol. 85, 125–136 (2019).
Lyden, P. D. et al. Stroke treatment with PAR-1 agents to decrease hemorrhagic transformation. Front. Neurol. 12, 593582 (2021).
Lyu, Z. et al. A neurovascular-unit-on-a-chip for the evaluation of the restorative potential of stem cell therapies for ischaemic stroke. Nat. Biomed. Eng. 5, 847–863 (2021).
Mansour, A. A. et al. An in vivo model of functional and vascularized human brain organoids. Nat. Biotechnol. 36, 432–441 (2018).
Vunjak-Novakovic, G., Ronaldson-Bouchard, K. & Radisic, M. Organs-on-a-chip models for biological research. Cell 184, 4597–4611 (2021).
Sorbie, A., Delgado Jimenez, R. & Benakis, C. Increasing transparency and reproducibility in stroke-microbiota research: a toolbox for microbiota analysis. iScience 25, 103998 (2022).
Wu, G. D. et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108 (2011).
Thaiss, C. A. et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 159, 514–529 (2014).
Lax, S. et al. Longitudinal analysis of microbial interaction between humans and the indoor environment. Science 345, 1048–1052 (2014).
Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214 (2012).
Wilmanski, T. et al. Blood metabolome predicts gut microbiome α-diversity in humans. Nat. Biotechnol. 37, 1217–1228 (2019).
Chu, H. et al. Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel disease. Science 352, 1116–1120 (2016).
Juul, F. E. et al. Fecal microbiota transplantation for primary Clostridium difficile infection. N. Engl. J. Med. 378, 2535–2536 (2018).
Chen, R. Y. et al. A microbiota-directed food intervention for undernourished children. N. Engl. J. Med. 384, 1517–1528 (2021).
Gehrig, J. L. et al. Effects of microbiota-directed foods in gnotobiotic animals and undernourished children. Science 365, eaau4732 (2019).
Kolarski, D. et al. Reversible modulation of circadian time with chronophotopharmacology. Nat. Commun. 12, 3164 (2021).
Heindl, S. et al. Chronic T cell proliferation in brains after stroke could interfere with the efficacy of immunotherapies. J. Exp. Med. 218, e20202411 (2021).
Acknowledgements
We thank C. Benakis (Institute for Stroke and Dementia Research, University hospital, Ludwig-Maximilians-Universität München, Germany) and M. Merrow (Institute of Medical Psychology, Ludwig-Maximilians-Universität München, Germany) for critical revision of parts of the manuscript. S.T. is supported by a grant from the Corona Foundation. A.M.B. is supported by a visiting fellowship from the Einstein Foundation Berlin. All authors are supported by a grant from the Leducq Foundation.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
A.M.B. is a co-founder of Brainomix. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neurology thanks D. Hermann, D. Michalski, S. Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Circadian rhythms
-
The ~24 h rhythmic temporal programmes found within almost all cells.
- Glymphatic system
-
The cerebral glial-associated functional homologue of the peripheral lymphatic system that clears waste from the brain.
- Perivascular spaces
-
The passageways surrounding cerebral microvessels that contribute to waste clearance.
- Zeitgebers
-
Regular environmental signals that entrain circadian clocks.
- Damage-associated molecular patterns
-
(DAMPS). The endogenous danger molecules that are released from damaged or dying cells.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tiedt, S., Buchan, A.M., Dichgans, M. et al. The neurovascular unit and systemic biology in stroke — implications for translation and treatment. Nat Rev Neurol 18, 597–612 (2022). https://doi.org/10.1038/s41582-022-00703-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-022-00703-z
This article is cited by
-
Macrophages and endothelial cells in the neurovascular unit
Acta Neuropathologica (2024)
-
A year in review: brain barriers and brain fluids research in 2022
Fluids and Barriers of the CNS (2023)
-
Combining atomic force microscopy and fluorescence-based techniques to explore mechanical properties of naive and ischemia-affected brain regions in mice
Scientific Reports (2023)
-
Feature extraction of EEG images by using soft computing methods
Soft Computing (2023)