Abstract
Evidence for a close bidirectional link between the brain and the gut has led to a paradigm shift in neurology, especially in the case of Parkinson disease (PD), in which gastrointestinal dysfunction is a prominent feature. Over the past decade, numerous high-quality preclinical and clinical publications have shed light on the highly complex relationship between the gut and the brain in PD, providing potential for the development of new biomarkers and therapeutics. With the advent of high-throughput sequencing, the role of the gut microbiome has been specifically highlighted. Here, we provide a critical review of the literature on the microbiome–gut–brain axis in PD and present perspectives that will be useful for clinical practice. We begin with an overview of the gut–brain axis in PD, including the potential roles and interrelationships of the vagus nerve, α-synuclein in the enteric nervous system, altered intestinal permeability and inflammation, and gut microbes and their metabolic activities. The sections that follow synthesize the proposed roles of gut-related factors in the development and progression of, in responses to PD treatment, and as therapeutic targets. Finally, we summarize current knowledge gaps and challenges and delineate future directions for the field.
Key points
-
Evidence is accumulating that the gut–brain axis has an important role in Parkinson disease (PD) risk and progression.
-
Key events include: ‘unhealthy’ alterations in gut microbiome structure and/or function; gut inflammation and hyperpermeability; and seeding and propagation of α-synuclein in the enteric nervous system. Ageing has an influence on all of these events.
-
Investigations into gut bacterial composition and activity have been greatly facilitated by ‘omics’ technologies; future progress will require greater collaboration to obtain large, longitudinal datasets and to develop harmonized research protocols.
-
Various gut disorders (including infections, dysbiosis, inflammation and dysmotility), gut interventions and dietary factors have been linked to PD development and progression, and have been shown to influence the response to PD medication.
-
Higher-resolution mechanistic studies are needed to understand the observed associations at the molecular, cellular and systems levels, and will aid the translation of microbiome science into new diagnostic, prognostic and therapeutic approaches.
-
Gut-related factors provide multiple potential targets for biomarker development and interventional strategies that should be explored in well-designed studies; microbiome-directed therapeutics to treat PD manifestations are already emerging.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Lim, S. Y. et al. Parkinson’s disease in the Western Pacific region. Lancet Neurol. 18, 865–879 (2019).
Bloem, B. R., Okun, M. S. & Klein, C. Parkinson’s disease. Lancet 397, 2284–2303 (2021).
Pfeiffer, R. F. Gastrointestinal dysfunction in Parkinson’s disease. Curr. Treat. Options Neurol. 20, 54 (2018).
Lynch, S. V. & Pedersen, O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 375, 2369–2379 (2016).
Cryan, J. F., O’Riordan, K. J., Sandhu, K., Peterson, V. & Dinan, T. G. The gut microbiome in neurological disorders. Lancet Neurol. 19, 179–194 (2020).
Fang, P., Kazmi, S. A., Jameson, K. G. & Hsiao, E. Y. The microbiome as a modifier of neurodegenerative disease risk. Cell Host Microbe 28, 201–222 (2020).
Willyard, C. How gut microbes could drive brain disorders. Nature 590, 22–25 (2021).
Tan, A. H. et al. Gut microbial ecosystem in Parkinson disease: new clinicobiological insights from multi-omics. Ann. Neurol. 89, 546–559 (2021).
Wakabayashi, K., Takahashi, H., Takeda, S., Ohama, E. & Ikuta, F. Parkinson’s disease: the presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta Neuropathol. 76, 217–221 (1988).
Braak, H. et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 24, 197–211 (2003).
Breen, D. P., Halliday, G. M. & Lang, A. E. Gut-brain axis and the spread of α-synuclein pathology: vagal highway or dead end? Mov. Disord. 34, 307–316 (2019).
Klingelhoefer, L. & Reichmann, H. Pathogenesis of Parkinson disease — the gut-brain axis and environmental factors. Nat. Rev. Neurol. 11, 625–636 (2015).
Horsager, J. et al. Brain-first versus body-first Parkinson’s disease: a multimodal imaging case-control study. Brain 143, 3077–3088 (2020).
Adams-Carr, K. L. et al. Constipation preceding Parkinson’s disease: a systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 87, 710–716 (2016).
Scott, G. D., Lim, M. M., Drake, M. G., Woltjer, R. & Quinn, J. F. Onset of skin, gut, and genitourinary prodromal Parkinson’s disease: a study of 1.5 million veterans. Mov. Disord. 36, 2094–2103 (2021).
Liu, B. et al. Vagotomy and Parkinson disease: a Swedish register-based matched-cohort study. Neurology 88, 1996–2002 (2017).
Svensson, E. et al. Vagotomy and subsequent risk of Parkinson’s disease. Ann. Neurol. 78, 522–529 (2015).
Engelender, S. & Isacson, O. The threshold theory for Parkinson’s disease. Trends Neurosci. 40, 4–14 (2017).
Tysnes, O. B. et al. Does vagotomy reduce the risk of Parkinson’s disease? Ann. Neurol. 78, 1011–1012 (2015).
Knudsen, K. et al. In-vivo staging of pathology in REM sleep behaviour disorder: a multimodality imaging case-control study. Lancet Neurol. 17, 618–628 (2018).
Hilton, D. et al. Accumulation of α-synuclein in the bowel of patients in the pre-clinical phase of Parkinson’s disease. Acta Neuropathol. 127, 235–241 (2014).
Shannon, K. M., Keshavarzian, A., Dodiya, H. B., Jakate, S. & Kordower, J. H. Is alpha-synuclein in the colon a biomarker for premotor Parkinson’s disease? Evidence from 3 cases. Mov. Disord. 27, 716–719 (2012).
Stokholm, M. G., Danielsen, E. H., Hamilton-Dutoit, S. J. & Borghammer, P. Pathological α-synuclein in gastrointestinal tissues from prodromal Parkinson disease patients. Ann. Neurol. 79, 940–949 (2016).
Beach, T. G. et al. Multi-organ distribution of phosphorylated α-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 119, 689–702 (2010).
Tanei, Z. I. et al. Lewy pathology of the esophagus correlates with the progression of Lewy body disease: a Japanese cohort study of autopsy cases. Acta Neuropathol. 141, 25–37 (2021).
Beach, T. G. et al. Vagus nerve and stomach synucleinopathy in Parkinson’s disease, incidental Lewy body disease, and normal elderly subjects: evidence against the “body-first” hypothesis. J. Parkinsons Dis. 11, 1833–1843 (2021).
Van Den Berge, N. et al. Evidence for bidirectional and trans-synaptic parasympathetic and sympathetic propagation of alpha-synuclein in rats. Acta Neuropathol. 138, 535–550 (2019).
Killinger, B. & Labrie, V. The appendix in Parkinson’s disease: from vestigial remnant to vital organ? J. Parkinsons Dis. 9, S345–S358 (2019).
Tsukita, K., Sakamaki-Tsukita, H., Tanaka, K., Suenaga, T. & Takahashi, R. Value of in vivo α-synuclein deposits in Parkinson’s disease: a systematic review and meta-analysis. Mov. Disord. 34, 1452–1463 (2019).
Annerino, D. M. et al. Parkinson’s disease is not associated with gastrointestinal myenteric ganglion neuron loss. Acta Neuropathol. 124, 665–680 (2012).
Holmqvist, S. et al. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 128, 805–820 (2014).
Kim, S. et al. Transneuronal propagation of pathologic α-synuclein from the gut to the brain models Parkinson’s disease. Neuron 103, 627–641 (2019).
Challis, C. et al. Gut-seeded α-synuclein fibrils promote gut dysfunction and brain pathology specifically in aged mice. Nat. Neurosci. 23, 327–336 (2020).
Van Den Berge, N. et al. Ageing promotes pathological alpha-synuclein propagation and autonomic dysfunction in wild-type rats. Brain 144, 1853–1868 (2021).
Rota, L. et al. Constipation, deficit in colon contractions and alpha-synuclein inclusions within the colon precede motor abnormalities and neurodegeneration in the central nervous system in a mouse model of alpha-synucleinopathy. Transl. Neurodegener. 8, 5 (2019).
Gries, M. et al. Parkinson mice show functional and molecular changes in the gut long before motoric disease onset. Mol. Neurodegener. 16, 34 (2021).
Chen, S. G. et al. Exposure to the functional bacterial amyloid protein curli enhances alpha-synuclein aggregation in aged fischer 344 rats and Caenorhabditis elegans. Sci. Rep. 6, 34477 (2016).
Sampson, T. R. et al. A gut bacterial amyloid promotes α-synuclein aggregation and motor impairment in mice. Elife 9, e53111 (2020).
Leclair-Visonneau, L., Neunlist, M., Derkinderen, P. & Lebouvier, T. The gut in Parkinson’s disease: bottom-up, top-down, or neither? Neurogastroenterol. Motil. 32, e13777 (2020).
Ulusoy, A. et al. Brain-to-stomach transfer of α-synuclein via vagal preganglionic projections. Acta Neuropathol. 133, 381–393 (2017).
Matteoli, G. & Boeckxstaens, G. E. The vagal innervation of the gut and immune homeostasis. Gut 62, 1214–1222 (2013).
Arotcarena, M. L. et al. Bidirectional gut-to-brain and brain-to-gut propagation of synucleinopathy in non-human primates. Brain 143, 1462–1475 (2020).
Kuan, W. L. et al. Systemic α-synuclein injection triggers selective neuronal pathology as seen in patients with Parkinson’s disease. Mol. Psychiatry 26, 556–567 (2021).
Pellegrini, C. et al. Alteration of colonic excitatory tachykininergic motility and enteric inflammation following dopaminergic nigrostriatal neurodegeneration. J. Neuroinflamm. 13, 146 (2016).
O’Donovan, S. M. et al. Nigral overexpression of α-synuclein in a rat Parkinson’s disease model indicates alterations in the enteric nervous system and the gut microbiome. Neurogastroenterol. Motil. 32, e13726 (2020).
Lionnet, A. et al. Does Parkinson’s disease start in the gut? Acta Neuropathol. 135, 1–12 (2018).
Forsyth, C. B. et al. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS ONE 6, e28032 (2011).
Kelly, L. P. et al. Progression of intestinal permeability changes and alpha-synuclein expression in a mouse model of Parkinson’s disease. Mov. Disord. 29, 999–1009 (2014).
Clairembault, T. et al. Structural alterations of the intestinal epithelial barrier in Parkinson’s disease. Acta Neuropathol. Commun. 3, 12 (2015).
Houser, M. C. et al. Stool immune profiles evince gastrointestinal inflammation in Parkinson’s disease. Mov. Disord. 33, 793–804 (2018).
Schwiertz, A. et al. Fecal markers of intestinal inflammation and intestinal permeability are elevated in Parkinson’s disease. Parkinsonism Relat. Disord. 50, 104–107 (2018).
Perez-Pardo, P. et al. Role of TLR4 in the gut-brain axis in Parkinson’s disease: a translational study from men to mice. Gut 68, 829–843 (2019).
Burgueno, J. F. & Abreu, M. T. Epithelial Toll-like receptors and their role in gut homeostasis and disease. Nat. Rev. Gastroenterol. Hepatol. 17, 263–278 (2020).
Kouli, A., Horne, C. B. & Williams-Gray, C. H. Toll-like receptors and their therapeutic potential in Parkinson’s disease and α-synucleinopathies. Brain Behav. Immun. 81, 41–51 (2019).
Devos, D. et al. Colonic inflammation in Parkinson’s disease. Neurobiol. Dis. 50, 42–48 (2013).
Clairembault, T. et al. Enteric GFAP expression and phosphorylation in Parkinson’s disease. J. Neurochem. 130, 805–815 (2014).
Benvenuti, L. et al. Enteric glia at the crossroads between intestinal immune system and epithelial barrier: implications for Parkinson disease. Int. J. Mol. Sci. 21, 9199 (2020).
Hor, J. W. et al. Fecal calprotectin in Parkinson’s disease and multiple system atrophy. J. Mov. Disord. https://doi.org/10.14802/jmd.21085 (2021).
Tan, E. K. et al. Parkinson disease and the immune system — associations, mechanisms and therapeutics. Nat. Rev. Neurol. 16, 303–318 (2020).
Houser, M. C. & Tansey, M. G. The gut-brain axis: is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? NPJ Parkinsons Dis. 3, 3 (2017).
Stolzenberg, E. B. et al. Role for neuronal alpha-synuclein in gastrointestinal immunity. J. Innate Immun. 9, 456–463 (2017).
Allen Reish, H. E. & Standaert, D. G. Role of α-synuclein in inducing innate and adaptive immunity in Parkinson disease. J. Parkinsons Dis. 5, 1–19 (2015).
Wang, W. et al. Caspase-1 causes truncation and aggregation of the Parkinson’s disease-associated protein α-synuclein. Proc. Natl Acad. Sci. USA 113, 9587–9592 (2016).
Grathwohl, S. et al. Specific immune modulation of experimental colitis drives enteric alpha-synuclein accumulation and triggers age-related Parkinson-like brain pathology. Free Neuropathol. 2, 13 (2021).
Resnikoff, H. et al. Colonic inflammation affects myenteric alpha-synuclein in nonhuman primates. J. Inflamm. Res. 12, 113–126 (2019).
Shannon, K. M. et al. Alpha-synuclein in colonic submucosa in early untreated Parkinson’s disease. Mov. Disord. 27, 709–715 (2012).
Prigent, A. et al. Enteric alpha-synuclein expression is increased in Crohn’s disease. Acta Neuropathol. 137, 359–361 (2019).
Chandra, R., Hiniker, A., Kuo, Y. M., Nussbaum, R. L. & Liddle, R. A. α-Synuclein in gut endocrine cells and its implications for Parkinson’s disease. JCI Insight 2, e92295 (2017).
Sampson, T. R. et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167, 1469–1480 (2016).
Matheoud, D. et al. Intestinal infection triggers Parkinson’s disease-like symptoms in Pink1(-/-) mice. Nature 571, 565–569 (2019).
Unger, M. M. et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat. Disord. 32, 66–72 (2016).
Rolli-Derkinderen, M. et al. Is Parkinson’s disease a chronic low-grade inflammatory bowel disease? J. Neurol. 267, 2207–2213 (2020).
Vancamelbeke, M. & Vermeire, S. The intestinal barrier: a fundamental role in health and disease. Expert. Rev. Gastroenterol. Hepatol. 11, 821–834 (2017).
Heintz-Buschart, A. & Wilmes, P. Human gut microbiome: function matters. Trends Microbiol. 26, 563–574 (2018).
Levy, M., Kolodziejczyk, A. A., Thaiss, C. A. & Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 17, 219–232 (2017).
Walter, J., Armet, A. M., Finlay, B. B. & Shanahan, F. Establishing or exaggerating causality for the gut microbiome: lessons from human microbiota-associated rodents. Cell 180, 221–232 (2020).
Schmidt, T. S. B., Raes, J. & Bork, P. The human gut microbiome: from association to modulation. Cell 172, 1198–1215 (2018).
David, L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563 (2014).
Yatsunenko, T. et al. Human gut microbiome viewed across age and geography. Nature 486, 222–227 (2012).
Falony, G. J. et al. Population level analysis of gut microbiome variation. Science 352, 560–564 (2020).
Maier, L. et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 555, 623–628 (2018).
Shanahan, F., Ghosh, T. S. & O’Toole, P. W. The healthy microbiome — what is the definition of a healthy gut microbiome? Gastroenterology 160, 483–494 (2021).
DeJong, E. N., Surette, M. G. & Bowdish, D. M. E. The gut microbiota and unhealthy aging: disentangling cause from consequence. Cell Host Microbe 28, 180–189 (2020).
Kundu, P., Blacher, E., Elinav, E. & Pettersson, S. Our gut microbiome: the evolving inner self. Cell 171, 1481–1493 (2017).
Claesson, M. J. et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 488, 178–184 (2012).
Franceschi, C., Garagnani, P., Parini, P., Giuliani, C. & Santoro, A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 14, 576–590 (2018).
Thevaranjan, N. et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe 21, 455–466 (2017).
Jeffery, I. B., Lynch, D. B. & O’Toole, P. W. Composition and temporal stability of the gut microbiota in older persons. ISME J. 10, 170–182 (2016).
Kong, F. et al. Gut microbiota signatures of longevity. Curr. Biol. 26, R832–R833 (2016).
Tuikhar, N. et al. Comparative analysis of the gut microbiota in centenarians and young adults shows a common signature across genotypically non-related populations. Mech. Ageing Dev. 179, 23–35 (2019).
Wu, L. et al. A cross-sectional study of compositional and functional profiles of gut microbiota in Sardinian centenarians. mSystems 4, e00325-19 (2019).
Rampelli, S. et al. Shotgun metagenomics of gut microbiota in humans with up to extreme longevity and the increasing role of xenobiotic degradation. mSystems 5, e00124-20 (2020).
Cirstea, M. S. et al. The gut mycobiome in Parkinson’s disease. J. Parkinsons Dis. 11, 153–158 (2021).
Bedarf, J. R. et al. Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naive Parkinson’s disease patients. Genome Med. 9, 39 (2017).
Hill-Burns, E. M. et al. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov. Disord. 32, 739–749 (2017).
Cirstea, M. S. et al. Microbiota composition and metabolism are associated with gut function in Parkinson’s disease. Mov. Disord. 35, 1208–1217 (2020).
Nishiwaki, H. et al. Meta-analysis of gut dysbiosis in Parkinson’s disease. Mov. Disord. 35, 1626–1635 (2020).
Barichella, M. et al. Unraveling gut microbiota in Parkinson’s disease and atypical parkinsonism. Mov. Disord. 34, 396–405 (2019).
Romano, S. et al. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinsons Dis. 7, 27 (2021).
Toh, T. S. et al. Gut microbiome in Parkinson’s disease: new insights from meta-analysis. Parkinsonism Relat. Disord. 94, 1–9 (2022).
Baldini, F. et al. Parkinson’s disease-associated alterations of the gut microbiome predict disease-relevant changes in metabolic functions. BMC Biol. 18, 62 (2020).
Wallen, Z. D. et al. Characterizing dysbiosis of gut microbiome in PD: evidence for overabundance of opportunistic pathogens. NPJ Parkinsons Dis. 6, 11 (2020).
Qian, Y. et al. Gut metagenomics-derived genes as potential biomarkers of Parkinson’s disease. Brain 143, 2474–2489 (2020).
Tan, A. H., Hor, J. W., Chong, C. W. & Lim, S. Y. Probiotics for Parkinson’s disease: current evidence and future directions. JGH Open. 5, 414–419 (2021).
Cani, P. D. Human gut microbiome: hopes, threats and promises. Gut 67, 1716–1725 (2018).
Tan, A. H. et al. Altered body composition, sarcopenia, frailty, and their clinico-biological correlates, in Parkinson’s disease. Parkinsonism Relat. Disord. 56, 58–64 (2018).
Yong, V. W. et al. Progressive and accelerated weight and body fat loss in Parkinson’s disease: a three-year prospective longitudinal study. Parkinsonism Relat. Disord. 77, 28–35 (2020).
Duvallet, C., Gibbons, S. M., Gurry, T., Irizarry, R. A. & Alm, E. J. Meta-analysis of gut microbiome studies identifies disease-specific and shared responses. Nat. Commun. 8, 1784 (2017).
Almeida, A. et al. A unified catalog of 204,938 reference genomes from the human gut microbiome. Nat. Biotechnol. 39, 105–114 (2021).
Vascellari, S. et al. Gut microbiota and metabolome alterations associated with Parkinson’s disease. mSystems 5, e00561-20 (2020).
Aho, V. T. E. et al. Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson’s disease. Mol. Neurodegener. 16, 6 (2021).
Dalile, B., Van Oudenhove, L., Vervliet, B. & Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 16, 461–478 (2019).
Sharma, S., Taliyan, R. & Singh, S. Beneficial effects of sodium butyrate in 6-OHDA induced neurotoxicity and behavioral abnormalities: modulation of histone deacetylase activity. Behav. Brain Res. 291, 306–314 (2015).
Rane, P. et al. The histone deacetylase inhibitor, sodium butyrate, alleviates cognitive deficits in pre-motor stage PD. Neuropharmacology 62, 2409–2412 (2012).
Srivastav, S. et al. Probiotics mixture increases butyrate, and subsequently rescues the nigral dopaminergic neurons from MPTP and rotenone-induced neurotoxicity. J. Nutr. Biochem. 69, 73–86 (2019).
Tan, A. H. et al. Altered gut microbiome and metabolome in patients with multiple system atrophy. Mov. Disord. 33, 174 (2018).
Rosario, D. et al. Systematic analysis of gut microbiome reveals the role of bacterial folate and homocysteine metabolism in Parkinson’s disease. Cell Rep. 34, 108807 (2021).
Pu, Y. et al. Antibiotic-induced microbiome depletion protects against MPTP-induced dopaminergic neurotoxicity in the brain. Aging 11, 6915–6929 (2019).
Xie, W. et al. Twice subacute MPTP administrations induced time-dependent dopaminergic neurodegeneration and inflammation in midbrain and ileum, as well as gut microbiota disorders in PD mice. Neurotoxicology 76, 200–212 (2020).
Zhu, Y. et al. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced Parkinson’s disease in mouse: potential association between neurotransmitter disturbance and gut microbiota dysbiosis. ACS Chem. Neurosci. 11, 3366–3376 (2020).
Lai, F. et al. Intestinal pathology and gut microbiota alterations in a methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson’s disease. Neurochem. Res. 43, 1986–1999 (2018).
Bhattarai, Y. et al. Role of gut microbiota in regulating gastrointestinal dysfunction and motor symptoms in a mouse model of Parkinson’s disease. Gut Microbes 13, 1866974 (2021).
Perez-Pardo, P. et al. Gut bacterial composition in a mouse model of Parkinson’s disease. Benef. Microbes 9, 799–814 (2018).
Yang, X., Qian, Y., Xu, S., Song, Y. & Xiao, Q. Longitudinal analysis of fecal microbiome and pathologic processes in a rotenone induced mice model of Parkinson’s disease. Front. Aging Neurosci. 9, 441 (2017).
Dodiya, H. B. et al. Chronic stress-induced gut dysfunction exacerbates Parkinson’s disease phenotype and pathology in a rotenone-induced mouse model of Parkinson’s disease. Neurobiol. Dis. 135, 104352 (2020).
Johnson, M. E., Stringer, A. & Bobrovskaya, L. Rotenone induces gastrointestinal pathology and microbiota alterations in a rat model of Parkinson’s disease. Neurotoxicology 65, 174–185 (2018).
Choi, J. G., Huh, E., Kim, N., Kim, D. H. & Oh, M. S. High-throughput 16S rRNA gene sequencing reveals that 6-hydroxydopamine affects gut microbial environment. PLoS ONE 14, e0217194 (2019).
Koutzoumis, D. N. et al. Alterations of the gut microbiota with antibiotics protects dopamine neuron loss and improve motor deficits in a pharmacological rodent model of Parkinson’s disease. Exp. Neurol. 325, 113159 (2020).
Nixon, A. M. et al. HFE genotype restricts the response to paraquat in a mouse model of neurotoxicity. J. Neurochem. 145, 299–311 (2018).
Singh, Y. et al. Enriched environmental conditions modify the gut microbiome composition and fecal markers of inflammation in Parkinson’s disease. Front. Neurosci. 13, 1032 (2019).
Ghaisas, S. et al. MitoPark transgenic mouse model recapitulates the gastrointestinal dysfunction and gut-microbiome changes of Parkinson’s disease. Neurotoxicology 75, 186–199 (2019).
Sun, M. F. et al. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav. Immun. 70, 48–60 (2018).
Erny, D. et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18, 965–977 (2015).
Mulak, A. & Bonaz, B. Brain-gut-microbiota axis in Parkinson’s disease. World J. Gastroenterol. 21, 10609–10620 (2015).
Goya, M. E. et al. Probiotic Bacillus subtilis protects against α-synuclein aggregation in C. elegans. Cell Rep. 30, 367–380 (2020).
Laukens, D., Brinkman, B. M., Raes, J., De Vos, M. & Vandenabeele, P. Heterogeneity of the gut microbiome in mice: guidelines for optimizing experimental design. FEMS Microbiol. Rev. 40, 117–132 (2016).
Abbott, R. D. et al. Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology 57, 456–462 (2001).
Song, E. M. et al. Long-term risks of Parkinson’s disease, surgery, and colorectal cancer in patients with slow-transit constipation. Clin. Gastroenterol. Hepatol. 19, 2577–2586 (2021).
Camacho, M. et al. Early constipation predicts faster dementia onset in Parkinson’s disease. NPJ Parkinsons Dis. 7, 45 (2021).
Jones, J. D., Rahmani, E., Garcia, E. & Jacobs, J. P. Gastrointestinal symptoms are predictive of trajectories of cognitive functioning in de novo Parkinson’s disease. Parkinsonism Relat. Disord. 72, 7–12 (2020).
Chen, Y. Y. et al. Clinical characteristics and peripheral T cell subsets in Parkinson’s disease patients with constipation. Int. J. Clin. Exp. Pathol. 8, 2495–2504 (2015).
Ivanov, I. I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Chen, M. L. & Sundrud, M. S. Cytokine networks and T-cell subsets in inflammatory bowel diseases. Inflamm. Bowel Dis. 22, 1157–1167 (2016).
Peter, I. et al. Anti-tumor necrosis factor therapy and incidence of Parkinson disease among patients with inflammatory bowel disease. JAMA Neurol. 75, 939–946 (2018).
Zhu, F. et al. The risk of Parkinson’s disease in inflammatory bowel disease: a systematic review and meta-analysis. Dig. Liver Dis. 51, 38–42 (2019).
Lee, H. S., Lobbestael, E., Vermeire, S., Sabino, J. & Cleynen, I. Inflammatory bowel disease and Parkinson’s disease: common pathophysiological links. Gut 70, 408–417 (2021).
Kang, X. et al. Tumor necrosis factor inhibition and Parkinson disease: a Mendelian randomization study. Neurology 96, e1672–e1679 (2021).
Kang, X. et al. Association between microscopic colitis and Parkinson’s disease in a Swedish population. Mov. Disord. 36, 1919–1926 (2021).
Herrick, M. K. & Tansey, M. G. Is LRRK2 the missing link between inflammatory bowel disease and Parkinson’s disease? NPJ Parkinsons Dis. 7, 26 (2021).
Hui, K. Y. et al. Functional variants in the LRRK2 gene confer shared effects on risk for Crohn’s disease and Parkinson’s disease. Sci. Transl. Med. 10, eaai7795 (2018).
Witoelar, A. et al. Genome-wide pleiotropy between Parkinson disease and autoimmune diseases. JAMA Neurol. 74, 780–792 (2017).
Lin, C. H. et al. Mild chronic colitis triggers parkinsonism in LRRK2 mutant mice through activating TNF-α pathway. Mov. Disord. 37, 745–757 (2022).
Black, C. J. & Ford, A. C. Global burden of irritable bowel syndrome: trends, predictions and risk factors. Nat. Rev. Gastroenterol. Hepatol. 17, 473–486 (2020).
Mertsalmi, T. H., But, A., Pekkonen, E. & Scheperjans, F. Irritable bowel syndrome and risk of Parkinson’s disease in Finland: a nationwide registry-based cohort study. J. Parkinsons Dis. 11, 641–651 (2021).
Lai, S. W., Liao, K. F., Lin, C. L. & Sung, F. C. Irritable bowel syndrome correlates with increased risk of Parkinson’s disease in Taiwan. Eur. J. Epidemiol. 29, 57–62 (2014).
Weimers, P. et al. Association between inflammatory bowel disease and Parkinson’s disease: seek and you shall find? Gut 68, 175–176 (2019).
Yoon, S. Y. et al. Irritable bowel syndrome and subsequent risk of Parkinson’s disease: a nationwide population-based matched-cohort study. J. Neurol. 269, 1404–1412 (2022).
Liu, B. et al. Irritable bowel syndrome and Parkinson’s disease risk: register-based studies. NPJ Parkinsons Dis. 7, 5 (2021).
Svensson, E. et al. Does vagotomy reduce the risk of Parkinson’s disease: the authors reply. Ann. Neurol. 78, 1012–1013 (2015).
Killinger, B. A. et al. The vermiform appendix impacts the risk of developing Parkinson’s disease. Sci. Transl. Med. 10, eaar5280 (2018).
Chen, Y. et al. Increased accumulation of α-synuclein in inflamed appendices of Parkinson’s disease patients. Mov. Disord. 36, 1911–1918 (2021).
Liu, B., Fang, F., Ye, W. & Wirdefeldt, K. Appendectomy, tonsillectomy and Parkinson’s disease risk: a Swedish register-based study. Front. Neurol. 11, 510 (2020).
Marras, C., Lang, A. E., Austin, P. C., Lau, C. & Urbach, D. R. Appendectomy in mid and later life and risk of Parkinson’s disease: a population-based study. Mov. Disord. 31, 1243–1247 (2016).
Svensson, E. et al. Appendectomy and risk of Parkinson’s disease: a nationwide cohort study with more than 10 years of follow-up. Mov. Disord. 31, 1918–1922 (2016).
Palacios, N., Hughes, K. C., Cereda, E., Schwarzschild, M. A. & Ascherio, A. Appendectomy and risk of Parkinson’s disease in two large prospective cohorts of men and women. Mov. Disord. 33, 1492–1496 (2018).
Li, P. et al. Gut microbiota dysbiosis is associated with elevated bile acids in Parkinson’s disease. Metabolites 11, 29 (2021).
Yao, X. & Smolka, A. J. Gastric parietal cell physiology and Helicobacter pylori-induced disease. Gastroenterology 156, 2158–2173 (2019).
Pimentel, M., Saad, R. J., Long, M. D. & Rao, S. S. C. ACG Clinical Guideline: small intestinal bacterial overgrowth. Am. J. Gastroenterol. 115, 165–178 (2020).
Nielsen, H. H., Qiu, J., Friis, S., Wermuth, L. & Ritz, B. Treatment for Helicobacter pylori infection and risk of Parkinson’s disease in Denmark. Eur. J. Neurol. 19, 864–869 (2012).
Huang, H. K. et al. Helicobacter pylori infection is associated with an increased risk of Parkinson’s disease: a population-based retrospective cohort study. Parkinsonism Relat. Disord. 47, 26–31 (2018).
Tan, A. H. et al. Helicobacter pylori infection is associated with worse severity of Parkinson’s disease. Parkinsonism Relat. Disord. 21, 221–225 (2015).
Rahne, K. E., Tagesson, C. & Nyholm, D. Motor fluctuations and Helicobacter pylori in Parkinson’s disease. J. Neurol. 260, 2974–2980 (2013).
Adike, A. & DiBaise, J. K. Small intestinal bacterial overgrowth: nutritional implications, diagnosis, and management. Gastroenterol. Clin. North. Am. 47, 193–208 (2018).
Tan, A. H. et al. Small intestinal bacterial overgrowth in Parkinson’s disease. Parkinsonism Relat. Disord. 20, 535–540 (2014).
Niu, X. L. et al. Prevalence of small intestinal bacterial overgrowth in Chinese patients with Parkinson’s disease. J. Neural Transm. 123, 1381–1386 (2016).
Lee, W. Y., Yoon, W. T., Shin, H. Y., Jeon, S. H. & Rhee, P. L. Helicobacter pylori infection and motor fluctuations in patients with Parkinson’s disease. Mov. Disord. 23, 1696–1700 (2008).
Fasano, A. et al. The role of small intestinal bacterial overgrowth in Parkinson’s disease. Mov. Disord. 28, 1241–1249 (2013).
Pierantozzi, M. et al. Helicobacter pylori eradication and l-dopa absorption in patients with PD and motor fluctuations. Neurology 66, 1824–1829 (2006).
McGee, D. J., Lu, X. H. & Disbrow, E. A. Stomaching the possibility of a pathogenic role for Helicobacter pylori in Parkinson’s disease. J. Parkinsons Dis. 8, 367–374 (2018).
Suwarnalata, G. et al. Augmentation of autoantibodies by Helicobacter pylori in Parkinson’s disease patients may be linked to greater severity. PLoS ONE 11, e0153725 (2016).
De Pablo-Fernandez, E. et al. Association of autonomic dysfunction with disease progression and survival in Parkinson disease. JAMA Neurol. 74, 970–976 (2017).
Heintz-Buschart, A. et al. The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov. Disord. 33, 88–98 (2018).
Nishiwaki, H. et al. Short-chain fatty acid-producing gut microbiota is decreased in Parkinson’s disease but not in rapid-eye-movement sleep behavior disorder. mSystems 5, e00797-20 (2020).
Heinzel, S. et al. Gut microbiome signatures of risk and prodromal markers of Parkinson disease. Ann. Neurol. 88, 320–331 (2020).
Lubomski, M. et al. Parkinson’s disease and the gastrointestinal microbiome. J. Neurol. 267, 2507–2523 (2020).
Vascellari, S. et al. Clinical phenotypes of Parkinson’s disease associate with distinct gut microbiota and metabolome enterotypes. Biomolecules 11, 144 (2021).
Scheperjans, F. et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord. 30, 350–358 (2015).
Cilia, R. et al. Does gut microbiota influence the course of Parkinson’s disease? A 3-year prospective exploratory study in de novo patients. J. Parkinsons Dis. 11, 159–170 (2021).
Minato, T. et al. Progression of Parkinson’s disease is associated with gut dysbiosis: two-year follow-up study. PLoS ONE 12, e0187307 (2017).
Aho, V. T. E. et al. Gut microbiota in Parkinson’s disease: temporal stability and relations to disease progression. EBioMedicine 44, 691–707 (2019).
Shin, C., Lim, Y., Lim, H. & Ahn, T. B. Plasma short-chain fatty acids in patients with Parkinson’s disease. Mov. Disord. 35, 1021–1027 (2020).
He, X. et al. Plasma short-chain fatty acids differences in multiple system atrophy from Parkinson’s disease. J. Parkinsons Dis. 11, 1167–1176 (2021).
Chen, S. J. et al. Association of fecal and plasma levels of short-chain fatty acids with gut microbiota and clinical severity in Parkinson disease patients. Neurology 98, e848–e858 (2022).
Janeiro, M. H., Ramirez, M. J., Milagro, F. I., Martinez, J. A. & Solas, M. Implication of trimethylamine N-oxide (TMAO) in disease: potential biomarker or new therapeutic target. Nutrients 10, 1398 (2018).
Chung, S. J. et al. Gut microbiota-derived metabolite trimethylamine N-oxide as a biomarker in early Parkinson’s disease. Nutrition 83, 111090 (2021).
Sankowski, B. et al. Higher cerebrospinal fluid to plasma ratio of p-cresol sulfate and indoxyl sulfate in patients with Parkinson’s disease. Clin. Chim. Acta 501, 165–173 (2020).
Chen, S. J. et al. The gut metabolite trimethylamine N-oxide is associated with Parkinson’s disease severity and progression. Mov. Disord. 35, 2115–2116 (2020).
Gentile, C. L. & Weir, T. L. The gut microbiota at the intersection of diet and human health. Science 362, 776–780 (2018).
Zmora, N., Suez, J. & Elinav, E. You are what you eat: diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 16, 35–56 (2019).
Boulos, C., Yaghi, N., El Hayeck, R., Heraoui, G. N. & Fakhoury-Sayegh, N. Nutritional risk factors, microbiota and Parkinson’s disease: what is the current evidence? Nutrients 11, 1896 (2019).
Wang, A., Lin, Y., Wu, Y. & Zhang, D. Macronutrients intake and risk of Parkinson’s disease: a meta-analysis. Geriatr. Gerontol. Int. 15, 606–616 (2015).
Marion-Letellier, R., Savoye, G. & Ghosh, S. Polyunsaturated fatty acids and inflammation. IUBMB Life 67, 659–667 (2015).
Ying, A. F. et al. Dietary antioxidants and risk of Parkinson’s disease in the Singapore Chinese Health Study. Mov. Disord. 35, 1765–1773 (2020).
Hantikainen, E. et al. Dietary antioxidants and the risk of Parkinson disease: the Swedish National March Cohort. Neurology 96, e895–e903 (2021).
Yang, F., Wolk, A., Hakansson, N., Pedersen, N. L. & Wirdefeldt, K. Dietary antioxidants and risk of Parkinson’s disease in two population-based cohorts. Mov. Disord. 32, 1631–1636 (2017).
Hughes, K. C. et al. Intake of antioxidant vitamins and risk of Parkinson’s disease. Mov. Disord. 31, 1909–1914 (2016).
Chang, M. C., Kwak, S. G. & Kwak, S. Effect of dietary vitamins C and E on the risk of Parkinson’s disease: a meta-analysis. Clin. Nutr. 40, 3922–3930 (2021).
Takeda, A. et al. Vitamin A and carotenoids and the risk of Parkinson’s disease: a systematic review and meta-analysis. Neuroepidemiology 42, 25–38 (2014).
de Lau, L. M. L., Koudstaal, P. J., Witteman, J. C., Hofman, A. & Breteler, M. M. Dietary folate, vitamin B12, and vitamin B6 and the risk of Parkinson disease. Neurology 67, 315–318 (2006).
Murakami, K. et al. Dietary intake of folate, vitamin B6, vitamin B12 and riboflavin and risk of Parkinson’s disease: a case-control study in Japan. Br. J. Nutr. 104, 757–764 (2010).
Shen, L. Associations between B vitamins and Parkinson’s disease. Nutrients 7, 7197–7208 (2015).
Chen, H. et al. Folate intake and risk of Parkinson’s disease. Am. J. Epidemiol. 160, 368–375 (2004).
Fan, X. et al. Role of homocysteine in the development and progression of Parkinson’s disease. Ann. Clin. Transl. Neurol. 7, 2332–2338 (2020).
Zhao, Y. et al. Investigating the causality of metabolites involved in one-carbon metabolism with the risk and age at onset of Parkinson’s disease: a two-sample Mendelian randomization study. Neurobiol. Aging 108, 196–199 (2021).
Christine, C. W. et al. Vitamin B12 and homocysteine levels predict different outcomes in early Parkinson’s disease. Mov. Disord. 33, 762–770 (2018).
Sleeman, I. et al. Urate and homocysteine: predicting motor and cognitive changes in newly diagnosed Parkinson’s disease. J. Parkinsons Dis. 9, 351–359 (2019).
Cheng, P. et al. Dietary intake of iron, zinc, copper, and risk of Parkinson’s disease: a meta-analysis. Neurol. Sci. 36, 2269–2275 (2015).
Palacios, N. et al. Caffeine and risk of Parkinson’s disease in a large cohort of men and women. Mov. Disord. 27, 1276–1282 (2012).
Qi, H. & Li, S. Dose-response meta-analysis on coffee, tea and caffeine consumption with risk of Parkinson’s disease. Geriatr. Gerontol. Int. 14, 430–439 (2014).
Chen, J. F. & Schwarzschild, M. A. Do caffeine and more selective adenosine A2A receptor antagonists protect against dopaminergic neurodegeneration in Parkinson’s disease? Parkinonism Relat. Disord. 80, S45–S53 (2020).
Iriondo-DeHond, A., Uranga, J. A., Del Castillo, M. D. & Abalo, R. Effects of coffee and its components on the gastrointestinal tract and the brain-gut axis. Nutrients 13, 88 (2020).
Postuma, R. B. et al. Risk factors for neurodegeneration in idiopathic rapid eye movement sleep behavior disorder: a multicenter study. Ann. Neurol. 77, 830–839 (2015).
Gao, X., Cassidy, A., Schwarzschild, M. A., Rimm, E. B. & Ascherio, A. Habitual intake of dietary flavonoids and risk of Parkinson disease. Neurology 78, 1138–1145 (2012).
Gao, X., O’ Reilly, É. J., Schwarzschild, M. A. & Ascherio, A. Prospective study of plasma urate and risk of Parkinson disease in men and women. Neurology 86, 520–526 (2016).
Gao, X. et al. Diet, urate, and Parkinson’s disease risk in men. Am. J. Epidemiol. 167, 831–838 (2008).
Chamorro, A. & Mir, P. Raising serum urate levels in Parkinson disease: a strategy only for women? Neurology 93, 611–612 (2019).
Hughes, K. C. et al. Intake of dairy foods and risk of Parkinson disease. Neurology 89, 46–52 (2017).
Jiang, W., Ju, C., Jiang, H. & Zhang, D. Dairy foods intake and risk of Parkinson’s disease: a dose-response meta-analysis of prospective cohort studies. Eur. J. Epidemiol. 29, 613–619 (2014).
Jimenez-Jimenez, F. J., Alonso-Navarro, H., Garcia-Martin, E. & Agundez, J. A. G. Alcohol consumption and risk for Parkinson’s disease: a systematic review and meta-analysis. J. Neurol. 266, 1821–1834 (2019).
Peters, S. et al. Alcohol consumption and risk of Parkinson’s disease: data from a large prospective European cohort. Mov. Disord. 35, 1258–1263 (2020).
Kim, I. Y. et al. Alcohol intake and Parkinson’s disease risk in the Million Women Study. Mov. Disord. 35, 443–449 (2020).
Kim, R., Yoo, D., Jung, Y. J., Han, K. & Lee, J. Y. Sex differences in smoking, alcohol consumption, and risk of Parkinson’s disease: a nationwide cohort study. Parkinsonism Relat. Disord. 71, 60–65 (2020).
Jacobs, B. M. et al. Parkinson’s disease determinants, prediction and gene–environment interactions in the UK Biobank. J. Neurol. Neurosurg. Psychiatry 91, 1046–1054 (2020).
Paul, K. C. et al. The association between lifestyle factors and Parkinson’s disease progression and mortality. Mov. Disord. 34, 58–66 (2019).
Kim, J. J., Bandres-Ciga, S., Blauwendraat, C. & Gan-Or, Z. International Parkinson’s Disease Genomics ConsortiumNo genetic evidence for involvement of alcohol dehydrogenase genes in risk for Parkinson’s disease. Neurobiol. Aging 87, 140.e19–140.e22 (2020).
Gao, X. et al. Prospective study of dietary pattern and risk of Parkinson disease. Am. J. Clin. Nutr. 86, 1486–1494 (2007).
Yin, W., Lof, M., Pedersen, N. L., Sandin, S. & Fang, F. Mediterranean dietary pattern at middle age and risk of Parkinson’s disease: a Swedish cohort study. Mov. Disord. 36, 255–260 (2021).
Maraki, M. I. et al. Mediterranean diet adherence is related to reduced probability of prodromal Parkinson’s disease. Mov. Disord. 34, 48–57 (2019).
Alcalay, R. N. et al. The association between Mediterranean diet adherence and Parkinson’s disease. Mov. Disord. 27, 771–774 (2012).
Metcalfe-Roach, A. et al. MIND and Mediterranean diets associated with later onset of Parkinson’s disease. Mov. Disord. 36, 977–984 (2021).
Cassani, E. et al. Dietary habits in Parkinson’s disease: adherence to Mediterranean diet. Parkinsonism Relat. Disord. 42, 40–46 (2017).
Tosti, V., Bertozzi, B. & Fontana, L. Health benefits of the Mediterranean diet: metabolic and molecular mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 73, 318–326 (2018).
Kolodziejczyk, A. A., Zheng, D. & Elinav, E. Diet–microbiota interactions and personalized nutrition. Nat. Rev. Microbiol. 17, 742–753 (2019).
Kline, E. M. et al. Genetic and environmental factors in Parkinson’s disease converge on immune function and inflammation. Mov. Disord. 36, 25–36 (2021).
Gardet, A. et al. LRRK2 is involved in the IFN-γ response and host response to pathogens. J. Immunol. 185, 5577–5585 (2010).
Cook, D. A. et al. LRRK2 levels in immune cells are increased in Parkinson’s disease. NPJ Parkinsons Dis. 3, 11 (2017).
Takagawa, T. et al. An increase in LRRK2 suppresses autophagy and enhances Dectin-1-induced immunity in a mouse model of colitis. Sci. Transl. Med. 10, eaan8162 (2018).
Menozzi, E., Macnaughtan, J. & Schapira, A. H. V. LRRK2 parkinsonism: does the response to gut bacteria mitigate the neurological picture? Mov. Disord. 36, 71–75 (2021).
Wallen, Z. D. et al. Exploring human-genome gut–microbiome interaction in Parkinson’s disease. NPJ Parkinsons Dis. 7, 74 (2021).
Tan, A. H. et al. PINK1 p.Leu347Pro mutations in Malays: prevalence and illustrative cases. Parkinsonism Relat. Disord. 79, 34–39 (2020).
Knudsen, K., Szwebs, M., Hansen, A. K. & Borghammer, P. Gastric emptying in Parkinson’s disease – a mini-review. Parkinsonism Relat. Disord. 55, 18–25 (2018).
Pfeiffer, R. F., Isaacson, S. H. & Pahwa, R. Clinical implications of gastric complications on levodopa treatment in Parkinson’s disease. Parkinsonism Relat. Disord. 76, 63–71 (2020).
Barboza, J. L., Okun, M. S. & Moshiree, B. The treatment of gastroparesis, constipation and small intestinal bacterial overgrowth syndrome in patients with Parkinson’s disease. Expert. Opin. Pharmacother. 16, 2449–2464 (2015).
Lim, S. Y. & Tan, A. H. in Parkinson’s Disease: Current and Future Therapeutics and Clinical Trials (eds Galvez-Jimenez, N. et al.) 93–109 (Cambridge Univ. Press, 2016).
Choi, J. H. et al. Double-blind, randomized, placebo-controlled trial of DA-9701 in Parkinson’s disease: PASS-GI Study. Mov. Disord. 35, 1966–1976 (2020).
Marrinan, S. L. et al. A randomized, double-blind, placebo-controlled trial of camicinal in Parkinson’s disease. Mov. Disord. 33, 329–332 (2018).
Safirstein, B. E. et al. Pharmacokinetics of inhaled levodopa administered with oral carbidopa in the fed state in patients with Parkinson’s disease. Clin. Ther. 42, 1034–1046 (2020).
Lipp, M. M., Batycky, R., Moore, J., Leinonen, M. & Freed, M. I. Preclinical and clinical assessment of inhaled levodopa for OFF episodes in Parkinson’s disease. Sci. Transl. Med. 8, 360ra136 (2016).
Baruzzi, A. C. et al. Influence of meal ingestion time on pharmacokinetics of orally administered levodopa in Parkinsonian patients. Clin. Neuropharmacol. 10, 527–537 (1987).
Trenkwalder, C., Kuoppamaki, M., Vahteristo, M., Muller, T. & Ellmen, J. Increased dose of carbidopa with levodopa and entacapone improves “off” time in a randomized trial. Neurology 92, e1487–e1496 (2019).
Tan, A. H. et al. Helicobacter pylori eradication in Parkinson’s disease: a randomized placebo-controlled trial. Mov. Disord. 35, 2250–2260 (2020).
Astarloa, R., Mena, M. A., Sánchez, V., de la Vega, L. & de Yébenes, J. G. Clinical and pharmacokinetic effects of a diet rich in insoluble fiber on Parkinson disease. Clin. Neuropharmacol. 15, 375–380 (1992).
Maini Rekdal, V., Bess, E. N., Bisanz, J. E., Turnbaugh, P. J. & Balskus, E. P. Discovery and inhibition of an interspecies gut bacterial pathway for levodopa metabolism. Science 364, eaau6323 (2019).
van Kessel, S. P. et al. Gut bacterial tyrosine decarboxylases restrict levels of levodopa in the treatment of Parkinson’s disease. Nat. Commun. 10, 310 (2019).
Limousin, P. & Foltynie, T. Long-term outcomes of deep brain stimulation in Parkinson disease. Nat. Rev. Neurol. 15, 234–242 (2019).
Arai, E. et al. Subthalamic deep brain stimulation can improve gastric emptying in Parkinson’s disease. Brain 135, 1478–1485 (2012).
Su, A., Gandhy, R., Barlow, C. & Triadafilopoulos, G. Utility of the wireless motility capsule and lactulose breath testing in the evaluation of patients with Parkinson’s disease who present with functional gastrointestinal symptoms. BMJ Open Gastroenterol. 4, e000132 (2017).
Shiina, S. et al. Levodopa does not worsen gastric emptying in Parkinson’s disease. J. Am. Geriatr. Soc. 63, 2185–2186 (2015).
Tan, Y. J. et al. Osteoporosis in Parkinson’s disease: relevance of distal radius dual-energy X-ray absorptiometry (DXA) and sarcopenia. J. Clin. Densitom. 24, 351–361 (2020).
Virmani, T., Tazan, S., Mazzoni, P., Ford, B. & Greene, P. E. Motor fluctuations due to interaction between dietary protein and levodopa in Parkinson’s disease. J. Clin. Mov. Disord. 3, 8 (2016).
Zimmermann, M., Zimmermann-Kogadeeva, M., Wegmann, R. & Goodman, A. L. Separating host and microbiome contributions to drug pharmacokinetics and toxicity. Science 363, eaat9931 (2019).
Zimmermann, M., Zimmermann-Kogadeeva, M., Wegmann, R. & Goodman, A. L. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature 570, 462–467 (2019).
Javdan, B. et al. Personalized mapping of drug metabolism by the human gut microbiome. Cell 181, 1661–1679 (2020).
Vizcarra, J. A., Wilson-Perez, H. E., Fasano, A. & Espay, A. J. Small intestinal bacterial overgrowth in Parkinson’s disease: tribulations of a trial. Parkinsonism Relat. Disord. 54, 110–112 (2018).
Fasano, A., Visanji, N. P., Liu, L. W. C., Lang, A. E. & Pfeiffer, R. F. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 14, 625–639 (2015).
Narozanska, E. et al. Pharmacokinetics of levodopa in patients with Parkinson disease and motor fluctuations depending on the presence of Helicobacter pylori infection. Clin. Neuropharmacol. 37, 96–99 (2014).
Sanders, M. E., Merenstein, D. J., Reid, G., Gibson, G. R. & Rastall, R. A. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 16, 605–616 (2019).
Suez, J., Zmora, N., Segal, E. & Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 25, 716–729 (2019).
Zmora, N. S. E. & Elinav, E. Transforming medicine with the microbiome. Sci. Transl. Med. 11, eaaw1815 (2019).
Harkins, C. P., Kong, H. H. & Segre, J. A. Manipulating the human microbiome to manage disease. JAMA 323, 303–304 (2019).
Phillips, M. C. L., Murtagh, D. K. J., Gilbertson, L. J., Asztely, F. J. S. & Lynch, C. D. P. Low-fat versus ketogenic diet in Parkinson’s disease: a pilot randomized controlled trial. Mov. Disord. 33, 1306–1314 (2018).
Hegelmaier, T. et al. Interventional influence of the intestinal microbiome through dietary intervention and bowel cleansing might improve motor symptoms in Parkinson’s disease. Cells 9, 376 (2020).
Postuma, R. B. et al. Caffeine as symptomatic treatment for Parkinson disease (Cafe-PD): a randomized trial. Neurology 89, 1795–1803 (2017).
Becker, A. et al. Effects of resistant starch on symptoms, fecal markers and gut microbiota in Parkinson’s disease–the RESISTA-PD trial. Genomics Proteom. Bioinforma. https://doi.org/10.1016/j.gpb.2021.08.009 (2021).
Barichella, M. et al. Probiotics and prebiotic fiber for constipation associated with Parkinson disease: an RCT. Neurology 87, 1274–1280 (2016).
Tan, A. H. et al. Probiotics for constipation in Parkinson disease: a randomized placebo-controlled study. Neurology 96, e772–e782 (2021).
Segal, A., Zlotnik, Y., Moyal-Atias, K., Abuhasira, R. & Ifergane, G. Fecal microbiota transplant as a potential treatment for Parkinson’s disease–a case series. Clin. Neurol. Neurosurg. 207, 106791 (2021).
Kuai, X. Y. et al. Evaluation of fecal microbiota transplantation in Parkinson’s disease patients with constipation. Microb. Cell Fact. 20, 98 (2021).
Aden, K. et al. Metabolic functions of gut microbes associate with efficacy of tumor necrosis factor antagonists in patients with inflammatory bowel diseases. Gastroenterology 157, 1279–1292 (2019).
Hauser, R. A. et al. Targeting neurons in the gastrointestinal tract to treat Parkinson’s disease. Clin. Parkinsonism Relat. Disord. 1, 2–7 (2019).
Lynch, S. V., Ng, S. C., Shanahan, F. & Tilg, H. Translating the gut microbiome: ready for the clinic? Nat. Rev. Gastroenterol. Hepatol. 16, 656–661 (2019).
Acknowledgements
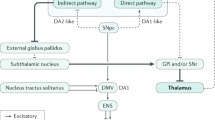
The authors gratefully acknowledge support from the University of Malaya Parkinson’s Disease and Movement Disorders Research Program Fund (PV035-2017), and M. G. Rudakewich (Synapse Visuals) for providing professional medical illustration services for the figure in Box 2 (the original figure done by the artist was modified by the journal).
Author information
Authors and Affiliations
Contributions
A.H.T. and S.Y.L. developed the concept and structure of the manuscript, conducted the literature review and prepared the first draft of the manuscript. A.E.L. provided critical review of the manuscript. All authors contributed to the revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neurology thanks M. Cirstea, who co-reviewed with S. Appel-Cresswell; A. Keshavarzian; and R. Pfeiffer for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Review criteria
We searched PubMed for articles published in English from 1 January 2010 to 4 October 2021, using the following main search terms: “Parkinson’s disease” and “gut–brain axis”, “microbiome”, “dysbiosis”, “gut metabolites”, “constipation”, “inflammatory bowel disease”, “irritable bowel syndrome”, “vagotomy”, “appendectomy”, “Helicobacter pylori”, “small intestinal bacterial overgrowth”, “diet”, “gut motility”, “levodopa pharmacokinetics”, “prebiotics”, “probiotics”, “faecal microbiota transplantation”, “antimicrobials” and “postbiotics”. Where relevant, our literature search was expanded to include studies in healthy or older adults and related neurodegenerative diseases. We also used references cited in the publications retrieved. The final reference list was generated on the basis of relevance to the topics covered in this Review, with a focus on publications within the past 5 years. For the section “Gut dysbiosis in PD: evidence from clinical studies”, we systematically reviewed PubMed articles published in any language from 1 January 2000 to 31 May 2021, using the following search terms: “microbiome” or “microbiota” or “microflora” or “dysbiosis” and “Parkinson” or “parkinsonism”. The inclusion criteria were human case–control studies involving patients with PD, and microbial DNA extraction from stool samples. The results of this literature search are summarized in Supplementary Table 1.
Supplementary information
Glossary
- Enteric nervous system
-
(ENS). An extensive neural network comprising neurons and glial cells embedded in the gut wall that coordinates the main functions of the gastrointestinal tract. Often referred to as the ‘second brain’, it is the largest of the three divisions of the autonomic nervous system, the other two being the sympathetic and the parasympathetic nervous system.
- Dorsal motor nucleus of the vagus
-
(DMNV). The cluster of neuronal cell bodies in the medulla oblongata of the lower brainstem that supplies efferent fibres of the vagus nerve, which is the main contributor to the parasympathetic nervous system. DMNV fibres innervate the gastrointestinal tract from the distal third of the oesophagus to approximately the splenic flexure of the colon.
- Microbiome
-
The collection of all the microorganisms (microbiota) living in a particular environment, including bacteria, fungi, protozoa and viruses. Sometimes, the term is used more specifically to denote the combined genetic material of these microorganisms.
- Metabolome
-
The collection of all low-molecular-weight metabolites within a biological system (cell, biofluid, tissue or organism).
- Lipopolysaccharide
-
An endotoxin and biologically active component of Gram-negative bacterial cell walls with potential immunostimulatory activity.
- Defaecatory dyssynergia
-
A disorder of paradoxical anal sphincter contraction during attempted defaecation in patients with constipation.
- Mendelian randomization
-
An observational research approach that uses variation in genes of known function as a proxy for an exposure (for example, to a drug), risk factor or phenotypic trait, so as to predict its causal effects on health outcomes. This method is less likely to be affected by confounding or reverse causation than are conventional observational studies.
- Helicobacter pylori infection
-
A common, usually chronic, bacterial infection of the stomach and duodenal epithelium that is found worldwide. It is associated with perturbation of the upper gut microbiome and has been directly linked particularly to peptic ulcers and non-ulcer dyspepsia.
- Small intestinal bacterial overgrowth
-
(SIBO). An acquired disorder defined by an excess of bacteria in the small intestine, often caused by colonization of coliform bacteria that usually reside in the colon.
- Hypochlorhydria
-
A condition of reduced production of hydrochloric acid, one of the components of gastric acid. A common cause is atrophic gastritis induced by Helicobacter pylori infection.
Rights and permissions
About this article
Cite this article
Tan, A.H., Lim, S.Y. & Lang, A.E. The microbiome–gut–brain axis in Parkinson disease — from basic research to the clinic. Nat Rev Neurol 18, 476–495 (2022). https://doi.org/10.1038/s41582-022-00681-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-022-00681-2
This article is cited by
-
The involvement of α-synucleinopathy in the disruption of microglial homeostasis contributes to the pathogenesis of Parkinson’s disease
Cell Communication and Signaling (2024)
-
Therapeutics for neurodegenerative diseases by targeting the gut microbiome: from bench to bedside
Translational Neurodegeneration (2024)
-
Doxycycline for transgene control disrupts gut microbiome diversity without compromising acute neuroinflammatory response
Journal of Neuroinflammation (2024)
-
Parkinson disease pathology in inflammatory bowel disease
Nature Reviews Neurology (2024)
-
Gastrointestinal and brain barriers: unlocking gates of communication across the microbiota–gut–brain axis
Nature Reviews Gastroenterology & Hepatology (2024)