Abstract
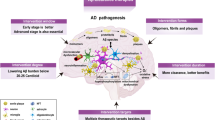
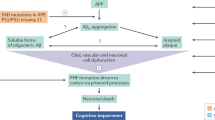
Alzheimer disease (AD) is the most common cause of dementia in older individuals (>65 years) and has a long presymptomatic phase. Preventive therapies for AD are not yet available, and potential disease-modifying therapies targeting amyloid-β plaques in symptomatic stages of AD have only just been approved in the United States. Small-molecule inhibitors of β-site amyloid precursor protein (APP)-cleaving enzyme 1 (BACE1; also known as β-secretase 1) reduce the production of amyloid-β peptide and are among the most advanced drug candidates for AD. However, to date all phase II and phase III clinical trials of BACE inhibitors were either concluded without benefit or discontinued owing to futility or the occurrence of adverse effects. Adverse effects included early, mild cognitive impairment that was associated with all but one inhibitor; preliminary results suggest that the cognitive effects are non-progressive and reversible. These discontinuations have raised questions regarding the suitability of BACE1 as a drug target for AD. In this Perspective, we discuss the status of BACE inhibitors and suggest ways in which the results of the discontinued trials can inform the development of future clinical trials of BACE inhibitors and related secretase modulators as preventative therapies. We also propose a series of experiments that should be performed to inform ‘go–no-go’ decisions in future trials with BACE inhibitors and consider the possibility that low levels of BACE1 inhibition could avoid adverse effects while achieving efficacy for AD prevention.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Selkoe, D. J. & Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 8, 595–608 (2016).
De Strooper, B. & Karran, E. The cellular phase of Alzheimer’s disease. Cell 164, 603–615 (2016).
Hussain, I. et al. Identification of a novel aspartic protease (Asp 2) as beta-secretase. Mol. Cell. Neurosci. 14, 419–427 (1999).
Sinha, S. et al. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature 402, 537–540 (1999).
Vassar, R. et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286, 735–741 (1999).
Yan, R. et al. Membrane-anchored aspartyl protease with Alzheimer’s disease beta-secretase activity. Nature 402, 533–537 (1999).
Das, B. & Yan, R. A close look at BACE1 inhibitors for Alzheimer’s disease treatment. CNS Drugs 33, 251–263 (2019).
Egan, M. F. et al. Randomized trial of verubecestat for prodromal Alzheimer’s disease. N. Engl. J. Med. 380, 1408–1420 (2019).
Henley, D. et al. Preliminary results of a trial of atabecestat in preclinical Alzheimer’s disease. N. Engl. J. Med. 380, 1483–1485 (2019).
Knopman, D. S. Lowering of amyloid-beta by beta-secretase inhibitors - some informative failures. N. Engl. J. Med. 380, 1476–1478 (2019).
Doody, R. S. et al. A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N. Engl. J. Med. 369, 341–350 (2013).
Coric, V. et al. Safety and tolerability of the gamma-secretase inhibitor avagacestat in a phase 2 study of mild to moderate Alzheimer disease. Arch. Neurol. 69, 1430–1440 (2012).
De Strooper, B. Lessons from a failed gamma-secretase Alzheimer trial. Cell 159, 721–726 (2014).
Kevin, D. et al. Preclinical validation of a potent γ-secretase modulator for Alzheimer’s disease prevention. J. Exp. Med. 218, e20202560 (2021).
Graf, A. et al. Umibecestat in the API Generation program: worsening in RBANS and/or CDR on treatment reverses after wash-out. Alzheimers Dement. 16, e041140 (2020).
Hampel, H. et al. Precision pharmacology for Alzheimer’s disease. Pharmacol. Res. 130, 331–365 (2018).
Eketjall, S. et al. AZD3293: a novel, orally active BACE1 inhibitor with high potency and permeability and markedly slow off-rate kinetics. J. Alzheimers Dis. 50, 1109–1123 (2016).
Kennedy, M. E. et al. The BACE1 inhibitor verubecestat (MK-8931) reduces CNS beta-amyloid in animal models and in Alzheimer’s disease patients. Sci. Transl Med. 8, 363ra150 (2016).
Neumann, U. et al. The BACE-1 inhibitor CNP520 for prevention trials in Alzheimer’s disease. EMBO Mol. Med. 10, e9316 (2018).
Egan, M. F. et al. Randomized trial of verubecestat for mild-to-moderate Alzheimer’s disease. N. Engl. J. Med. 378, 1691–1703 (2018).
Wessels, A. M. et al. Efficacy and safety of lanabecestat for treatment of early and mild Alzheimer disease: the AMARANTH and DAYBREAK-ALZ randomized clinical trials. JAMA Neurol. 77, 199–209 (2019).
Mintun, M. A. et al. Donanemab in early Alzheimer’s disease. N. Engl. J. Med. 384, 1691–1704 (2021).
Koskinas, K. C. et al. Effect of statins and non-statin LDL-lowering medications on cardiovascular outcomes in secondary prevention: a meta-analysis of randomized trials. Eur. Heart J. 39, 1172–1180 (2018).
Zuhl, A. M. et al. Chemoproteomic profiling reveals that cathepsin D off-target activity drives ocular toxicity of beta-secretase inhibitors. Nat. Commun. 7, 13042 (2016).
Cai, J. et al. β-Secretase (BACE1) inhibition causes retinal pathology by vascular dysregulation and accumulation of age pigment. EMBO Mol. Med. 4, 980–991 (2012).
Eli Lilly & Company. Lilly voluntarily terminates phase II study for LY2886721, a beta secretase inhibitor being investigated as a treatment for Alzheimer’s disease. Lilly https://investor.lilly.com/static-files/32b60234-ea3c-4461-875d-87167528f516 (2013).
Esterhazy, D. et al. Bace2 is a beta cell-enriched protease that regulates pancreatic beta cell function and mass. Cell Metab. 14, 365–377 (2011).
Stutzer, I. et al. Systematic proteomic analysis identifies beta-site amyloid precursor protein cleaving enzyme 2 and 1 (BACE2 and BACE1) substrates in pancreatic beta-cells. J. Biol. Chem. 288, 10536–10547 (2013).
Voytyuk, I. et al. BACE2 distribution in major brain cell types and identification of novel substrates. Life Sci. Alliance 1, e201800026 (2018).
Farzan, M., Schnitzler, C. E., Vasilieva, N., Leung, D. & Choe, H. BACE2, a beta -secretase homolog, cleaves at the beta site and within the amyloid-beta region of the amyloid-beta precursor protein. Proc. Natl Acad. Sci. USA 97, 9712–9717 (2000).
Yan, R., Munzner, J. B., Shuck, M. E. & Bienkowski, M. J. BACE2 functions as an alternative alpha-secretase in cells. J. Biol. Chem. 276, 34019–34027 (2001).
Rochin, L. et al. BACE2 processes PMEL to form the melanosome amyloid matrix in pigment cells. Proc. Natl Acad. Sci. USA 110, 10658–10663 (2013).
Cebers, G. et al. Reversible and species-specific depigmentation effects of AZD3293, a BACE inhibitor for the treatment of Alzheimer’s disease, are related to BACE2 inhibition and confined to epidermis and hair. J. Prev. Alzheimers Dis. 3, 202–218 (2016).
Shimshek, D. R. et al. Pharmacological BACE1 and BACE2 inhibition induces hair depigmentation by inhibiting PMEL17 processing in mice. Sci. Rep. 6, 21917 (2016).
Kuhn, P. H. et al. Secretome protein enrichment identifies physiological BACE1 protease substrates in neurons. EMBO J. 31, 3157–3168 (2012).
Zhou, L. et al. The neural cell adhesion molecules L1 and CHL1 are cleaved by BACE1 protease in vivo. J. Biol. Chem. 287, 25927–25940 (2012).
Hemming, M. L., Elias, J. E., Gygi, S. P. & Selkoe, D. J. Identification of beta-secretase (BACE1) substrates using quantitative proteomics. PLoS ONE 4, e8477 (2009).
Tüshaus, J. et al. An optimized quantitative proteomics method establishes the cell type-resolved mouse brain secretome. EMBO J. 39, e105693 (2020).
Rogers, M. B. Bump in the road or disaster? BACE inhibitors worsen cognition. AlzForum https://www.alzforum.org/news/conference-coverage/bump-road-or-disaster-bace-inhibitors-worsen-cognition (2018).
Egan, M. F. et al. Further analyses of the safety of verubecestat in the phase 3 EPOCH trial of mild-to-moderate Alzheimer’s disease. Alzheimers Res. Ther. 11, 68 (2019).
Lopez Lopez, C. et al. The Alzheimer’s Prevention Initiative Generation Program: study design of two randomized controlled trials for individuals at risk for clinical onset of Alzheimer’s disease. Alzheimers Dement. 5, 216–227 (2019).
Sperling, R. et al. Findings of efficacy, safety, and biomarker outcomes of atabecestat in preclinical Alzheimer disease: a truncated randomized phase 2b/3 clinical trial. JAMA Neurol. 78, 293–301 (2021).
Wessels, A. M. et al. Cognitive outcomes in trials of two BACE inhibitors in Alzheimer’s disease. Alzheimers Dement. 16, 1483–1492 (2020).
Timmers, M. et al. Pharmacodynamics of atabecestat (JNJ-54861911), an oral BACE1 inhibitor in patients with early Alzheimer’s disease: randomized, double-blind, placebo-controlled study. Alzheimers Res. Ther. 10, 85 (2018).
Lynch, S. Y. et al. Elenbecestat, a BACE inhibitor: results from a phase 2 study in subjects with mild cognitive impairment and mild-to-moderate dementia due to Alzheimer’s disease [abstract P4-389]. Alzheimers Dement. 14, 1623 (2018).
Willis, B. et al. Pharmacokinetics, pharmacodynamics, safety, and tolerability of LY3202626, a novel BACE1 inhibitor, in healthy subjects and patients with Alzheimer’s disease [abstract P1-044]. Alzheimers Dement. 12, 418 (2016).
Rogers, M. B. Picking through the rubble, field tries to salvage BACE inhibitors. AlzForum https://www.alzforum.org/news/conference-coverage/picking-through-rubble-field-tries-salvage-bace-inhibitors (2019).
Reiman, E. M. et al. The API Generation program: umibecestat treatment and discontinuation effects on hippocampal and whole brain volumes in the overall population and amyloid-negative APOE4 homozygotes. Alzheimers Dement. 16, e041142 (2020).
Sur, C. et al. BACE inhibition causes rapid, regional, and non-progressive volume reduction in Alzheimer’s disease brain. Brain 143, 3816–3826 (2020).
McConlogue, L. et al. Partial reduction of BACE1 has dramatic effects on Alzheimer plaque and synaptic pathology in APP transgenic mice. J. Biol. Chem. 282, 26326–26334 (2007).
Cao, L., Rickenbacher, G. T., Rodriguez, S., Moulia, T. W. & Albers, M. W. The precision of axon targeting of mouse olfactory sensory neurons requires the BACE1 protease. Sci. Rep. 2, 231 (2012).
Dominguez, D. et al. Phenotypic and biochemical analyses of BACE1- and BACE2-deficient mice. J. Biol. Chem. 280, 30797–30806 (2005).
Laird, F. M. et al. BACE1, a major determinant of selective vulnerability of the brain to amyloid-beta amyloidogenesis, is essential for cognitive, emotional, and synaptic functions. J. Neurosci. 25, 11693–11709 (2005).
Hu, X., Das, B., Hou, H., He, W. & Yan, R. BACE1 deletion in the adult mouse reverses preformed amyloid deposition and improves cognitive functions. J. Exp. Med. 215, 927–940 (2018).
Lombardo, S. et al. BACE1 partial deletion induces synaptic plasticity deficit in adult mice. Sci. Rep. 9, 19877 (2019).
Barao, S. et al. Antagonistic effects of BACE1 and APH1B-gamma-secretase control axonal guidance by regulating growth cone collapse. Cell Rep. 12, 1367–1376 (2015).
Jonsson, T. et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature 488, 96–99 (2012).
Martiskainen, H. et al. Decreased plasma beta-amyloid in the Alzheimer’s disease APP A673T variant carriers. Ann. Neurol. 82, 128–132 (2017).
Maloney, J. A. et al. Molecular mechanisms of Alzheimer disease protection by the A673T allele of amyloid precursor protein. J. Biol. Chem. 289, 30990–31000 (2014).
Das, P. et al. Transient pharmacologic lowering of Abeta production prior to deposition results in sustained reduction of amyloid plaque pathology. Mol. Neurodegener. 7, 39 (2012).
Jack, C. R. et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 14, 535–562 (2018).
Sevigny, J. et al. The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature 537, 50–56 (2016).
Mills, S. M. et al. Preclinical trials in autosomal dominant AD: implementation of the DIAN-TU trial. Rev. Neurol. 169, 737–743 (2013).
Reiman, E. M. et al. Alzheimer’s Prevention Initiative: a plan to accelerate the evaluation of presymptomatic treatments. J. Alzheimers Dis. 26, 321–329 (2011).
Sperling, R. A. et al. The A4 study: stopping AD before symptoms begin? Sci. Transl Med. 6, 228fs213 (2014).
Tariot, P. N. et al. The generation program: baseline characteristics of cognitively unimpaired APOE4 carriers recruited for Generation study 1 and Generation study 2. Alzheimers Dement. 16, e041139 (2020).
Rouzade-Dominguez, M.-L. et al. The API Generation program: biomarker phenotyping of cognitively unimpaired participants screened in Generation study 1 and Generation study 2. Alzheimers Dement. 16, e041143 (2020).
Karlnoski, R. A. et al. Suppression of amyloid deposition leads to long-term reductions in Alzheimer’s pathologies in Tg2576 mice. J. Neurosci. 29, 4964–4971 (2009).
Uhlmann, R. E. et al. Acute targeting of pre-amyloid seeds in transgenic mice reduces Alzheimer-like pathology later in life. Nat. Neurosci. 23, 1580–1588 (2020).
Mortamais, M. et al. Detecting cognitive changes in preclinical Alzheimer’s disease: a review of its feasibility. Alzheimers Dement. 13, 468–492 (2017).
Brown, M. S. & Goldstein, J. L. A tribute to Akira Endo, discoverer of a “penicillin” for cholesterol. Atheroscler. Suppl. 5, 13–16 (2004).
Golde, T. E., Schneider, L. S. & Koo, E. H. Anti-aβ therapeutics in Alzheimer’s disease: the need for a paradigm shift. Neuron 69, 203–213 (2011).
Cummings, J., Lee, G., Ritter, A., Sabbagh, M. & Zhong, K. Alzheimer’s disease drug development pipeline: 2019. Alzheimers Dement. 5, 272–293 (2019).
Lewcock, J. W. et al. Emerging microglia biology defines novel therapeutic approaches for Alzheimer’s disease. Neuron 108, 801–821 (2020).
Panza, F., Lozupone, M., Logroscino, G. & Imbimbo, B. P. A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 15, 73–88 (2019).
Donohue, M. C. et al. The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol. 71, 961–970 (2014).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02569398 (2020).
Wang, J. et al. ADCOMS: A composite clinical outcome for prodromal Alzheimer’s disease trials. J. Neurol. Neurosurg. Psychiatry 87, 993–999 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02956486 (2021).
Mohs, R. C. et al. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer’s Disease Assessment Scale that broaden its scope. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis. Assoc. Disord. 11, S13–S21 (1997).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02245737 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02783573 (2019).
McKinzie, D. et al. Nonclinical pharmacological characterization of the BACE1 inhibitor LY3202626. Alzheimers Dement. 12, P432–P433 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02791191 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03367403 (2021).
Hsiao, C. C., Rombouts, F. & Gijsen, H. J. M. New evolutions in the BACE1 inhibitor field from 2014 to 2018. Bioorg. Med. Chem. Lett. 29, 761–777 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02565511 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01739348 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01953601 (2019).
Navitsky, M. et al. Standardization of amyloid quantitation with florbetapir standardized uptake value rations to the Centiloid scale. Alzheimers Dement. 14, 1565–1571 (2018).
Chávez-Gutiérrez, L. & Szaruga, M. Mechanisms of neurodegeneration - insights from familial Alzheimer’s disease. Semin. Cell Dev. Biol. 105, 75–85 (2020).
Güner, G. & Lichtenthaler, S. F. The substrate repertoire of γ-secretase/presenilin. Semin. Cell Dev. Biol. 105, 27–42 (2020).
Lichtenthaler, S. F., Lemberg, M. K. & Fluhrer, R. Proteolytic ectodomain shedding of membrane proteins in mammals-hardware, concepts, and recent developments. EMBO J. 37, e99456 (2018).
Jorissen, E. et al. The disintegrin/metalloproteinase ADAM10 is essential for the establishment of the brain cortex. J. Neurosci. 30, 4833–4844 (2010).
Kuhn, P. H. et al. ADAM10 is the physiologically relevant, constitutive alpha-secretase of the amyloid precursor protein in primary neurons. EMBO J. 29, 3020–3032 (2010).
Lammich, S. et al. Constitutive and regulated alpha-secretase cleavage of Alzheimer’s amyloid precursor protein by a disintegrin metalloprotease. Proc. Natl Acad. Sci. USA 96, 3922–3927 (1999).
Willem, M. et al. η-Secretase processing of APP inhibits neuronal activity in the hippocampus. Nature 526, 443–447 (2015).
Zhang, Z. et al. Delta-secretase cleaves amyloid precursor protein and regulates the pathogenesis in Alzheimer’s disease. Nat. Commun. 6, 8762 (2015).
Saftig, P. & Lichtenthaler, S. P. The alpha secretase ADAM10: a metalloprotease with multiple functions in the brain. Prog. Neurobiol. 135, 1–20 (2015).
Ou-Yang, M. H. et al. Axonal organization defects in the hippocampus of adult conditional BACE1 knockout mice. Sci. Transl Med. 10, e5620 (2018).
Cheret, C. et al. Bace1 and neuregulin-1 cooperate to control formation and maintenance of muscle spindles. EMBO J. 32, 2015–2028 (2013).
Fleck, D. et al. Dual cleavage of neuregulin 1 type III by BACE1 and ADAM17 liberates its EGF-like domain and allows paracrine signaling. J. Neurosci. 33, 7856–7869 (2013).
Willem, M. et al. Control of peripheral nerve myelination by the beta-secretase BACE1. Science 314, 664–666 (2006).
Hu, X. et al. Bace1 modulates myelination in the central and peripheral nervous system. Nat. Neurosci. 9, 1520–1525 (2006).
Hu, X., He, W., Luo, X., Tsubota, K. E. & Yan, R. BACE1 regulates hippocampal astrogenesis via the Jagged1-Notch pathway. Cell Rep. 4, 40–49 (2013).
Zhu, K. et al. Beta-site amyloid precursor protein cleaving enzyme 1 inhibition impairs synaptic plasticity via seizure protein 6. Biol. Psychiatry 83, 428–437 (2018).
Pigoni, M. et al. Seizure protein 6 and its homolog seizure 6-like protein are physiological substrates of BACE1 in neurons. Mol. Neurodegener. 11, 67 (2016).
Hitt, B. et al. beta-Site amyloid precursor protein (APP)-cleaving enzyme 1 (BACE1)-deficient mice exhibit a close homolog of L1 (CHL1) loss-of-function phenotype involving axon guidance defects. J. Biol. Chem. 287, 38408–38425 (2012).
Muller, T. et al. Neuregulin 3 promotes excitatory synapse formation on hippocampal interneurons. EMBO J. 37, e98858 (2018).
Wang, Y. N. et al. Controlling of glutamate release by neuregulin3 via inhibiting the assembly of the SNARE complex. Proc. Natl Acad. Sci. USA 115, 2508–2513 (2018).
Acknowledgements
The authors thank the many thousands of participants and study partners worldwide who have participated in β-site amyloid precursor protein (APP)-cleaving enzyme (BACE) inhibitor clinical trials. Without their altruism and bravery, the understanding of BACE inhibition as a therapeutic strategy for Alzheimer disease would not have progressed nearly so rapidly.
Author information
Authors and Affiliations
Contributions
E.M., R.V. and S.F.L. researched data for the article, contributed substantially to the discussion of the content, wrote the article and reviewed and/or edited the manuscript before submission. I.V. and R.Y. contributed substantially to the discussion of the content, wrote the article and reviewed and/or edited the manuscript before submission. E.M.R., R.J.B., B.D.S., C.H., R.S., P.N.T. and C.L.M. contributed substantially to the discussion of the content and reviewed and/or edited the manuscript before submission. M.C.C. and P.A. reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
There were no sources of funding that directly supported this work. E.M. reports serving on a data safety committee for Eli Lilly and Company, personal honoraria for continuing medical education activities for Eisai and Eli Lilly and Company and UpToDate, and institutional grant support from Eli Lilly and Company, Hoffman-La Roche and Janssen. P.A. has research grants from Eisai, Eli Lilly and Company and Janssen pharmaceuticals, and has received consulting fees from Biogen, Hoffman-La Roche, ImmunoBrain Checkpoint, Merck & Co. and Samus. R.J.B. serves as the principal investigator of the Dominantly Inherited Alzheimer Network Trial Unit (DIAN-TU), which is supported by the Alzheimer’s Association, the GHR Foundation, an anonymous organization and the DIAN-TU Pharma Consortium (active: Biogen, Eisai, Eli Lilly and Company/Avid Radiopharmaceuticals, Hoffman-La Roche/Genentech, Janssen and United Neuroscience; previously: Abbvie, Amgen, AstraZeneca, Forum, Mithridion, Novartis, Pfizer and Sanofi). The DIAN-TU-001 clinical trial is supported by pharmaceutical partners Eli Lilly and Company, Hoffman-La Roche and Janssen, the Alzheimer’s Association, Avid Radiopharmaceuticals, the GHR Foundation, US National Institutes of Health (NIH) project U01AG042791, NIH project U01AG42791-S1 (Foundation for the National Institutes of Health Accelerating Medicines Partnership), NIH project R01AG046179, NIH project R56AG053267, NIH project R01AG053267, NIH project R01AG053267-S1, NIH project R01AG053267-S2, NIH project U01AG059798 and an anonymous organization. In-kind support has been received from Bracket and CogState for DIAN-TU-001. R.J.B. has received honoraria as a speaker and/or consultant and/or advisory board member from Amgen, AC Immune, Eisai, Hoffman-LaRoche, Janssen and Pfizer and reimbursement of travel expenses from AC Immune, Hoffman-La Roche and Janssen. R.J.B. receives laboratory research funding from the Alzheimer’s Association, the Association for Frontotemporal Degeneration, the BrightFocus Foundation, the Cure Alzheimer’s Fund, Centene Corporation, the NIH, the Rainwater Foundation Tau Consortium, the Tau SILK Consortium (AbbVie, Biogen and Eli Lilly and Company) and an anonymous foundation. R.J.B. is a cofounder of and serves on the scientific advisory board for C2N Diagnostics LLC. Washington University also holds equity in C2N Diagnostics. C2N Diagnostics has licensed certain anti-tau antibodies to AbbVie for therapeutic development. Washington University, R.J.B. and D. Holtzman have equity ownership interest in C2N Diagnostics and receive royalty income based on technology (stable isotope labelling kinetics and blood plasma assay) licensed by Washington University to C2N Diagnostics. R.J.B. receives income from C2N Diagnostics for serving on the scientific advisory board. Washington University, with R.J.B. as a co-inventor, has submitted the US non-provisional patent application “Methods for measuring the metabolism of CNS derived biomolecules in vivo” and the provisional patent application “Plasma based methods for detecting CNS amyloid deposition”. B.D.S. has received research support from Esai and Janssen pharmaceuticals. C.H. reports collaborations with Denali Therapeutics Inc., has participated in one advisory board meeting of Biogen and has received a speaker honorarium from Novartis and Roche. C.H. is the chief adviser to ISAR Bioscience. E.M.R. is one of the leaders of the Alzheimer’s Prevention Initiative, which worked closely with Amgen and Novartis in Alzheimer’s Prevention Initiative Generation Study 1 and Generation Study 2. These trials were funded by Amgen, the US National Institute on Aging, Novartis and philanthropy. E.M.R. is an unpaid scientific adviser to Avid Radiopharmaceuticals, a subsidiary of Eli Lilly and Company. R.S. has research grants from Eli Lilly and Company, Eisai and Janssen pharmaceuticals. P.N.T. has received consulting fees from Acadia, AC Immune, Axsome, BioXcel, Boehringer-Ingelheim, Brain Test Inc., Eisai, eNOVA, the Gerontological Society of America, Otuska & Astex and Syneos, consulting fees and research support from Abbvie, Avanir, Biogen, Cortexyme, Genentech, Eli Lilly and Company, Merck & Co. and Roche and research support from Novartis and has stock ownership in Adamas Pharmaceuticals, which has no past, current or known future involvement in any β-site amyloid precursor protein (APP)-cleaving enzyme (BACE) inhibitor-related therapies or clinical trials. R.V. has stock options in Alector given for his service on the scientific advisory board. R.V.’s advisory relationship with Alector does not involve BACE1 inhibition as a therapeutic strategy. R.V. has consulted for Eisai and Novartis in the past, but is not doing so currently. S.F.L. reports research funding from Novartis and Shionogi. The other authors report no relevant disclosures.
Additional information
Peer review information
Nature Reviews Neurology thanks M. Boada, T. Golde, C. Ritchie and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
McDade, E., Voytyuk, I., Aisen, P. et al. The case for low-level BACE1 inhibition for the prevention of Alzheimer disease. Nat Rev Neurol 17, 703–714 (2021). https://doi.org/10.1038/s41582-021-00545-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-021-00545-1
This article is cited by
-
N1-methylation of adenosine (m1A) in ND5 mRNA leads to complex I dysfunction in Alzheimer’s disease
Molecular Psychiatry (2024)
-
Combination strategy employing BACE1 inhibitor and memantine to boost cognitive benefits in Alzheimer’s disease therapy
Psychopharmacology (2024)
-
The Alzheimer’s disease-linked protease BACE1 modulates neuronal IL-6 signaling through shedding of the receptor gp130
Molecular Neurodegeneration (2023)
-
BACE1 regulates expression of Clusterin in astrocytes for enhancing clearance of β-amyloid peptides
Molecular Neurodegeneration (2023)
-
Detection and treatment of Alzheimer’s disease in its preclinical stage
Nature Aging (2023)