Abstract
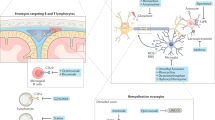
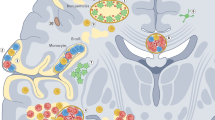
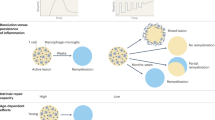
The rapidly evolving therapeutic landscape of multiple sclerosis (MS) has contributed to paradigm shifts in our understanding of the biological mechanisms that contribute to CNS injury and in treatment philosophies. Opportunities remain to further improve treatment of relapsing–remitting MS, but two major therapeutic gaps are the limiting of progressive disease mechanisms and the repair of CNS injury. In this Review, we provide an overview of selected emerging therapies that predominantly target processes within the CNS that are thought to be involved in limiting non-relapsing, progressive disease injury or promoting tissue repair. Among these, we consider agents that modulate adaptive and innate CNS-compartmentalized inflammation, which can be mediated by infiltrating immune cells and/or resident CNS cells, including microglia and astrocytes. We also discuss agents that target degenerative disease mechanisms, agents that might confer neuroprotection, and agents that create a more favourable environment for or actively contribute to oligodendrocyte precursor cell differentiation, remyelination and axonal regeneration. We focus on agents that are novel for MS, that are known to or are presumed to penetrate the CNS, and that have already entered early stages of development in MS clinical trials.
Key points
-
Therapeutic agents that limit progressive disease mechanisms and repair CNS injury are an important unmet need in multiple sclerosis (MS) clinical practice.
-
A number of emerging therapies target compartmentalized inflammation, remyelination and neuroprotection, and could be beneficial in progressive MS and CNS repair.
-
Emerging therapies that target CNS inflammation include Bruton tyrosine kinase inhibitors, CD40 ligand antibodies and α-lipoic acid.
-
Emerging therapies that provide neuroprotection include masitinib, ibudilast, metformin, clomipramine and receptor-interacting protein kinase 1.
-
Emerging therapies that disinhibit CNS repair include elezanumab and opicinumab, and those that promote CNS repair include proteoglycans, niacin, muscarinic receptor antagonists and temelimab.
-
Many candidate drugs require validation in large clinical trials, but their emergence opens the door to new treatment algorithms and personalized therapy in MS.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Thompson, A. J., Baranzini, S. E., Geurts, J., Hemmer, B. & Ciccarelli, O. Multiple sclerosis. Lancet 391, 1622–1636 (2018).
Reich, D. S., Lucchinetti, C. F. & Calabresi, P. A. Multiple sclerosis. N. Engl. J. Med. 378, 169–180 (2018).
Bar-Or, A. & Li, R. Cellular immunology of relapsing multiple sclerosis: interactions, checks, and balances. Lancet Neurol. 20, 470–483 (2021).
Absinta, M., Lassmann, H. & Trapp, B. D. Mechanisms underlying progression in multiple sclerosis. Curr. Opin. Neurol. 33, 277–285 (2020).
Mahad, D. H., Trapp, B. D. & Lassmann, H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 14, 183–193 (2015).
Fadda, G. et al. A surface-in gradient of thalamic damage evolves in pediatric multiple sclerosis. Ann. Neurol. 85, 340–351 (2019).
Pardini, M., Brown, J. W. L., Magliozzi, R., Reynolds, R. & Chard, D. T. Surface-in pathology in multiple sclerosis: a new view on pathogenesis? Brain 144, 1646–1654 (2021).
Dal-Bianco, A. et al. Long-term evolution of multiple sclerosis iron rim lesions in 7T MRI. Brain 144, 833–847 (2021).
Maggi, P. et al. Paramagnetic rim lesions are specific to multiple sclerosis: an international multicenter 3T MRI study. Ann. Neurol. 88, 1034–1042 (2020).
Absinta, M. et al. A lymphocyte-microglia-astrocyte axis in chronic active multiple sclerosis. Nature 597, 709–714 (2021).
Lisak, R. P. et al. Secretory products of multiple sclerosis B cells are cytotoxic to oligodendroglia in vitro. J. Neuroimmunol. 246, 85–95 (2012).
Magliozzi, R. et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 130, 1089–1104 (2007).
Haider, L. et al. The topograpy of demyelination and neurodegeneration in the multiple sclerosis brain. Brain 139, 807–815 (2016).
Hauser, S. L. & Cree, B. A. C. Treatment of multiple sclerosis: a review. Am. J. Med. 133, 1380–1390.e2 (2020).
Freedman, M. S. et al. Treatment optimization in multiple sclerosis: Canadian MS working group recommendations. Can. J. Neurol. Sci. 47, 437–455 (2020).
Montalban, X. et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Mult. Scler. 24, 96–120 (2018).
Hartkamp, L. M. et al. Btk inhibition suppresses agonist-induced human macrophage activation and inflammatory gene expression in RA synovial tissue explants. Ann. Rheum. Dis. 74, 1603–1611 (2015).
Corneth, O. B. J. et al. Enhanced Bruton’s tyrosine kinase activity in peripheral blood B lymphocytes from patients with autoimmune disease. Arthritis Rheumatol. 69, 1313–1324 (2017).
Mano, H. The Tec family protein-tyrosine kinases: a subset of kinases for a subset of signalings. Int. J. Hematol. 69, 6–12 (1999).
Carnero Contentti, E. & Correale, J. Bruton’s tyrosine kinase inhibitors: a promising emerging treatment option for multiple sclerosis. Expert. Opin. Emerg. Drugs 25, 377–381 (2020).
Machado-Santos, J. et al. The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells. Brain 141, 2066–2082 (2018).
Torke, S. et al. Inhibition of Bruton’s tyrosine kinase interferes with pathogenic B-cell development in inflammatory CNS demyelinating disease. Acta Neuropathol. 140, 535–548 (2020).
Li, R. et al. BTK inhibition limits B-cell-T-cell interaction through modulation of B-cell metabolism: implications for multiple sclerosis therapy. Acta Neuropathol. 143, 505–521 (2022). This study delineated important mechanisms underlying the therapeutic potential of BTK inhibitors in MS.
Owens, T. D. et al. Phase 1 clinical trial evaluating safety, exposure and pharmacodynamics of BTK inhibitor tolebrutinib (PRN2246, SAR442168). Clin. Transl. Sci. 15, 442–450 (2022).
Mangla, A. et al. Pleiotropic consequences of Bruton tyrosine kinase deficiency in myeloid lineages lead to poor inflammatory responses. Blood 104, 1191–1197 (2004).
Hata, D. et al. Involvement of Bruton’s tyrosine kinase in FcεRI-dependent mast cell degranulation and cytokine production. J. Exp. Med. 187, 1235–1247 (1998).
Montalban, X. et al. Placebo-controlled trial of an oral BTK inhibitor in multiple sclerosis. N. Engl. J. Med. 380, 2406–2417 (2019). This was the first phase II clinical trial that demonstrated the efficacy of a BTK inhibitor (evobrutinib) on MRI measures of interest in relapsing–remitting MS.
Smith P. F., et al. Phase 1 clinical trial of PRN2246 (SAR441268), a covalent BTK inhibitor demonstrates safety, CNS exposure and therapeutic levels of BTK occupancy. Mult. Scler. 25, 157–165 (2019).
Reich, D. S. et al. Safety and efficacy of tolebrutinib, an oral brain-penetrant BTK inhibitor, in relapsing multiple sclerosis: a phase 2b, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 20, 729–738 (2021). This phase II clinical trial demonstrated the efficacy of tolebrutinib on MRI measures of interest in relapsing–remitting MS using a unique clinical trial design.
Cohen, S. et al. Fenebrutinib versus placebo or adalimumab in rheumatoid arthritis: a randomized, double-blind, phase II trial (ANDES study). Arthritis Rheumatol. https://doi.org/10.1002/art.41275 (2020).
Peters, A. L., Stunz, L. L. & Bishop, G. A. CD40 and autoimmunity: the dark side of a great activator. Semin. Immunol. 21, 293–300 (2009).
D’Aversa, T. G., Weidenheim, K. M. & Berman, J. W. CD40-CD40L interactions induce chemokine expression by human microglia: implications for human immunodeficiency virus encephalitis and multiple sclerosis. Am. J. Pathol. 160, 559–567 (2002).
de Goer de Herve, M. G., Delfraissy, J. F. & Taoufik, Y. Following direct CD40 activation, human primary microglial cells produce IL-12 p40 but not bioactive IL-12 p70. Cytokine 14, 88–96 (2001).
Girvin, A. M., Dal Canto, M. C. & Miller, S. D. CD40/CD40L interaction is essential for the induction of EAE in the absence of CD28-mediated co-stimulation. J. Autoimmun. 18, 83–94 (2002).
Aarts, S. A. et al. Macrophage CD40 signaling drives experimental autoimmune encephalomyelitis. J. Pathol. 247, 471–480 (2019).
Du, L., Chang, H., Wei, Y., Zhang, X. & Yin, L. Different roles of soluble CD40 ligand in central nervous system damage. Neurol. Res. 42, 372–378 (2020).
Davidson, D. C. et al. Excess soluble CD40L contributes to blood brain barrier permeability in vivo: implications for HIV-associated neurocognitive disorders. PLoS ONE 7, e51793 (2012).
Masuda, H. et al. Soluble CD40 ligand contributes to blood-brain barrier breakdown and central nervous system inflammation in multiple sclerosis and neuromyelitis optica spectrum disorder. J. Neuroimmunol. 305, 102–107 (2017).
Couzin, J. Drug discovery. Magnificent obsession. Science 307, 1712–1715 (2005).
Sidiropoulos, P. I. & Boumpas, D. T. Lessons learned from anti-CD40L treatment in systemic lupus erythematosus patients. Lupus 13, 391–397 (2004).
Vaitaitis, G. M., Yussman, M. G. & Wagner, D. H. Jr. A CD40 targeting peptide prevents severe symptoms in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 332, 8–15 (2019). This study demonstrated the potential therapeutic benefit of targeting CD40 ligand in EAE.
Chamberlain, C. et al. Repeated administration of dapirolizumab pegol in a randomised phase I study is well tolerated and accompanied by improvements in several composite measures of systemic lupus erythematosus disease activity and changes in whole blood transcriptomic profiles. Ann. Rheum. Dis. 76, 1837–1844 (2017).
Furie, R. A. et al. Phase 2, randomized, placebo-controlled trial of dapirolizumab pegol in patients with moderate-to-severe active systemic lupus erythematosus. Rheumatology 60, 5397–5407 (2021).
Morini, M. et al. α-Lipoic acid is effective in prevention and treatment of experimental autoimmune encephalomyelitis. J. Neuroimmunol. 148, 146–153 (2004). This study demonstrated the potential therapeutic benefit of α-lipoic acid in EAE.
Marracci, G. H., Jones, R. E., McKeon, G. P. & Bourdette, D. N. Alpha lipoic acid inhibits T cell migration into the spinal cord and suppresses and treats experimental autoimmune encephalomyelitis. J. Neuroimmunol. 131, 104–114 (2002).
Chaudhary, P. et al. Effects of lipoic acid on primary murine microglial cells. J. Neuroimmunol. 334, 576972 (2019).
Yadav, V. et al. Lipoic acid in multiple sclerosis: a pilot study. Mult. Scler. 11, 159–165 (2005).
Khalili, M. et al. Does lipoic acid consumption affect the cytokine profile in multiple sclerosis patients: a double-blind, placebo-controlled, randomized clinical trial. Neuroimmunomodulation 21, 291–296 (2014).
Spain, R. et al. Lipoic acid in secondary progressive MS: a randomized controlled pilot trial. Neurol. Neuroimmunol. Neuroinflamm. 4, e374 (2017). This phase II clinical trial demonstrated the efficacy of lipoic acid in reducing annual brain atrophy in secondary progressive MS.
Falardeau, J. et al. Oral lipoic acid as a treatment for acute optic neuritis: a blinded, placebo controlled randomized trial. Mult. Scler. J. Exp. Transl. Clin. 5, 2055217319850193 (2019).
Elieh-Ali-Komi, D. & Cao, Y. Role of mast cells in the pathogenesis of multiple sclerosis and experimental autoimmune encephalomyelitis. Clin. Rev. Allergy Immunol. 52, 436–445 (2017).
Conti, P. & Kempuraj, D. Important role of mast cells in multiple sclerosis. Mult. Scler. Relat. Disord. 5, 77–80 (2016).
Dubreuil, P. et al. Masitinib (AB1010), a potent and selective tyrosine kinase inhibitor targeting KIT. PLoS ONE 4, e7258 (2009).
Christy, A. L. & Brown, M. A. The multitasking mast cell: positive and negative roles in the progression of autoimmunity. J. Immunol. 179, 2673–2679 (2007).
Bebo, B. F. Jr., Yong, T., Orr, E. L. & Linthicum, D. S. Hypothesis: a possible role for mast cells and their inflammatory mediators in the pathogenesis of autoimmune encephalomyelitis. J. Neurosci. Res. 45, 340–348 (1996).
Sayed, B. A., Christy, A. L., Walker, M. E. & Brown, M. A. Meningeal mast cells affect early T cell central nervous system infiltration and blood-brain barrier integrity through TNF: a role for neutrophil recruitment? J. Immunol. 184, 6891–6900 (2010).
Pinke, K. H., Zorzella-Pezavento, S. F. G., Lara, V. S. & Sartori, A. Should mast cells be considered therapeutic targets in multiple sclerosis. Neural Regen. Res. 15, 1995–2007 (2020).
Skaper, S. D., Facci, L., Romanello, S. & Leon, A. Mast cell activation causes delayed neurodegeneration in mixed hippocampal cultures via the nitric oxide pathway. J. Neurochem. 66, 1157–1166 (1996).
Bidri, M. et al. Mast cells as a source and target for nitric oxide. Int. Immunopharmacol. 1, 1543–1558 (2001).
Vermersch, P. et al. Masitinib treatment in patients with progressive multiple sclerosis: a randomized pilot study. BMC Neurol. 12, 36 (2012). This pilot study demonstrated possible clinical efficacy of masitinib in primary progressive MS, prompting further clinical trials.
Vermersch, P. et al. Efficacy and safety of masitinib in progressive forms of multiple sclerosis: a randomized, phase 3, clinical trial. Neurol. Neuroimmunol. Neuroinflamm. https://doi.org/10.1212/NXI.0000000000001148 (2022). This phase II clinical trial demonstrated the efficacy of masitinib in reducing disability progression in people with primary progressve MS and non-active secondary progressive MS.
Fox, R. J. et al. Phase 2 trial of ibudilast in progressive multiple sclerosis. N. Engl. J. Med. 379, 846–855 (2018). This phase II clinical trial demonstrated the efficacy of ibudilast in reducing the rate of brain atrophy in people with progressive MS.
Ruiz-Perez, D. et al. The effects of the toll-like receptor 4 antagonist, ibudilast, on sevoflurane’s minimum alveolar concentration and the delayed remifentanil-induced increase in the minimum alveolar concentration in rats. Anesth. Analg. 122, 1370–1376 (2016).
Miranda-Hernandez, S. & Baxter, A. G. Role of toll-like receptors in multiple sclerosis. Am. J. Clin. Exp. Immunol. 2, 75–93 (2013).
Suzumura, A., Ito, A., Yoshikawa, M. & Sawada, M. Ibudilast suppresses TNFα production by glial cells functioning mainly as type III phosphodiesterase inhibitor in the CNS. Brain Res. 837, 203–212 (1999).
Gibson, L. C. et al. The inhibitory profile of Ibudilast against the human phosphodiesterase enzyme family. Eur. J. Pharmacol. 538, 39–42 (2006).
Knott, E. P., Assi, M., Rao, S. N., Ghosh, M. & Pearse, D. D. Phosphodiesterase inhibitors as a therapeutic approach to neuroprotection and repair. Int. J. Mol. Sci. https://doi.org/10.3390/ijms18040696 (2017).
Cho, Y. et al. Allosteric inhibition of macrophage migration inhibitory factor revealed by ibudilast. Proc. Natl Acad. Sci. USA 107, 11313–11318 (2010).
Naismith, R. T. et al. Effects of ibudilast on MRI measures in the phase 2 SPRINT-MS study. Neurology 96, e491–e500 (2021).
Barkhof, F. et al. Ibudilast in relapsing-remitting multiple sclerosis: a neuroprotectant? Neurology 74, 1033–1040 (2010).
Sormani, M. P., Tur, C. & Barkhof, F. Ibudilast: a paradigm shift for progressive multiple sclerosis? Neurology 96, 141–142 (2021).
Nath, N. et al. Metformin attenuated the autoimmune disease of the central nervous system in animal models of multiple sclerosis. J. Immunol. 182, 8005–8014 (2009). This study demonstrated the potential therapeutic benefit of metformin in preclincal models of MS.
Cunniffe, N. et al. Systematic approach to selecting licensed drugs for repurposing in the treatment of progressive multiple sclerosis. J. Neurol. Neurosurg. Psychiatry https://doi.org/10.1136/jnnp-2020-324286 (2020). This study systematically evaluated existing drugs that have the potential to be repurposed as treatments for progressive MS and identified a short list of potential candidates.
Meng, X. et al. Metformin protects neurons against oxygen-glucose deprivation/reoxygenation-induced injury by down-regulating MAD2B. Cell Physiol. Biochem. 40, 477–485 (2016).
Neumann, B. et al. Metformin restores CNS remyelination capacity by rejuvenating aged stem cells. Cell Stem Cell 25, 473–485.e8 (2019).
Negrotto, L., Farez, M. F. & Correale, J. Immunologic effects of metformin and pioglitazone treatment on metabolic syndrome and multiple sclerosis. JAMA Neurol. 73, 520–528 (2016). This pilot study demonstrated the potential benefit of metformin and pioglitazone on MRI and biological measures of MS disease activity.
Faissner, S. et al. Systematic screening of generic drugs for progressive multiple sclerosis identifies clomipramine as a promising therapeutic. Nat. Commun. 8, 1990 (2017). This study systematically evaluated existing drugs that have the potential to be repurposed as treatments for progressive MS and identified a short list of potential candidates, including clomipramine.
Comi, G. et al. Role of B cells in multiple sclerosis and related disorders. Ann. Neurol. 89, 13–23 (2021).
Degterev, A. et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 1, 112–119 (2005).
Wallach, D., Kang, T. B., Dillon, C. P. & Green, D. R. Programmed necrosis in inflammation: toward identification of the effector molecules. Science 352, aaf2154 (2016).
Zheng, T. S. & Flavell, R. A. Divinations and surprises: genetic analysis of caspase function in mice. Exp. Cell Res. 256, 67–73 (2000).
Shutinoski, B. et al. K45A mutation of RIPK1 results in poor necroptosis and cytokine signaling in macrophages, which impacts inflammatory responses in vivo. Cell Death Differ. 23, 1628–1637 (2016).
Yuan, J., Amin, P. & Ofengeim, D. Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat. Rev. Neurosci. 20, 19–33 (2019).
Ofengeim, D. et al. Activation of necroptosis in multiple sclerosis. Cell Rep. 10, 1836–1849 (2015). This study provided important insights into the role of necroptosis and relevant molecules in MS pathophysiology.
Zhu, K. et al. Necroptosis promotes cell-autonomous activation of proinflammatory cytokine gene expression. Cell Death Dis. 9, 500 (2018).
Kim, S. J. & Li, J. Caspase blockade induces RIP3-mediated programmed necrosis in Toll-like receptor-activated microglia. Cell Death Dis. 4, e716 (2013).
Yoshikawa, M. et al. Discovery of 7-Oxo-2,4,5,7-tetrahydro-6 H-pyrazolo[3,4- c]pyridine derivatives as potent, orally available, and brain-penetrating receptor interacting protein 1 (RIP1) kinase inhibitors: analysis of structure-kinetic relationships. J. Med. Chem. q, 2384–2409 (2018).
Chen, Y. et al. Necrostatin-1 improves long-term functional recovery through protecting oligodendrocyte precursor cells after transient focal cerebral ischemia in mice. Neuroscience 371, 229–241 (2018).
& Weisel, K. et al. Randomized clinical study of safety, pharmacokinetics, and pharmacodynamics of RIPK1 inhibitor GSK2982772 in healthy volunteers. Pharmacol. Res. Perspect. 5, e00365 (2017).
Grievink, H. W. et al. DNL104, a centrally penetrant RIPK1 inhibitor, inhibits RIP1 kinase phosphorylation in a randomized phase I ascending dose study in healthy volunteers. Clin. Pharmacol. Ther. 107, 406–414 (2020). This phase I clinical trial evaluated a centrally penetrant RIPK1 inhibitor at multiple doses in healthy volunteers.
Muramatsu, R. et al. RGMa modulates T cell responses and is involved in autoimmune encephalomyelitis. Nat. Med. 17, 488–494 (2011).
Demicheva, E. et al. Targeting repulsive guidance molecule A to promote regeneration and neuroprotection in multiple sclerosis. Cell Rep. 10, 1887–1898 (2015). This study demonstrated the therapeutic regenerative potential of RGMa antibodies in preclinical models of MS.
Hata, K. et al. RGMa inhibition promotes axonal growth and recovery after spinal cord injury. J. Cell Biol. 173, 47–58 (2006).
Tanabe, S., Fujita, Y., Ikuma, K. & Yamashita, T. Inhibiting repulsive guidance molecule-a suppresses secondary progression in mouse models of multiple sclerosis. Cell Death Dis. 9, 1061 (2018).
Ziemann, A., Rosebraugh, M., Barger, B. & Cree, B. A phase 1, multiple-dose study of elezanumab (ABT-555) in patients with relapsing forms of multiple sclerosis [abstract]. Neurology 92 (Suppl. 15), S56.001 (2019). This phase I trial evaluated RGMa antibodies in people with MS.
Mi, S. et al. LINGO-1 negatively regulates myelination by oligodendrocytes. Nat. Neurosci. 8, 745–751 (2005). This study demonstrated in vitro and in animal models the role that LINGO1 plays in preventing remyelination by oligodendrocytes.
Mi, S., Pepinsky, R. B. & Cadavid, D. Blocking LINGO-1 as a therapy to promote CNS repair: from concept to the clinic. CNS Drugs 27, 493–503 (2013).
Mi, S. et al. LINGO-1 antagonist promotes spinal cord remyelination and axonal integrity in MOG-induced experimental autoimmune encephalomyelitis. Nat. Med. 13, 1228–1233 (2007). This study demonstrated the therapeutic remyelinating potential of LINGO1 antagonism in EAE.
Zhang, Y. et al. Inhibition of LINGO-1 promotes functional recovery after experimental spinal cord demyelination. Exp. Neurol. 266, 68–73 (2015).
Ranger, A. et al. Anti-LINGO-1 has no detectable immunomodulatory effects in preclinical and phase 1 studies. Neurol. Neuroimmunol. Neuroinflamm. 5, e417 (2018).
Tran, J. Q. et al. Randomized phase I trials of the safety/tolerability of anti-LINGO-1 monoclonal antibody BIIB033. Neurol. Neuroimmunol. Neuroinflamm 1, e18 (2014).
Cadavid, D. et al. Safety and efficacy of opicinumab in acute optic neuritis (RENEW): a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 16, 189–199 (2017). This was the first phase II clinical trial to evaluate opicinumab in people with MS and acute optic neuritis.
Cadavid, D. et al. Safety and efficacy of opicinumab in patients with relapsing multiple sclerosis (SYNERGY): a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 18, 845–856 (2019).
Zhu, B. et al. Phase 2 AFFINITY trial evaluates opicinumab in a targeted population of patients with relapsing multiple sclerosis: rationale, design and baseline characteristics [abstract]. Neurology 92 (Suppl. 15), P3.2-072 (2019).
Figueiredo, M. Biogen discontinues development of opicinumab for MS. Multiple Sclerosis News Today https://multiplesclerosisnewstoday.com/news-posts/2020/10/26/biogen-discontinues-development-opicinumab-data-affinity-trial/ (2020).
Huntemann, N. et al. Failed, interrupted, or inconclusive trials on neuroprotective and neuroregenerative treatment strategies in multiple sclerosis: update 2015–2020. Drugs 81, 1031–1063 (2021).
Keough, M. B. et al. An inhibitor of chondroitin sulfate proteoglycan synthesis promotes central nervous system remyelination. Nat. Commun. 7, 11312 (2016). This study demonstrated the role of CSPGs in preventing remyelination and the therapeutic remyelinating potential of a novel CSPG inhibitor in vitro and in preclinical models of demyelination.
Asher, R. A. et al. Neurocan is upregulated in injured brain and in cytokine-treated astrocytes. J. Neurosci. 20, 2427–2438 (2000).
Beggah, A. T. et al. Lesion-induced differential expression and cell association of neurocan, brevican, versican V1 and V2 in the mouse dorsal root entry zone. Neuroscience 133, 749–762 (2005).
Pu, A., Stephenson, E. L. & Yong, V. W. The extracellular matrix: focus on oligodendrocyte biology and targeting CSPGs for remyelination therapies. Glia 66, 1809–1825 (2018).
Galtrey, C. M. & Fawcett, J. W. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res. Rev. 54, 1–18 (2007).
Lau, L. W. et al. Chondroitin sulfate proteoglycans in demyelinated lesions impair remyelination. Ann. Neurol. 72, 419–432 (2012).
Stephenson, E. L. et al. Chondroitin sulfate proteoglycans as novel drivers of leucocyte infiltration in multiple sclerosis. Brain 141, 1094–1110 (2018).
Pu, A. et al. The glycosyltransferase EXTL2 promotes proteoglycan deposition and injurious neuroinflammation following demyelination. J. Neuroinflamm. 17, 220 (2020).
Stephenson, E. L. et al. Targeting the chondroitin sulfate proteoglycans: evaluating fluorinated glucosamines and xylosides in screens pertinent to multiple sclerosis. ACS Cent. Sci. 5, 1223–1234 (2019).
Kumar, N. Nutrients and neurology. Continuum 23, 822–861 (2017).
Zhang, J. et al. Niaspan treatment improves neurological functional recovery in experimental autoimmune encephalomyelitis mice. Neurobiol. Dis. 32, 273–280 (2008).
Kaneko, S. et al. Protecting axonal degeneration by increasing nicotinamide adenine dinucleotide levels in experimental autoimmune encephalomyelitis models. J. Neurosci. 26, 9794–9804 (2006).
Rawji, K. S. et al. Niacin-mediated rejuvenation of macrophage/microglia enhances remyelination of the aging central nervous system. Acta Neuropathol. 139, 893–909 (2020). This study demonstrated the therapeutic remyelinating potential of niacin in vitro and in preclinical models of demyelination.
Metz, L. M. & Eliasziw, M. Trial of minocycline in clinically isolated syndrome of multiple sclerosis. N. Engl. J. Med. 377, 788–789 (2017).
Mei, F. et al. Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nat. Med. 20, 954–960 (2014). This study demonstrated the utility of a high-throughput screening platform to identify drugs with remyelinating potential, including anti-muscarinic agents.
Deshmukh, V. A. et al. A regenerative approach to the treatment of multiple sclerosis. Nature 502, 327–332 (2013). This study used an image-based screen to identify drugs with regenerative potential and demonstrated the therapeutic remyelinating potential of benztropine in preclinical models of MS.
Green, A. J. et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial. Lancet 390, 2481–2489 (2017). This phase II clinical trial demonstrated the efficacy of clemastine fumarate in chronic demyelinating optic neuropathy.
Morandi, E. et al. The association between human endogenous retroviruses and multiple sclerosis: a systematic review and meta-analysis. PLoS ONE 12, e0172415 (2017).
Levet, S. et al. An ancestral retroviral protein identified as a therapeutic target in type-1 diabetes. JCI Insight https://doi.org/10.1172/jci.insight.94387 (2017).
Perron, H. & Lang, A. The human endogenous retrovirus link between genes and environment in multiple sclerosis and in multifactorial diseases associating neuroinflammation. Clin. Rev. Allergy Immunol. 39, 51–61 (2010).
Faucard, R. et al. Human endogenous retrovirus and neuroinflammation in chronic inflammatory demyelinating polyradiculoneuropathy. EBioMedicine 6, 190–198 (2016).
Rolland, A. et al. The envelope protein of a human endogenous retrovirus-W family activates innate immunity through CD14/TLR4 and promotes Th1-like responses. J. Immunol. 176, 7636–7644 (2006).
Perron, H. et al. Human endogenous retrovirus type W envelope expression in blood and brain cells provides new insights into multiple sclerosis disease. Mult. Scler. 18, 1721–1736 (2012).
Mameli, G. et al. Brains and peripheral blood mononuclear cells of multiple sclerosis (MS) patients hyperexpress MS-associated retrovirus/HERV-W endogenous retrovirus, but not human herpesvirus 6. J. Gen. Virol. 88, 264–274 (2007).
Garcia-Montojo, M. et al. The DNA copy number of human endogenous retrovirus-W (MSRV-type) is increased in multiple sclerosis patients and is influenced by gender and disease severity. PLoS ONE 8, e53623 (2013).
Porchet, H., Vidal, V., Kornmann, G., Malpass, S. & Curtin, F. A high-dose pharmacokinetic study of a new IgG4 monoclonal antibody temelimab/GNbAC1 antagonist of an endogenous retroviral protein pHERV-W Env. Clin. Ther. 41, 1737–1746 (2019).
Curtin, F. et al. GNbAC1, a humanized monoclonal antibody against the envelope protein of multiple sclerosis-associated endogenous retrovirus: a first-in-humans randomized clinical study. Clin. Ther. 34, 2268–2278 (2012).
Kornmann, G. & Curtin, F. Temelimab, an IgG4 anti-human endogenous retrovirus monoclonal antibody: an early development safety review. Drug Saf. 43, 1287–1296 (2020).
Derfuss, T. et al. A phase IIa randomized clinical study testing GNbAC1, a humanized monoclonal antibody against the envelope protein of multiple sclerosis associated endogenous retrovirus in multiple sclerosis patients–a twelve month follow-up. J. Neuroimmunol. 285, 68–70 (2015).
Derfuss, T. et al. A phase IIa randomised clinical study of GNbAC1, a humanised monoclonal antibody against the envelope protein of multiple sclerosis-associated endogenous retrovirus in multiple sclerosis patients. Mult. Scler. 21, 885–893 (2015).
Hartung, H. P. et al. Efficacy and safety of temelimab in multiple sclerosis: results of a randomized phase 2b and extension study. Mult. Scler. 28, 429–440 (2022). This phase II clinical trial and extension did not meet its primary end point, but demonstrated possible beneficial effects of temelimab on MRI measures, suggesting less neuroaxonal destruction as well as less demyelination and/or improved remyelination.
Faissner, S., Plemel, J. R., Gold, R. & Yong, V. W. Progressive multiple sclerosis: from pathophysiology to therapeutic strategies. Nat. Rev. Drug Discov. 18, 905–922 (2019).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
J.O. holds the Waugh Family Chair in MS Research and has received research funding from the Barford/Love MS Fund of St. Michael’s Hospital, Biogen-Idec, Brain Canada, EMD-Serono, the MS Society of Canada, the National MS Society, the National Institutes of Health, and Roche. She has received compensation for consulting or speaking from Biogen-Idec, BMS, EMD-Serono, Novartis, Roche and Sanofi-Genzyme. A.B.-O. holds the Melissa and Paul Anderson Chair. He has received research funding from the Canadian Institutes of Health Research, the Juvenile Diabetes Research Foundation, Multiple Sclerosis Society of Canada, the Multiple Sclerosis Scientific Foundation, the National Institutes of Health and the National MS Society. He has participated as a speaker in meetings sponsored by and received consulting fees from Accure, Atara Biotherapeutics, Biogen, BMS/Celgene/Receptos, GlaxoSmithKline, Gossamer, Janssen/Actelion, Medimmune, Merck/EMD Serono, Novartis, Roche/Genentech and Sanofi-Genzyme. He has received grant support to the University of Pennsylvania from Biogen Idec, Merck/EMD Serono, Novartis and Roche/Genentech.
Peer review
Peer review information
Nature Reviews Neurology thanks A. Bertolotto; D. Centonze, who co-reviewed with A. Gentile; and B. Weinstock-Guttman for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Oh, J., Bar-Or, A. Emerging therapies to target CNS pathophysiology in multiple sclerosis. Nat Rev Neurol 18, 466–475 (2022). https://doi.org/10.1038/s41582-022-00675-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-022-00675-0
This article is cited by
-
TREM-2 Drives Development of Multiple Sclerosis by Promoting Pathogenic Th17 Polarization
Neuroscience Bulletin (2024)
-
Emerging imaging markers in radiologically isolated syndrome: implications for earlier treatment initiation
Neurological Sciences (2024)
-
The astrocyte-produced growth factor HB-EGF limits autoimmune CNS pathology
Nature Immunology (2024)
-
Ferroptosis induces detrimental effects in chronic EAE and its implications for progressive MS
Acta Neuropathologica Communications (2023)
-
Inflammation in multiple sclerosis: consequences for remyelination and disease progression
Nature Reviews Neurology (2023)