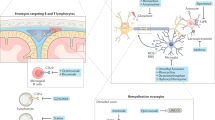
Abstract
In contrast to the multiple disease-modifying therapies that are available for relapsing–remitting multiple sclerosis (MS), the therapeutic options for progressive MS (PMS) are limited. Recent advances in our understanding of the neuroimmunology of PMS, including the mechanisms that drive slowly expanding lesions, have fuelled optimism for improved treatment of this condition. In this Review, we highlight the commonly observed neuropathology of PMS and discuss the associated mechanisms of CNS injury. We then apply this knowledge to formulate criteria for therapeutic efficacy in PMS, beginning with the need for early treatment owing to the substantial neuropathology that is already present at the initial clinical presentation. Other requirements include: antagonism of neuroaxonal injury mediators such as pro-inflammatory microglia and lymphocytes; remediation of oxidative stress resulting from iron deposition and mitochondrial dysfunction; and promotion of neuroprotection through remyelination. We consider whether current disease-modifying therapies for relapsing–remitting MS meet the criteria for successful therapeutics in PMS and suggest that the evidence favours the early introduction of sphingosine 1-phosphate receptor modulators. Finally, we weigh up emerging medications, including repurposed generic medications and Bruton’s tyrosine kinase inhibitors, against these fundamental criteria. In this new therapeutic era in PMS, success depends collectively on understanding disease mechanisms, drug characteristics (including brain penetration) and rational use.
Key points
-
Prominent pathological features of progressive multiple sclerosis (PMS) include global brain atrophy, slowly expanding lesions and a predominantly microglia/macrophage-mediated inflammatory response.
-
Neurodegeneration in MS seems to be driven by a complex interplay between compartmentalized neuroinflammation, oxidative stress, iron toxicity and mitochondrial dysfunction, and occurs as early as the radiologically and clinically isolated syndrome stages.
-
Proposed criteria for efficacious therapeutics in PMS include penetration across the blood–brain barrier, antagonism of compartmentalized lymphocytes and microglia/macrophages, and amelioration of oxidative stress.
-
Conferral of direct neuroprotection and remyelination, with the new myelin itself also exerting protective effects, are also desired features of effective treatments for PMS.
-
Emerging therapeutics for PMS that are discussed in this Review include α-lipoic acid, Bruton’s tyrosine kinase inhibitors, ibudilast and statins.
-
Repurposing of oral drugs for use in PMS is a promising area of research, and potential add-on therapeutics include hydroxychloroquine, metformin and niacin.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lublin, F. D. et al. The 2013 clinical course descriptors for multiple sclerosis: a clarification. Neurology 94, 1088–1092 (2020).
MS International Federation. Treatments and therapies. https://www.msif.org/living-with-ms/treatments/ (2021).
Thompson, A. J., Baranzini, S. E., Geurts, J., Hemmer, B. & Ciccarelli, O. Multiple sclerosis. Lancet 391, 1622–1636 (2018).
Lassmann, H., van Horssen, J. & Mahad, D. Progressive multiple sclerosis: pathology and pathogenesis. Nat. Rev. Neurol. 8, 647–656 (2012).
Lassmann, H. Pathogenic mechanisms associated with different clinical courses of multiple sclerosis. Front. Immunol. 9, 3116 (2018).
Weiner, H. L. A shift from adaptive to innate immunity: a potential mechanism of disease progression in multiple sclerosis. J. Neurol. 255 (Suppl. 1), 3–11 (2008).
Kutzelnigg, A. et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 128, 2705–2712 (2005).
Magliozzi, R. et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 130, 1089–1104 (2007).
Fransen, N. L. et al. Tissue-resident memory T cells invade the brain parenchyma in multiple sclerosis white matter lesions. Brain 143, 1714–1730 (2020).
Komori, M. et al. Cerebrospinal fluid markers reveal intrathecal inflammation in progressive multiple sclerosis. Ann. Neurol. 78, 3–20 (2015).
Choi, S. R. et al. Meningeal inflammation plays a role in the pathology of primary progressive multiple sclerosis. Brain 135, 2925–2937 (2012).
Antel, J., Antel, S., Caramanos, Z., Arnold, D. L. & Kuhlmann, T. Primary progressive multiple sclerosis: part of the MS disease spectrum or separate disease entity? Acta Neuropathol. 123, 627–638 (2012).
Zurawski, J. et al. 7T MRI cerebral leptomeningeal enhancement is common in relapsing-remitting multiple sclerosis and is associated with cortical and thalamic lesions. Mult. Scler. 26, 177–187 (2020).
Lucchinetti, C. F. et al. Inflammatory cortical demyelination in early multiple sclerosis. N. Engl. J. Med. 365, 2188–2197 (2011).
Faissner, S., Plemel, J. R., Gold, R. & Yong, V. W. Progressive multiple sclerosis: from pathophysiology to therapeutic strategies. Nat. Rev. Drug Discov. 18, 905–922 (2019).
He, A. et al. Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol. 19, 307–316 (2020).
Stys, P. K., Zamponi, G. W., van Minnen, J. & Geurts, J. J. Will the real multiple sclerosis please stand up? Nat. Rev. Neurosci. 13, 507–514 (2012).
Koch, M., Kingwell, E., Rieckmann, P. & Tremlett, H., UBC MS Clinic Neurologists. The natural history of secondary progressive multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 81, 1039–1043 (2010).
Tsutsui, S. et al. Multiple sclerosis brain transmits pathology to humanized transgenic mice potentially via protein misfolding pathway (278874, abstr. P514). Presented at the ECTRIMS Congress, 2019.
Luchicchi, A. et al. Axon–myelin unit blistering as early event in MS normal appearing white matter. Ann. Neurol. 89, 711–725 (2021).
De Stefano, N. et al. Axonal damage correlates with disability in patients with relapsing-remitting multiple sclerosis. Results of a longitudinal magnetic resonance spectroscopy study. Brain 121, 1469–1477 (1998).
Bjartmar, C., Kidd, G., Mork, S., Rudick, R. & Trapp, B. D. Neurological disability correlates with spinal cord axonal loss and reduced N-acetyl aspartate in chronic multiple sclerosis patients. Ann. Neurol. 48, 893–901 (2000).
Eshaghi, A. et al. Progression of regional grey matter atrophy in multiple sclerosis. Brain 141, 1665–1677 (2018).
Eshaghi, A. et al. Deep gray matter volume loss drives disability worsening in multiple sclerosis. Ann. Neurol. 83, 210–222 (2018).
Matthews, P. M. et al. Assessment of lesion pathology in multiple sclerosis using quantitative MRI morphometry and magnetic resonance spectroscopy. Brain 119, 715–722 (1996).
Bitsch, A., Schuchardt, J., Bunkowski, S., Kuhlmann, T. & Bruck, W. Acute axonal injury in multiple sclerosis. Correlation with demyelination and inflammation. Brain 123, 1174–1183 (2000).
Seewann, A. et al. Diffusely abnormal white matter in chronic multiple sclerosis: imaging and histopathologic analysis. Arch. Neurol. 66, 601–609 (2009).
Absinta, M., Lassmann, H. & Trapp, B. D. Mechanisms underlying progression in multiple sclerosis. Curr. Opin. Neurol. 33, 277–285 (2020).
Plemel, J. R., Liu, W. Q. & Yong, V. W. Remyelination therapies: a new direction and challenge in multiple sclerosis. Nat. Rev. Drug Discov. 16, 617–634 (2017).
Calabrese, M. et al. Exploring the origins of grey matter damage in multiple sclerosis. Nat. Rev. Neurosci. 16, 147–158 (2015).
Fischer, M. T. et al. NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain 135, 886–899 (2012).
Frischer, J. M. et al. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann. Neurol. 78, 710–721 (2015).
Luchetti, S. et al. Progressive multiple sclerosis patients show substantial lesion activity that correlates with clinical disease severity and sex: a retrospective autopsy cohort analysis. Acta Neuropathol. 135, 511–528 (2018).
Dal-Bianco, A. et al. Long-term evolution of multiple sclerosis iron rim lesions in 7 T MRI. Brain 144, 833–847 (2021).
Dal-Bianco, A. et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol. 133, 25–42 (2017).
Absinta, M. et al. Association of chronic active multiple sclerosis lesions with disability in vivo. JAMA Neurol. 76, 1474–1483 (2019).
Elliott, C. et al. Slowly expanding/evolving lesions as a magnetic resonance imaging marker of chronic active multiple sclerosis lesions. Mult. Scler. 25, 1915–1925 (2019).
Campbell, G. R. et al. Mitochondrial DNA deletions and neurodegeneration in multiple sclerosis. Ann. Neurol. 69, 481–492 (2011).
Jackle, K. et al. Molecular signature of slowly expanding lesions in progressive multiple sclerosis. Brain 143, 2073–2088 (2020).
Bottcher, C. et al. Single-cell mass cytometry reveals complex myeloid cell composition in active lesions of progressive multiple sclerosis. Acta Neuropathol. Commun. 8, 136 (2020).
Ludwin, S. K., Rao, V., Moore, C. S. & Antel, J. P. Astrocytes in multiple sclerosis. Mult. Scler. 22, 1114–1124 (2016).
Lebrun, C. et al. Anomalies characteristic of central nervous system demyelination: radiologically isolated syndrome. Neurol. Clin. 36, 59–68 (2018).
Alcaide-Leon, P. et al. Quantitative spinal cord MRI in radiologically isolated syndrome. Neurol. Neuroimmunol. Neuroinflamm 5, e436 (2018).
Azevedo, C. J. et al. Early CNS neurodegeneration in radiologically isolated syndrome. Neurol. Neuroimmunol. Neuroinflamm 2, e102 (2015).
George, I. C. et al. Cerebellar volume loss in radiologically isolated syndrome. Mult. Scler. 27, 130–133 (2021).
Kantarci, O. H. et al. Primary progressive multiple sclerosis evolving from radiologically isolated syndrome. Ann. Neurol. 79, 288–294 (2016).
Stromillo, M. L. et al. Brain metabolic changes suggestive of axonal damage in radiologically isolated syndrome. Neurology 80, 2090–2094 (2013).
Bjornevik, K. et al. Serum neurofilament light chain levels in patients with presymptomatic multiple sclerosis. JAMA Neurol. 77, 58–64 (2020).
Matute-Blanch, C. et al. Neurofilament light chain and oligoclonal bands are prognostic biomarkers in radiologically isolated syndrome. Brain 141, 1085–1093 (2018).
Mendiola, A. S. et al. Transcriptional profiling and therapeutic targeting of oxidative stress in neuroinflammation. Nat. Immunol. 21, 513–524 (2020).
Dong, Y. & Yong, V. W. When encephalitogenic T cells collaborate with microglia in multiple sclerosis. Nat. Rev. Neurol. 15, 704–717 (2019).
Serafini, B., Rosicarelli, B., Magliozzi, R., Stigliano, E. & Aloisi, F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 14, 164–174 (2004).
Howell, O. W. et al. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain 134, 2755–2771 (2011).
Lisak, R. P. et al. B cells from patients with multiple sclerosis induce cell death via apoptosis in neurons in vitro. J. Neuroimmunol. 309, 88–99 (2017).
Androdias, G. et al. Meningeal T cells associate with diffuse axonal loss in multiple sclerosis spinal cords. Ann. Neurol. 68, 465–476 (2010).
Prineas, J. W. et al. Immunopathology of secondary-progressive multiple sclerosis. Ann. Neurol. 50, 646–657 (2001).
Nikić, I. et al. A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat. Med. 17, 495–499 (2011).
Singh, S. et al. Microglial nodules in early multiple sclerosis white matter are associated with degenerating axons. Acta Neuropathol. 125, 595–608 (2013).
Giannetti, P. et al. Increased PK11195-PET binding in normal-appearing white matter in clinically isolated syndrome. Brain 138, 110–119 (2015).
Sucksdorff, M. et al. Brain TSPO-PET predicts later disease progression independent of relapses in multiple sclerosis. Brain 143, 3318–3330 (2020).
Brown, G. C. & Vilalta, A. How microglia kill neurons. Brain Res. 1628, 288–297 (2015).
Yong, H. Y. F., Rawji, K. S., Ghorbani, S., Xue, M. & Yong, V. W. The benefits of neuroinflammation for the repair of the injured central nervous system. Cell Mol. Immunol. 16, 540–546 (2019).
Berghoff, S. A. et al. Microglia facilitate repair of demyelinated lesions via post-squalene sterol synthesis. Nat. Neurosci. 24, 47–60 (2021).
Zrzavy, T. et al. Loss of ‘homeostatic’ microglia and patterns of their activation in active multiple sclerosis. Brain 140, 1900–1913 (2017).
Linnerbauer, M., Wheeler, M. A. & Quintana, F. J. Astrocyte crosstalk in CNS inflammation. Neuron 108, 608–622 (2020).
Lassmann, H. & van Horssen, J. Oxidative stress and its impact on neurons and glia in multiple sclerosis lesions. Biochim. Biophys. Acta 1862, 506–510 (2016).
van Horssen, J. et al. Severe oxidative damage in multiple sclerosis lesions coincides with enhanced antioxidant enzyme expression. Free Radic. Biol. Med. 45, 1729–1737 (2008).
Fischer, M. T. et al. Disease-specific molecular events in cortical multiple sclerosis lesions. Brain 136, 1799–1815 (2013).
Kemp, K. et al. Oxidative injury in multiple sclerosis cerebellar grey matter. Brain Res. 1642, 452–460 (2016).
Haider, L. et al. Oxidative damage in multiple sclerosis lesions. Brain 134, 1914–1924 (2011).
Choi, I. Y., Lee, P., Hughes, A. J., Denney, D. R. & Lynch, S. G. Longitudinal changes of cerebral glutathione (GSH) levels associated with the clinical course of disease progression in patients with secondary progressive multiple sclerosis. Mult. Scler. 23, 956–962 (2017).
Choi, I. Y. et al. In vivo evidence of oxidative stress in brains of patients with progressive multiple sclerosis. Mult. Scler. 24, 1029–1038 (2018).
Campbell, G. & Mahad, D. J. Mitochondrial dysfunction and axon degeneration in progressive multiple sclerosis. FEBS Lett. 592, 1113–1121 (2018).
Licht-Mayer, S. et al. Enhanced axonal response of mitochondria to demyelination offers neuroprotection: implications for multiple sclerosis. Acta Neuropathol. 140, 143–167 (2020).
Mahad, D. H., Trapp, B. D. & Lassmann, H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 14, 183–193 (2015).
Dutta, R. et al. Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann. Neurol. 59, 478–489 (2006).
Stephenson, E., Nathoo, N., Mahjoub, Y., Dunn, J. F. & Yong, V. W. Iron in multiple sclerosis: roles in neurodegeneration and repair. Nat. Rev. Neurol. 10, 459–468 (2014).
Lee, N. J. et al. Potential role of iron in repair of inflammatory demyelinating lesions. J. Clin. Invest. 129, 4365–4376 (2019).
Cronin, S. J. F., Woolf, C. J., Weiss, G. & Penninger, J. M. The role of iron regulation in immunometabolism and immune-related disease. Front. Mol. Biosci. 6, 116 (2019).
Urrutia, P. et al. Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells. J. Neurochem. 126, 541–549 (2013).
Faissner, S. et al. Systematic screening of generic drugs for progressive multiple sclerosis identifies clomipramine as a promising therapeutic. Nat. Commun. 8, 1990 (2017).
Filippi, M. et al. Association between pathological and MRI findings in multiple sclerosis. Lancet Neurol. 18, 198–210 (2019).
Hagemeier, J. et al. Iron-related gene variants and brain iron in multiple sclerosis and healthy individuals. Neuroimage Clin. 17, 530–540 (2018).
Elkady, A. M., Cobzas, D., Sun, H., Blevins, G. & Wilman, A. H. Progressive iron accumulation across multiple sclerosis phenotypes revealed by sparse classification of deep gray matter. J. Magn. Reson. Imaging 46, 1464–1473 (2017).
Raz, E. et al. Relationship between iron accumulation and white matter injury in multiple sclerosis: a case-control study. J. Neurol. 262, 402–409 (2015).
Haider, L. et al. Multiple sclerosis deep grey matter: the relation between demyelination, neurodegeneration, inflammation and iron. J. Neurol. Neurosurg. Psychiatry 85, 1386–1395 (2014).
Bergsland, N. et al. White matter tract injury is associated with deep gray matter iron deposition in multiple sclerosis. J. Neuroimaging 27, 107–113 (2017).
Zivadinov, R. et al. Brain iron at quantitative MRI is associated with disability in multiple sclerosis. Radiology 289, 487–496 (2018).
Hametner, S. et al. Iron and neurodegeneration in the multiple sclerosis brain. Ann. Neurol. 74, 848–861 (2013).
Bagnato, F. et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain 134, 3602–3615 (2011).
Stangel, M., Kuhlmann, T., Matthews, P. M. & Kilpatrick, T. J. Achievements and obstacles of remyelinating therapies in multiple sclerosis. Nat. Rev. Neurol. 13, 742–754 (2017).
Lubetzki, C., Zalc, B., Williams, A., Stadelmann, C. & Stankoff, B. Remyelination in multiple sclerosis: from basic science to clinical translation. Lancet Neurol. 19, 678–688 (2020).
Micu, I., Plemel, J. R., Caprariello, A. V., Nave, K. A. & Stys, P. K. Axo-myelinic neurotransmission: a novel mode of cell signalling in the central nervous system. Nat. Rev. Neurosci. 19, 49–58 (2018).
Kornek, B. et al. Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am. J. Pathol. 157, 267–276 (2000).
Bodini, B. et al. Dynamic imaging of individual remyelination profiles in multiple sclerosis. Ann. Neurol. 79, 726–738 (2016).
Franklin, R. J. M., Frisen, J. & Lyons, D. A. Revisiting remyelination: towards a consensus on the regeneration of CNS myelin. Semin. Cell Dev. Biol. 116, 3–9 (2021).
Boyd, A., Zhang, H. & Williams, A. Insufficient OPC migration into demyelinated lesions is a cause of poor remyelination in MS and mouse models. Acta Neuropathol. 125, 841–859 (2013).
Kuhlmann, T. et al. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain 131, 1749–1758 (2008).
Patrikios, P. et al. Remyelination is extensive in a subset of multiple sclerosis patients. Brain 129, 3165–3172 (2006).
Goldschmidt, T., Antel, J., Konig, F. B., Bruck, W. & Kuhlmann, T. Remyelination capacity of the MS brain decreases with disease chronicity. Neurology 72, 1914–1921 (2009).
Bramow, S. et al. Demyelination versus remyelination in progressive multiple sclerosis. Brain 133, 2983–2998 (2010).
Strijbis, E. M. M., Kooi, E. J., van der Valk, P. & Geurts, J. J. G. Cortical remyelination is heterogeneous in multiple sclerosis. J. Neuropathol. Exp. Neurol. 76, 390–401 (2017).
Nicaise, A. M. et al. Cellular senescence in progenitor cells contributes to diminished remyelination potential in progressive multiple sclerosis. Proc. Natl Acad. Sci. USA 116, 9030–9039 (2019).
Starost, L. et al. Extrinsic immune cell-derived, but not intrinsic oligodendroglial factors contribute to oligodendroglial differentiation block in multiple sclerosis. Acta Neuropathol. 140, 715–736 (2020).
Cunniffe, N. & Coles, A. Promoting remyelination in multiple sclerosis. J. Neurol. 268, 30–44 (2021).
Faissner, S. & Gold, R. Progressive multiple sclerosis: latest therapeutic developments and future directions. Ther. Adv. Neurol. Disord. 12, 1756286419878323 (2019).
Villoslada, P. & Steinman, L. New targets and therapeutics for neuroprotection, remyelination and repair in multiple sclerosis. Expert Opin. Investig. Drugs 29, 443–459 (2020).
Macrez, R., Stys, P. K., Vivien, D., Lipton, S. A. & Docagne, F. Mechanisms of glutamate toxicity in multiple sclerosis: biomarker and therapeutic opportunities. Lancet Neurol. 15, 1089–1102 (2016).
Woo, M. S. et al. Neuronal metabotropic glutamate receptor 8 protects against neurodegeneration in CNS inflammation. J. Exp. Med. 218, e20201290 (2021).
Gallego-Delgado, P. et al. Neuroinflammation in the normal-appearing white matter (NAWM) of the multiple sclerosis brain causes abnormalities at the nodes of Ranvier. PLoS Biol. 18, e3001008 (2020).
Schattling, B. et al. Bassoon proteinopathy drives neurodegeneration in multiple sclerosis. Nat. Neurosci. 22, 887–896 (2019).
Tintore, M., Vidal-Jordana, A. & Sastre-Garriga, J. Treatment of multiple sclerosis — success from bench to bedside. Nat. Rev. Neurol. 15, 53–58 (2019).
Rommer, P. S. et al. Immunological aspects of approved MS therapeutics. Front. Immunol. 10, 1564 (2019).
Groves, A., Kihara, Y. & Chun, J. Fingolimod: direct CNS effects of sphingosine 1-phosphate (S1P) receptor modulation and implications in multiple sclerosis therapy. J. Neurol. Sci. 328, 9–18 (2013).
Dubey, D. et al. Dimethyl fumarate in relapsing-remitting multiple sclerosis: rationale, mechanisms of action, pharmacokinetics, efficacy and safety. Expert Rev. Neurother. 15, 339–346 (2015).
Baker, D., Pryce, G., Herrod, S. S. & Schmierer, K. Potential mechanisms of action related to the efficacy and safety of cladribine. Mult. Scler. Relat. Disord. 30, 176–186 (2019).
Yong, V. W. Differential mechanisms of action of interferon-β and glatiramer aetate in MS. Neurology 59, 802–808 (2002).
Brundula, V., Rewcastle, N. B., Metz, L. M., Bernard, C. C. & Yong, V. W. Targeting leukocyte MMPs and transmigration: minocycline as a potential therapy for multiple sclerosis. Brain 125, 1297–1308 (2002).
Banks, W. A. Characteristics of compounds that cross the blood-brain barrier. BMC Neurol. 9 (Suppl. 1), S3 (2009).
Montalban, X. et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N. Engl. J. Med. 376, 209–220 (2017).
Tallantyre, E., Evangelou, N. & Constantinescu, C. S. Spotlight on teriflunomide. Int. MS J. 15, 62–68 (2008).
Gottle, P. et al. Teriflunomide promotes oligodendroglial differentiation and myelination. J. Neuroinflammation 15, 76 (2018).
Singh, V., Voss, E. V., Benardais, K. & Stangel, M. Effects of 2-chlorodeoxyadenosine (cladribine) on primary rat microglia. J. Neuroimmune Pharmacol. 7, 939–950 (2012).
Linker, R. A. et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 134, 678–692 (2011).
Pagani, F. et al. Dimethyl fumarate reduces microglia functional response to tissue damage and favors brain iron homeostasis. Neuroscience 439, 241–254 (2020).
Arnon, R. & Aharoni, R. Glatiramer acetate: from bench to bed and back. Isr. Med. Assoc. J. 21, 151–157 (2019).
O’Sullivan, S. & Dev, K. K. Sphingosine-1-phosphate receptor therapies: advances in clinical trials for CNS-related diseases. Neuropharmacology 113, 597–607 (2017).
Kim, H. J. et al. Neurobiological effects of sphingosine 1-phosphate receptor modulation in the cuprizone model. FASEB J. 25, 1509–1518 (2011).
Noda, H., Takeuchi, H., Mizuno, T. & Suzumura, A. Fingolimod phosphate promotes the neuroprotective effects of microglia. J. Neuroimmunol. 256, 13–18 (2013).
Rossi, S. et al. Oral fingolimod rescues the functional deficits of synapses in experimental autoimmune encephalomyelitis. Br. J. Pharmacol. 165, 861–869 (2012).
Yazdi, A., Ghasemi-Kasman, M. & Javan, M. Possible regenerative effects of fingolimod (FTY720) in multiple sclerosis disease: an overview on remyelination process. J. Neurosci. Res. 98, 524–536 (2020).
Miron, V. E. et al. FTY720 modulates human oligodendrocyte progenitor process extension and survival. Ann. Neurol. 63, 61–71 (2008).
Lublin, F. et al. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet 387, 1075–1084 (2016).
Ward, L. A. et al. Siponimod therapy implicates Th17 cells in a preclinical model of subpial cortical injury. JCI Insight 5, e132522 (2020).
Gentile, A. et al. Siponimod (BAF312) prevents synaptic neurodegeneration in experimental multiple sclerosis. J. Neuroinflammation 13, 207 (2016).
Mannioui, A. et al. The Xenopus tadpole: an in vivo model to screen drugs favoring remyelination. Mult. Scler. 24, 1421–1432 (2018).
Kappos, L. et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet 391, 1263–1273 (2018).
Cree, B. A. et al. Siponimod: disentangling disability and relapses in secondary progressive multiple sclerosis. Mult. Scler. 27, 1564–1576 (2021).
Benedict, R. H. B. et al. Siponimod and cognition in secondary progressive multiple sclerosis: EXPAND secondary analyses. Neurology 96, e376–e386 (2021).
Kalincik, T. et al. Treatment effectiveness of alemtuzumab compared with natalizumab, fingolimod, and interferon beta in relapsing-remitting multiple sclerosis: a cohort study. Lancet Neurol. 16, 271–281 (2017).
Brown, J. W. L. et al. Association of initial disease-modifying therapy with later conversion to secondary progressive multiple sclerosis. JAMA 321, 175–187 (2019).
Narayan, R. N., Forsthuber, T. & Stuve, O. Emerging drugs for primary progressive multiple sclerosis. Expert Opin. Emerg. Drugs 23, 97–110 (2018).
Biewenga, G. P., Haenen, G. R. & Bast, A. The pharmacology of the antioxidant lipoic acid. Gen. Pharmacol. 29, 315–331 (1997).
Packer, L., Tritschler, H. J. & Wessel, K. Neuroprotection by the metabolic antioxidant α-lipoic acid. Free Radic. Biol. Med. 22, 359–378 (1997).
Whiteman, M., Tritschler, H. & Halliwell, B. Protection against peroxynitrite-dependent tyrosine nitration and α1-antiproteinase inactivation by oxidized and reduced lipoic acid. FEBS Lett. 379, 74–76 (1996).
Lovell, M. A., Xie, C., Xiong, S. & Markesbery, W. R. Protection against amyloid beta peptide and iron/hydrogen peroxide toxicity by alpha lipoic acid. J. Alzheimers Dis. 5, 229–239 (2003).
George, J. D., Kim, E., Spain, R., Bourdette, D. & Salinthone, S. Effects of lipoic acid on migration of human B cells and monocyte-enriched peripheral blood mononuclear cells in relapsing remitting multiple sclerosis. J. Neuroimmunol. 315, 24–27 (2018).
Fiedler, S. E., Spain, R. I., Kim, E. & Salinthone, S. Lipoic acid modulates inflammatory responses of monocytes and monocyte-derived macrophages from healthy and relapsing-remitting multiple sclerosis patients. Immunol. Cell Biol. 99, 107–115 (2021).
Marracci, G. H., Jones, R. E., McKeon, G. P. & Bourdette, D. N. Alpha lipoic acid inhibits T cell migration into the spinal cord and suppresses and treats experimental autoimmune encephalomyelitis. J. Neuroimmunol. 131, 104–114 (2002).
Sanadgol, N. et al. Alpha lipoic acid mitigates toxic-induced demyelination in the corpus callosum by lessening of oxidative stress and stimulation of polydendrocytes proliferation. Metab. Brain Dis. 33, 27–37 (2018).
Spain, R. et al. Lipoic acid in secondary progressive MS: a randomized controlled pilot trial. Neurol. Neuroimmunol. Neuroinflamm 4, e374 (2017).
Loy, B. D., Fling, B. W., Horak, F. B., Bourdette, D. N. & Spain, R. I. Effects of lipoic acid on walking performance, gait, and balance in secondary progressive multiple sclerosis. Complement. Ther. Med. 41, 169–174 (2018).
Rip, J., Van Der Ploeg, E. K., Hendriks, R. W. & Corneth, O. B. J. The role of Bruton’s tyrosine kinase in immune cell signaling and systemic autoimmunity. Crit. Rev. Immunol. 38, 17–62 (2018).
Torke, S. et al. Inhibition of Bruton’s tyrosine kinase interferes with pathogenic B-cell development in inflammatory CNS demyelinating disease. Acta Neuropathol. 140, 535–548 (2020).
Crofford, L. J., Nyhoff, L. E., Sheehan, J. H. & Kendall, P. L. The role of Bruton’s tyrosine kinase in autoimmunity and implications for therapy. Expert Rev. Clin. Immunol. 12, 763–773 (2016).
Bhargava, P. et al. Imaging meningeal inflammation in CNS autoimmunity identifies a therapeutic role for BTK inhibition. Brain 144, 1396–1408 (2021).
Montalban, X. et al. Placebo-controlled trial of an oral BTK inhibitor in multiple sclerosis. N. Engl. J. Med. 380, 2406–2417 (2019).
Weber, A. N. R. et al. Bruton’s tyrosine kinase: an emerging key player in innate immunity. Front. Immunol. 8, 1454 (2017).
Ni Gabhann, J. et al. Btk regulates macrophage polarization in response to lipopolysaccharide. PLoS ONE 9, e85834 (2014).
Nam, H. Y. et al. Ibrutinib suppresses LPS-induced neuroinflammatory responses in BV2 microglial cells and wild-type mice. J. Neuroinflammation 15, 271 (2018).
Goldwirt, L., Beccaria, K., Ple, A., Sauvageon, H. & Mourah, S. Ibrutinib brain distribution: a preclinical study. Cancer Chemother. Pharmacol. 81, 783–789 (2018).
Gruber, R. C. et al. Poster P0311: Decoding Bruton’s tyrosine kinase signaling in neuroinflammation. Presented at the 8th Joint ACTRIMS-ECTRIMS Meeting (MSVirtual2020) (2020).
Al-Harbi, N. O. et al. Therapeutic treatment with Ibrutinib attenuates imiquimod-induced psoriasis-like inflammation in mice through downregulation of oxidative and inflammatory mediators in neutrophils and dendritic cells. Eur. J. Pharmacol. 877, 173088 (2020).
Mangla, A. et al. Pleiotropic consequences of Bruton tyrosine kinase deficiency in myeloid lineages lead to poor inflammatory responses. Blood 104, 1191–1197 (2004).
Martin, E. et al. Bruton’s tyrosine kinase inhibition promotes myelin repair. Brain Plast. 5, 123–133 (2020).
Dolgin, E. BTK blockers make headway in multiple sclerosis. Nat. Biotechnol. 39, 3–5 (2021).
Suzumura, A., Ito, A., Yoshikawa, M. & Sawada, M. Ibudilast suppresses TNFα production by glial cells functioning mainly as type III phosphodiesterase inhibitor in the CNS. Brain Res. 837, 203–212 (1999).
Mizuno, T. et al. Neuroprotective role of phosphodiesterase inhibitor ibudilast on neuronal cell death induced by activated microglia. Neuropharmacology 46, 404–411 (2004).
Fujimoto, T., Sakoda, S., Fujimura, H. & Yanagihara, T. Ibudilast, a phosphodiesterase inhibitor, ameliorates experimental autoimmune encephalomyelitis in Dark August rats. J. Neuroimmunol. 95, 35–42 (1999).
Fox, R. J. et al. Phase 2 trial of ibudilast in progressive multiple sclerosis. N. Engl. J. Med. 379, 846–855 (2018).
Naismith, R. T. et al. Effects of ibudilast on MRI measures in the phase 2 SPRINT-MS study. Neurology 96, e491–e500 (2021).
Fox, R. J. et al. Neurofilament light chain in a phase 2 clinical trial of ibudilast in progressive multiple sclerosis. Mult. Scler. https://doi.org/10.1177/1352458520986956 (2021).
Botti, R. E., Triscari, J., Pan, H. Y. & Zayat, J. Concentrations of pravastatin and lovastatin in cerebrospinal fluid in healthy subjects. Clin. Neuropharmacol. 14, 256–261 (1991).
Youssef, S. et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature 420, 78–84 (2002).
Zhang, X., Tao, Y., Troiani, L. & Markovic-Plese, S. Simvastatin inhibits IFN regulatory factor 4 expression and Th17 cell differentiation in CD4+ T cells derived from patients with multiple sclerosis. J. Immunol. 187, 3431–3437 (2011).
Lawman, S., Mauri, C., Jury, E. C., Cook, H. T. & Ehrenstein, M. R. Atorvastatin inhibits autoreactive B cell activation and delays lupus development in New Zealand black/white F1 mice. J. Immunol. 173, 7641–7646 (2004).
Lindberg, C., Crisby, M., Winblad, B. & Schultzberg, M. Effects of statins on microglia. J. Neurosci. Res. 82, 10–19 (2005).
Guasti, L. et al. Prolonged statin-associated reduction in neutrophil reactive oxygen species and angiotensin II type 1 receptor expression: 1-year follow-up. Eur. Heart J. 29, 1118–1126 (2008).
Wagner, A. H., Kohler, T., Ruckschloss, U., Just, I. & Hecker, M. Improvement of nitric oxide-dependent vasodilatation by HMG-CoA reductase inhibitors through attenuation of endothelial superoxide anion formation. Arterioscler. Thromb. Vasc. Biol. 20, 61–69 (2000).
Tong, H. et al. Simvastatin inhibits activation of NADPH oxidase/p38 MAPK pathway and enhances expression of antioxidant protein in Parkinson disease models. Front. Mol. Neurosci. 11, 165 (2018).
Paintlia, A. S., Paintlia, M. K., Singh, A. K. & Singh, I. Inhibition of rho family functions by lovastatin promotes myelin repair in ameliorating experimental autoimmune encephalomyelitis. Mol. Pharmacol. 73, 1381–1393 (2008).
Dolga, A. M. et al. Lovastatin induces neuroprotection through tumor necrosis factor receptor 2 signaling pathways. J. Alzheimers Dis. 13, 111–122 (2008).
Chataway, J. et al. Effect of high-dose simvastatin on brain atrophy and disability in secondary progressive multiple sclerosis (MS-STAT): a randomised, placebo-controlled, phase 2 trial. Lancet 383, 2213–2221 (2014).
Chan, D. et al. Effect of high-dose simvastatin on cognitive, neuropsychiatric, and health-related quality-of-life measures in secondary progressive multiple sclerosis: secondary analyses from the MS-STAT randomised, placebo-controlled trial. Lancet Neurol. 16, 591–600 (2017).
Wei, Y., Nygard, G. A., Ellertson, S. L. & Khalil, S. K. Stereoselective disposition of hydroxychloroquine and its metabolite in rats. Chirality 7, 598–604 (1995).
Koch, M. W. et al. Hydroxychloroquine reduces microglial activity and attenuates experimental autoimmune encephalomyelitis. J. Neurol. Sci. 358, 131–137 (2015).
Faissner, S. et al. Unexpected additive effects of minocycline and hydroxychloroquine in models of multiple sclerosis: prospective combination treatment for progressive disease? Mult. Scler. 24, 1543–1556 (2018).
Brown, D., Moezzi, D., Dong, Y., Koch, M. & Yong, V. W. Combination of hydroxychloroquine and indapamide attenuates neurodegeneration in models relevant to multiple sclerosis. Neurotherapeutics 18, 387–400 (2021).
Koch, M. W. et al. Hydroxychloroquine for primary progressive multiple sclerosis. Ann. Neurol. https://doi.org/10.1002/ana.26239 (2021).
Sun, Y. et al. Metformin ameliorates the development of experimental autoimmune encephalomyelitis by regulating T helper 17 and regulatory T cells in mice. J. Neuroimmunol. 292, 58–67 (2016).
Nath, N. et al. Metformin attenuated the autoimmune disease of the central nervous system in animal models of multiple sclerosis. J. Immunol. 182, 8005–8014 (2009).
Algire, C. et al. Metformin reduces endogenous reactive oxygen species and associated DNA damage. Cancer Prev. Res. 5, 536–543 (2012).
Neumann, B. et al. Metformin restores CNS remyelination capacity by rejuvenating aged stem cells. Cell Stem Cell 25, 473–485.e8 (2019).
Nakashima, Y. & Suzue, R. Effect of nicotinic acid on myelin lipids in brain of developing rat. J. Nutr. Sci. Vitaminol. 28, 491–500 (1982).
Zhang, J. et al. Niaspan treatment improves neurological functional recovery in experimental autoimmune encephalomyelitis mice. Neurobiol. Dis. 32, 273–280 (2008).
Rawji, K. S. et al. Niacin-mediated rejuvenation of macrophage/microglia enhances remyelination of the aging central nervous system. Acta Neuropathol. 139, 893–909 (2020).
Kaneko, S. et al. Protecting axonal degeneration by increasing nicotinamide adenine dinucleotide levels in experimental autoimmune encephalomyelitis models. J. Neurosci. 26, 9794–9804 (2006).
Acknowledgements
V.W.Y. is funded by operating research grants from the MS Society of Canada and the Canadian Institutes of Health Research. We thank F. Yong for schematizing Fig. 1.
Review criteria
A full search of the PubMed database was conducted, combining ‘progressive multiple sclerosis’ with the following terms: ‘pathology and mechanisms’, ‘inflammation OR microglia OR oxidative injury OR mitochondria dysfunction OR iron’, ‘neurodegeneration’, ‘remyelination’ and ‘disease-modifying therapy(ies) OR immunomodulator OR clinical trial OR drug OR therapy’. For therapeutics, we used the search term ‘randomized controlled clinical drugs trials in progressive MS’. Other papers were discovered by hand-searching the references of reviews of disease-modifying therapies in progressive multiple sclerosis. We further analysed the reference lists of several key papers to identify additional papers and cross-references.
Author information
Authors and Affiliations
Contributions
H.Y.F.Y. prepared the first draft including Figs 2–4 and the tables, and revised the manuscript. V.W.Y. supervised the initial draft, revised the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
V.W.Y. has received honoraria from Biogen, Novartis, Roche, Sanofi-Genzyme and Teva for industry-sponsored talks, and consulting fees from EMD Serono, Novartis, Roche, Sanofi-Genzyme and Teva. He is the recipient of unrestricted educational grants from Biogen, EMD Serono, Novartis, Roche, Sanofi-Genzyme and Teva to support educational activities of the Alberta MS Network, which he directs. H.Y.F.Y. declares no competing interests.
Additional information
Peer review information
Nature Reviews Neurology thanks H. Lassmann, A. Thompson and R. Reynolds for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yong, H.Y.F., Yong, V.W. Mechanism-based criteria to improve therapeutic outcomes in progressive multiple sclerosis. Nat Rev Neurol 18, 40–55 (2022). https://doi.org/10.1038/s41582-021-00581-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-021-00581-x
This article is cited by
-
Myeloid cell replacement is neuroprotective in chronic experimental autoimmune encephalomyelitis
Nature Neuroscience (2024)
-
Evaluation of the quality and the productivity of neuroradiological reading of multiple sclerosis follow-up MRI scans using an intelligent automation software
Neuroradiology (2024)
-
Drugs Targeting CD20 in Multiple Sclerosis: Pharmacology, Efficacy, Safety, and Tolerability
Drugs (2024)
-
Granzyme B + CD8 + T cells with terminal differentiated effector signature determine multiple sclerosis progression
Journal of Neuroinflammation (2023)
-
Potentially toxic elements in the brains of people with multiple sclerosis
Scientific Reports (2023)