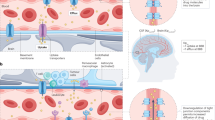
Abstract
The incidence of brain metastases has markedly increased in the past 20 years owing to progress in the treatment of malignant solid tumours, earlier diagnosis by MRI and an ageing population. Although local therapies remain the mainstay of treatment for many patients with brain metastases, a growing number of systemic options are now available and/or are under active investigation. HER2-targeted therapies (lapatinib, neratinib, tucatinib and trastuzumab emtansine), alone or in combination, yield a number of intracranial responses in patients with HER2-positive breast cancer brain metastases. New inhibitors are being investigated in brain metastases from ER-positive or triple-negative breast cancer. Several generations of EGFR and ALK inhibitors have shown activity on brain metastases from EGFR and ALK mutant non-small-cell lung cancer. Immune-checkpoint inhibitors (ICIs) hold promise in patients with non-small-cell lung cancer without druggable mutations and in patients with triple-negative breast cancer. The survival of patients with brain metastases from melanoma has substantially improved after the advent of BRAF inhibitors and ICIs (ipilimumab, nivolumab and pembrolizumab). The combination of targeted agents or ICIs with stereotactic radiosurgery could further improve the response rates and survival but the risk of radiation necrosis should be monitored. Advanced neuroimaging and liquid biopsy will hopefully improve response evaluation.
Key points
-
The blood–brain, blood–tumour and blood–cerebral fluid barriers limit the effective delivery of water-soluble drugs and macromolecules, such as monoclonal antibodies, to the CNS.
-
Brain metastases from EGFR-mutant and ALK-rearranged non-small-cell lung cancer (NSCLC), HER2-positive breast cancer and BRAF-mutant melanoma can be successfully targeted with specific inhibitors.
-
Immune-checkpoint inhibitors (ipilimumab, nivolumab, pembrolizumab and atezolizumab) have clearly improved the outcome of patients with brain metastases from melanoma and now show promising efficacy in patients with brain metastases from non-druggable subtypes of NSCLC and triple-negative breast cancer.
-
The combination of targeted agents and immune-checkpoint inhibitors with stereotactic radiosurgery might yield better results over single modalities but the risk of radionecrosis is still debated.
-
New druggable targets are being investigated in brain metastases from NSCLC (ROS1 rearrangement, NTRK fusions, BRAF and KRAS mutations), breast cancer (DNA repair, CDK4/CDK6 and ER signalling pathways) and melanoma (MEK resistance pathway).
-
Advanced neuroimaging modalities and liquid biopsy represent more precise tools than standard MRI to evaluate early response or progression following targeted therapies or immunotherapy, while phase 0 trials will give the opportunity for in vivo testing of new compounds before entering phase II or III clinical trials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Kromer, C. et al. Estimating the annual frequency of synchronous brain metastasis in the United States 2010-2013: a population-based study. J. Neurooncol. 134, 55–64 (2017).
Soffietti, R. et al. Diagnosis and treatment of brain metastases from solid tumors: guidelines from the European Association of Neuro-Oncology (EANO). Neuro Oncol. 19, 162–174 (2017).
National Comprehensive Cancer Network. Central nervous system cancers: extensive brain metastases. v.4.2012. http://www.nccn.org (2019).
Brastianos, P. K. et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 5, 1164–1177 (2015).
Shih, D. J. H. et al. Genomic characterization of human brain metastases identifies drivers of metastatic lung adenocarcinoma. Nat. Genet. 52, 371–377 (2020).
Kim, M. et al. Barriers to effective drug treatment for brain metastases: a multifactorial problem in the delivery of precision medicine. Pharm. Res. 35, 177 (2018).
Sprowls, S. A. et al. Improving CNS delivery to brain metastases by blood-tumor barrier disruption. Trends Cancer 5, 495–505 (2019).
Lockman, P. R. et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin. Cancer Res. 16, 5664–5678 (2010).
Morikawa, A. et al. Capecitabine and lapatinib uptake in surgically resected brain metastases from metastatic breast cancer patients: a prospective study. Neuro Oncol. 17, 289–295 (2015).
Pardridge, W. M. CSF, blood-brain barrier, and brain drug delivery. Expert. Opin. Drug Deliv. 13, 963–975 (2016).
Noone A. M. et al. SEER Cancer Statistics Review, 1975-2015. (National Cancer Institute, 2015).
Sørensen, J. B., Hansen, H. H., Hansen, M. & Dombernowsky, P. Brain metastases in adenocarcinoma of the lung: frequency, risk groups, and prognosis. J. Clin. Oncol. 6, 1474–1480 (1988).
Sperduto, P. W. et al. Estimating survival in patients with lung cancer and brain metastases: an update of the graded prognostic assessment for lung cancer using molecular markers (Lung-MolGPA). JAMA Oncol. 3, 827–831 (2017).
Kris, M. G. et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 311, 1998–2006 (2014).
Dong, J., Li, B., Lin, D., Zhou, Q. & Huang, D. Advances in targeted therapy and immunotherapy for non-small cell lung cancer based on accurate molecular typing. Front. Pharmacol. 10, 230 (2019).
Rosell, R. et al. Screening for epidermal growth factor receptor mutations in lung cancer. N. Engl. J. Med. 361, 958–967 (2009).
Pao, W. et al. EGF receptor gene mutations are common in lung cancers from ‘Never Smokers’ and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc. Natl Acad. Sci. USA 101, 13306–13311 (2004).
Eichler, A. F. et al. EGFR mutation status and survival after diagnosis of brain metastasis in nonsmall cell lung cancer. Neuro Oncol. 12, 1193–1199 (2010).
Wu, Y. L. et al. Erlotinib as second-line treatment in patients with advanced non-small-cell lung cancer and asymptomatic brain metastases: a phase II study (CTONG–0803). Ann. Oncol. 24, 993–999 (2013).
Welsh, J. W. et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J. Clin. Oncol. 31, 895–902 (2013).
Iuchi, T. et al. Phase II trial of gefitinib alone without radiation therapy for japanese patients with brain metastases from EGFR-mutant lung adenocarcinoma. Lung Cancer 82, 282–287 (2013).
Yang, J. J. et al. Icotinib versus Whole-Brain Irradiation in Patients with EGFR-Mutant Non-Small-Cell Lung Cancer and Multiple Brain Metastases (BRAIN): A Multicentre, Phase 3, Open-Label, Parallel, Randomised Controlled Trial. Lancet Respir. Med. 5, 707–716 (2017).
Zhao, J. et al. Cerebrospinal fluid concentrations of gefitinib in patients with lung adenocarcinoma. Clin. Lung Cancer 14, 188–193 (2013).
Deng, Y. et al. The concentration of erlotinib in the cerebrospinal fluid of patients with brain metastasis from non-small-cell lung cancer. Mol. Clin. Oncol. 2, 116–120 (2014).
Grommes, C. et al. ‘Pulsatile’ high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol. 13, 1364–1369 (2011).
How, J., Mann, J., Laczniak, A. N. & Baggstrom, M. Q. Pulsatile erlotinib in EGFR-positive non-small-cell lung cancer patients with leptomeningeal and brain metastases: review of the literature. Clin. Lung Cancer 18, 354–363 (2017).
Yu, H. A. et al. Phase 1 study of twice weekly pulse dose and daily low-dose erlotinib as initial treatment for patients with EGFR-mutant lung cancers. Ann. Oncol. 28, 278–284 (2017).
Kobayashi, S. et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 352, 786–792 (2005).
Sequist, L. V. et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci. Transl Med. 3, 75ra26 (2011).
Camidge, D. R., Pao, W. & Sequist, L. V. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat. Rev. Clin. Oncol. 11, 473–481 (2014).
Sequist, L. V. et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J. Clin. Oncol. 31, 3327–3334 (2013).
Wu, Y. L. et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 15, 213–222 (2014).
Herbst, R. S., Morgensztern, D. & Boshoff, C. The biology and management of non-small cell lung cancer. Nature 553, 446–454 (2018).
Jänne, P. A. et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N. Engl. J. Med. 372, 1689–1699 (2015).
Soria, J. C. et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N. Engl. J. Med. 378, 113–125 (2018).
Reungwetwattana, T. et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J. Clin. Oncol. 36, 3290–3297 (2018).
Ramalingam, S. S. et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N. Engl. J. Med. 382, 41–50 (2020).
Wu, Y. L. et al. CNS efficacy of osimertinib in patients with T790M-positive advanced non-small-cell lung cancer: data from a randomized phase III trial (AURA3). J. Clin. Oncol. 36, 2702–2709 (2018).
Takeuchi, K. et al. Multiplex reverse transcription-PCR screening for EML4-ALK fusion transcripts. Clin. Cancer Res. 14, 6618–6624 (2008).
Wong, D. W. et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer 115, 1723–1733 (2009).
Shaw, A. T. et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N. Engl. J. Med. 368, 2385–2394 (2013).
Solomon, B. J. et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N. Engl. J. Med. 371, 2167–2177 (2014).
Solomon, B. J. et al. Intracranial efficacy of crizotinib versus chemotherapy in patients with advanced ALK-positive non-small-cell lung cancer: results from PROFILE 1014. J. Clin. Oncol. 34, 2858–2865 (2016).
Shaw, A. T. et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol. 17, 234–242 (2016).
Kim, D. W. et al. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: a randomized, multicenter phase II trial. J. Clin. Oncol. 35, 2490–2498 (2017).
Nishio, M. et al. Final overall survival and other efficacy and safety results from ASCEND-3: phase II study of ceritinib in ALKi-naive patients with ALK-rearranged NSCLC. J. Thorac. Oncol. 15, 609–617 (2020).
Gadgeel, S. M. et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol. 15, 1119–1128 (2014).
Peters, S. et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N. Engl. J. Med. 377, 829–838 (2017).
Solomon, B. J. et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol. 19, 1654–1667 (2018).
Russo, A. et al. New targets in lung cancer (Excluding EGFR, ALK, ROS1). Curr. Oncol. Rep. 22, 48 (2020).
Planchard, D. et al. Dabrafenib plus trametinib in patients with previously treated BRAFV600E-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol. 17, 984–993 (2016).
Drilon, A. et al. Activity of larotrectinib in TRK fusion lung cancer. Ann. Oncol. 30 (Suppl. 2), ii48–ii49 (2019).
Drilon, A. et al. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three phase 1–2 trials. Lancet Oncol. 21, 261–270 (2020).
Scheel, A. H. et al. PDL-1 expression in non-small cell lung cancer: correlations with genetic alterations. Oncoimmunology 5, e1131379 (2016).
Takamori, S. et al. Clinical significance of PDL-1 expression in brain metastases from non-small cell lung cancer. Anticancer Res. 38, 553–557 (2018).
Mansfield, A. S. et al. Temporal and spatial discordance of programmed cell death-ligand 1 expression and lymphocyte tumor infiltration between paired primary lesions and brain metastases in lung cancer. Ann. Oncol. 27, 1953–1958 (2016).
Goldberg, S. B. et al. Pembrolizumab for management of patients with NSCLC and brain metastases: long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancet Oncol. 21, 655–663 (2020).
Davis, A. A. & Patel, V. G. The role of PDL-1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J. Immunother. Cancer 7, 278–285 (2019).
Gadgeel, S. M. et al. Atezolizumab in patients with advanced non-small cell lung cancer and history of asymptomatic, treated brain metastases: exploratory analyses of the phase III OAK study. Lung Cancer 128, 105–112 (2019).
Gong, X. et al. Combined radiotherapy and Anti-PDL-1 antibody synergistically enhances antitumor effect in non-small cell lung cancer. J. Thorac. Oncol. 12, 1085–1097 (2017).
Singh, C., Qian, J. M., Yu, J. B. & Chiang, V. L. Local tumor response and survival outcomes after combined stereotactic radiosurgery and immunotherapy in non-small cell lung cancer with brain metastases. J. Neurosurg. 132, 512–517 (2019).
Chen, L. et al. Concurrent immune checkpoint inhibitors and stereotactic radiosurgery for brain metastases in non-small cell lung cancer, melanoma, and renal cell carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 100, 916–925 (2018).
Kotecha, R. et al. The impact of sequencing PD-1/PDL-1 inhibitors and stereotactic radiosurgery for patients with brain metastasis. Neuro Oncol. 21, 1060–1068 (2019).
Lin, N. U. et al. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer 118, 5463–5472 (2012).
Olson, E. M. et al. Incidence and risk of central nervous system metastases as site of first recurrence in patients with HER2-positive breast cancer treated with adjuvant trastuzumab. Ann. Oncol. 24, 1526–1533 (2013).
Pestalozzi, B. C. et al. CNS relapses in patients with HER2-positive early breast cancer who have and have not received adjuvant trastuzumab: a retrospective substudy of the HERA trial (BIG 1-01). Lancet Oncol. 14, 244–248 (2013).
Dawood, S. et al. Incidence of and survival following brain metastases among women with inflammatory breast cancer. Ann. Oncol. 21, 2348–2355 (2010).
Warren, L. E. et al. Inflammatory breast cancer and development of brain metastases: risk factors and outcomes. Breast Cancer Res. Treat. 151, 225–232 (2015).
Lin, N. U. et al. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer 113, 2638–2645 (2008).
Kennecke, H. et al. Metastatic behavior of breast cancer subtypes. J. Clin. Oncol. 28, 3271–3277 (2010).
Ramakrishna, N. et al. Recommendations on disease management for patients with advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: ASCO clinical practice guideline update. J. Clin. Oncol. 36, 2804–2807 (2018).
Olson, E. M. et al. Clinical outcomes and treatment practice patterns of patients with HER2-positive metastatic breast cancer in the post-trastuzumab era. Breast 22, 525–531 (2013).
von Minckwitz, G. et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N. Engl. J. Med. 380, 617–628 (2019).
Bendell, J. C. et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer 97, 2972–2977 (2003).
Sperduto, P. W. et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J. Clin. Oncol. 30, 419–425 (2012).
Cagney, D. N. et al. Breast cancer subtype and intracranial recurrence patterns after brain-directed radiation for brain metastases. Breast Cancer Res. Treat. 176, 171–179 (2019).
Gori, S. et al. The HERBA study: a retrospective multi-institutional Italian study on patients with brain metastases from HER2-positive breast cancer. Clin. Breast Cancer 19, e501–e510 (2019).
Palmieri, D. et al. Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res. 67, 4190–4198 (2007).
Taskar, K. S. et al. Lapatinib distribution in HER2 overexpressing experimental brain metastases of breast cancer. Pharm. Res. 29, 770–781 (2012).
Lin, N. U. et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J. Clin. Oncol. 26, 1993–1999 (2008).
Lin, N. U. et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin. Cancer Res. 15, 1452–1459 (2009).
Sutherland, S. et al. Treatment of HER2-positive metastatic breast cancer with lapatinib and capecitabine in the lapatinib expanded access programme, including efficacy in brain metastases–the UK experience. Br. J. Cancer 102, 995–1002 (2010).
Lin, N. U. et al. Randomized phase II study of lapatinib plus capecitabine or lapatinib plus topotecan for patients with HER2-positive breast cancer brain metastases. J. Neurooncol. 105, 613–620 (2011).
Metro, G. et al. Clinical outcome of patients with brain metastases from HER2-positive breast cancer treated with lapatinib and capecitabine. Ann. Oncol. 22, 625–630 (2011).
Bachelot, T. et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 14, 64–71 (2013).
Freedman, R. A. et al. Pre- and postoperative neratinib for HER2-positive breast cancer brain metastases: translational breast cancer research consortium 022. Clin. Breast Cancer 20, 145–151.e2 (2020).
Freedman, R. A. et al. Translational breast cancer research consortium (TBCRC) 022: A phase II trial of neratinib for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J. Clin. Oncol. 34, 945–952 (2016).
Freedman, R. A. et al. TBCRC 022: a phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J. Clin. Oncol. 37, 1081–1089 (2019).
Metzger-Filho, O. et al. Phase I dose-escalation trial of ONT-380 in combination with trastuzumab in participants with brain metastases from HER2+ breast cancer. J. Clin. Oncol. 32 (Suppl. 15), https://doi.org/10.1200/jco.2014.32.15_suppl.tps660 (2014).
Murthy, R. et al. Tucatinib with capecitabine and trastuzumab in advanced HER2-positive metastatic breast cancer with and without brain metastases: a non-randomised, open-label, phase 1b study. Lancet Oncol. 19, 880–888 (2018).
Murthy, R. K. et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N. Engl. J. Med. 382, 597–609 (2020).
Dijkers, E. C. et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin. Pharmacol. Ther. 87, 586–592 (2010).
Lewis Phillips, G. D. et al. Trastuzumab uptake and its relation to efficacy in an animal model of HER2-positive breast cancer brain metastasis. Breast Cancer Res. Treat. 164, 581–591 (2017).
Lin, N. U. et al. Planned interim analysis of PATRICIA: an open-label, single-arm, phase II study of pertuzumab (P) with high-dose trastuzumab (H) for the treatment of central nervous system (CNS) progression post radiotherapy (RT) in patients (pts) with HER2-positive metastatic breast cancer (MBC). J. Clin. Oncol. 35 (Suppl. 15), 2074 (2017).
Bartsch, R. et al. Activity of T-DM1 in Her2-positive breast cancer brain metastases. Clin. Exp. Metastasis 32, 729–737 (2015).
Jacot, W. et al. Efficacy and safety of trastuzumab emtansine (T-DM1) in patients with HER2-positive breast cancer with brain metastases. Breast Cancer Res. Treat. 157, 307–318 (2016).
Askoxylakis, V., Kodack, D. P., Ferraro, G. B. & Jain, R. K. Antibody-based therapies for the treatment of brain metastases from HER2-positive breast cancer: time to rethink the importance of the BBB? Breast Cancer Res. Treat. 165, 467–468 (2017).
Fabi, A. et al. T-DM1 and brain metastases: clinical outcome in HER2-positive metastatic breast cancer. Breast 41, 137–143 (2018).
Modi, S. et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N. Engl. J. Med. 382, 610–621 (2020).
Lin, N. U., Bellon, J. R. & Winer, E. P. CNS metastases in breast cancer. J. Clin. Oncol. 22, 3608–3617 (2004).
Raub, T. J. et al. Brain exposure of two selective dual CDK4 and CDK6 inhibitors and the antitumor activity of CDK4 and CDK6 inhibition in combination with temozolomide in an intracranial glioblastoma xenograft. Drug Metab. Dispos. 43, 1360–1371 (2015).
Anders, C. K. et al. A phase 2 study of abemaciclib in patients (pts) with brain metastases (BM) secondary to HR+, HER2- metastatic breast cancer (MBC). J. Clin. Oncol. 37, 1017 (2019).
Patel, H. K. & Bihani, T. Selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) in cancer treatment. Pharmacol. Ther. 186, 1–24 (2018).
Ni, J. et al. Combination inhibition of PI3K and mTORC1 yields durable remissions in mice bearing orthotopic patient-derived xenografts of HER2-positive breast cancer brain metastases. Nat. Med. 22, 723–726 (2016).
Kodack, D. P. et al. The brain microenvironment mediates resistance in luminal breast cancer to PI3K inhibition through HER3 activation. Sci. Transl Med. 9, 391 (2017).
Ippen, F. M. et al. Targeting the PI3K/Akt/mTOR-pathway with the pan-Akt inhibitor GDC-0068 in PIK3CA-mutant breast cancer brain metastases. Neuro Oncol. 21, 1401–1411 (2019).
Song, Y. et al. Patterns of recurrence and metastasis in BRCA1/BRCA2-associated breast cancers. Cancer 126, 271–280 (2020).
Puri, A., Reddy, T. P., Patel, T. A. & Chang, J. C. Metastatic triple-negative breast cancer: established and emerging treatments. Breast J. https://doi.org/10.1111/tbj.13946 (2020).
Litton, J. K. et al. Neoadjuvant talazoparib for patients with operable breast cancer with a germline BRCA pathogenic variant. J. Clin. Oncol. 38, 388–394 (2020).
Karginova, O. et al. Efficacy of carboplatin alone and in combination with ABT888 in intracranial murine models of BRCA-mutated and BRCA-wild-type triple-negative breast cancer. Mol. Cancer Ther. 14, 920–930 (2015).
Voutouri, C. et al. Experimental and computational analyses reveal dynamics of tumor vessel cooption and optimal treatment strategies. Proc. Natl Acad. Sci. USA 116, 2662–2671 (2019).
Kodack, D. P. et al. Combined targeting of HER2 and VEGFR2 for effective treatment of HER2-amplified breast cancer brain metastases. Proc. Natl Acad. Sci. USA 109, E3119–E3127 (2012).
Lin, N. U. et al. Phase II trial of carboplatin (C) and bevacizumab (BEV) in patients (pts) with breast cancer brain metastases (BCBM). J. Clin. Oncol. 31 (Suppl. 15), 513 (2013).
Lu, Y. S. et al. Bevacizumab preconditioning followed by etoposide and cisplatin is highly effective in treating brain metastases of breast cancer progressing from whole-brain radiotherapy. Clin. Cancer Res. 21, 1851–1858 (2015).
Wagner, A. D., Thomssen, C., Haerting, J. & Unverzagt, S. Vascular-endothelial-growth-factor (VEGF) targeting therapies for endocrine refractory or resistant metastatic breast cancer. Cochrane Database Syst. Rev. 7, CD008941 (2012).
Schmid, P. et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 21, 44–59 (2020).
Duchnowska, R. et al. Immune response in breast cancer brain metastases and their microenvironment: the role of the PD-1/PD-L axis. Breast Cancer Res. 18, 43 (2016).
Ogiya, R. et al. Comparison of immune microenvironments between primary tumors and brain metastases in patients with breast cancer. Oncotarget 8, 103671–103681 (2017).
Yomo, S., Hayashi, M. & Cho, N. Impacts of HER2-overexpression and molecular targeting therapy on the efficacy of stereotactic radiosurgery for brain metastases from breast cancer. J. Neurooncol. 112, 199–207 (2013).
Kim, J. M. et al. Stereotactic radiosurgery with concurrent HER2-directed therapy is associated with improved objective response for breast cancer brain metastasis. Neuro Oncol. 21, 659–668 (2019).
Parsai, S. et al. Stereotactic radiosurgery with concurrent lapatinib is associated with improved local control for HER2-positive breast cancer brain metastases. J. Neurosurg. 132, 503–511 (2019).
Lin, N. U. et al. A phase I study of lapatinib with whole brain radiotherapy in patients with Human Epidermal Growth Factor Receptor 2 (HER2)-positive breast cancer brain metastases. Breast Cancer Res. Treat. 142, 405–414 (2013).
Stumpf, P. K. et al. Combination of trastuzumab emtansine and stereotactic radiosurgery results in high rates of clinically significant radionecrosis and dysregulation of aquaporin-4. Clin. Cancer Res. 25, 3946–3953 (2019).
Geraud, A., Xu, H. P., Beuzeboc, P. & Kirova, Y. M. Preliminary experience of the concurrent use of radiosurgery and T-DM1 for brain metastases in HER2-positive metastatic breast cancer. J. Neurooncol. 131, 69–72 (2017).
Rosner, D., Nemoto, T. & Lane, W. W. Chemotherapy induces regression of brain metastases in breast carcinoma. Cancer 58, 832–839 (1986).
Franciosi, V. et al. Front-line chemotherapy with cisplatin and etoposide for patients with brain metastases from breast carcinoma, nonsmall cell lung carcinoma, or malignant melanoma: a prospective study. Cancer 85, 1599–1605 (1999).
Christodoulou, C. et al. Temozolomide (TMZ) combined with cisplatin (CDDP) in patients with brain metastases from solid tumors: a Hellenic Cooperative Oncology Group (HeCOG) phase II study. J. Neurooncol. 71, 61–65 (2005).
Caraglia, M. et al. Phase II study of temozolomide plus pegylated liposomal doxorubicin in the treatment of brain metastases from solid tumours. Cancer Chemother. Pharmacol. 57, 34–39 (2006).
Rivera, E. et al. Phase I study of capecitabine in combination with temozolomide in the treatment of patients with brain metastases from breast carcinoma. Cancer 107, 1348–1354 (2006).
Linot, B. et al. Use of liposomal doxorubicin-cyclophosphamide combination in breast cancer patients with brain metastases: a monocentric retrospective study. J. Neurooncol. 117, 253–259 (2014).
Anders, C. et al. TBCRC 018: phase II study of iniparib in combination with irinotecan to treat progressive triple negative breast cancer brain metastases. Breast Cancer Res. Treat. 146, 557–566 (2014).
Melisko, M. E. et al. Phase II study of irinotecan and temozolomide in breast cancer patients with progressing central nervous system disease. Breast Cancer Res. Treat. 177, 401–408 (2019).
Shah, N. et al. Investigational chemotherapy and novel pharmacokinetic mechanisms for the treatment of breast cancer brain metastases. Pharmacol. Res. 132, 47–68 (2018).
Cortés, J. et al. Prolonged survival in patients with breast cancer and a history of brain metastases: results of a preplanned subgroup analysis from the randomized phase III BEACON trial. Breast Cancer Res. Treat. 165, 329–341 (2017).
Anders, C. et al. Phase 1 expansion study of irinotecan liposome injection (nal-IRI) in patients with metastatic breast cancer (mBC): findings from the cohort with active brain metastasis (BM). Neuro-oncol. Adv. 1 (Suppl. 1), https://doi.org/10.1093/noajnl/vdz014.039 (2019).
Kumthekar, P. et al. ANG1005, a novel brain-penetrant taxane derivative, for the treatment of recurrent brain metastases and leptomeningeal carcinomatosis from breast cancer. J. Clin. Oncol. 34 (Suppl. 15), 2004 (2016).
James, J., Tang, K. & Wei, T. Tesetaxel, a novel, oral taxane, crosses intact blood-brain barrier (BBB) at therapeutically relevant concentrations [abstract 3078]. Cancer Res. 79 (Suppl. 13), https://doi.org/10.1158/1538-7445.AM2019-3078 (2019).
Seidman, A. D. et al. Activity of tesetaxel, an oral taxane, given as a single-agent in patients (Pts) with HER2-, hormone receptor+ (HR+) locally advanced or metastatic breast cancer (MBC) in a phase 2 study. J. Clin. Oncol. https://doi.org/10.1200/JCO.2018.36.15_suppl.1042 (2018).
Zakrzewski, J. et al. Clinical variables and primary tumor characteristics predictive of the development of melanoma brain metastases and post-brain metastases survival. Cancer 117, 1711–1720 (2011).
Jakob, J. A. et al. NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer 118, 4014–4023 (2012).
Bucheit, A. D. et al. Clinical characteristics and outcomes with specific BRAF and NRAS mutations in patients with metastatic melanoma. Cancer 119, 3821–3829 (2013).
Hanniford, D. et al. A miRNA-based signature detected in primary melanoma tissue predicts development of brain metastasis. Clin. Cancer Res. 21, 4903–4912 (2015).
Fischer, G. M. et al. Molecular profiling reveals unique immune and metabolic features of melanoma brain metastases. Cancer Discov. 9, 628–645 (2019).
Davies, M. A. et al. Prognostic factors for survival in melanoma patients with brain metastases. Cancer 117, 1687–1696 (2011).
Sperduto, P. W. et al. Estimating survival in melanoma patients with brain metastases: an update of the graded prognostic assessment for melanoma using molecular markers (Melanoma-molGPA). Int. J. Radiat. Oncol. Biol. Phys. 99, 812–816 (2017).
Sloot, S. et al. Improved survival of patients with melanoma brain metastases in the era of targeted BRAF and immune checkpoint therapies. Cancer 124, 297–305 (2018).
Iorgulescu, J. B. et al. Improved risk-adjusted survival for melanoma brain metastases in the era of checkpoint blockade immunotherapies: results from a national cohort. Cancer Immunol. Res. 6, 1039–1045 (2018).
Schvartsman, G. et al. Incidence, patterns of progression and outcomes of preexisting and newly discovered brain metastases during treatment with anti-PD-1 in patients with metastatic melanoma. Cancer 125, 4193–4202 (2019).
Colombino, M. et al. BRAF/NRAS mutation frequencies among primary tumors and metastases in patients with melanoma. J. Clin. Oncol. 30, 2522–2529 (2012).
Chapman, P. B. et al. BRIM-3 study group. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 364, 2507–2516 (2011).
Dummer, R. et al. Vemurafenib in patients with BRAF(V600) mutation-positive melanoma with symptomatic brain metastases: final results of an open-label pilot study. Eur. J. Cancer 50, 611–621 (2014).
McArthur, G. A. et al. Vemurafenib in metastatic melanoma patients with brain metastases: an open-label, single-arm, phase 2, multicentre study. Ann. Oncol. 28, 634–641 (2017).
Long, G. V. et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 13, 1087–1095 (2012).
Mittapalli, R. K., Vaidhyanathan, S., Dudek, A. Z. & Elmquist, W. F. Mechanisms limiting distribution of the threonine-protein kinase B-RaF(V600E) inhibitor dabrafenib to the brain: implications for the treatment of melanoma brain metastases. J. Pharmacol. Exp. Ther. 344, 655–664 (2013).
Davies, M. A. et al. Dabrafenib plus trametinib in patients with BRAF(V600)-mutant melanoma brain metastases (COMBI-MB): a multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol. 18, 863–873 (2017).
Vaidhyanathan, S., Mittapalli, R. K., Sarkaria, J. N. & Elmquist, W. F. Factors influencing the CNS distribution of a novel MEK-1/2 inhibitor: implications for combination therapy for melanoma brain metastases. Drug Metab. Dispos. 42, 1292–1300 (2014).
Drago, J. Z. et al. Clinical experience with combination BRAF/MEK inhibitors for melanoma with brain metastases: a real-life multicenter study. Melanoma Res. 29, 65–69 (2019).
Babiker, H. M. et al. E6201, an intravenous MEK1 inhibitor, achieves an exceptional response in BRAF V600E-mutated metastatic malignant melanoma with brain metastases. Invest. N. Drugs 37, 636–645 (2019).
Niessner, H. et al. Targeting hyperactivation of the AKT survival pathway to overcome therapy resistance of melanoma brain metastases. Cancer Med. 2, 76–85 (2013).
Chen, G. et al. Molecular profiling of patient-matched brain and extracranial melanoma metastases implicates the PI3K pathway as a therapeutic target. Clin. Cancer Res. 20, 5537–5546 (2014).
Gopal, Y. N. et al. Inhibition of mTORC1/2 overcomes resistance to MAPK pathway inhibitors mediated by PGC1α and oxidative phosphorylation in melanoma. Cancer Res. 74, 7037–7047 (2014).
Tolcher, A. W. et al. A phase IB trial of the oral MEK inhibitor trametinib (GSK1120212) in combination with everolimus in patients with advanced solid tumors. Ann. Oncol. 26, 58–64 (2015).
Haueis, S. A. et al. Does the distribution pattern of brain metastases during BRAF inhibitor therapy reflect phenotype switching? Melanoma Res. 27, 231–237 (2017).
Margolin, K. et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 13, 459–465 (2012).
Di Giacomo, A. M. et al. Three-year follow-up of advanced melanoma patients who received ipilimumab plus fotemustine in the Italian Network for Tumor Biotherapy (NIBIT)-M1 phase II study. Ann. Oncol. 26, 798–803 (2015).
Kluger, H. M. et al. Long-term survival of patients with melanoma with active brain metastases treated with pembrolizumab on a phase II trial. J. Clin. Oncol. 37, 52–60 (2019).
Tawbi, H. A. et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N. Engl. J. Med. 379, 722–730 (2018).
Tawbi, H. A. et al. Efficacy and safety of the combination of nivolumab (NIVO) plus ipilimumab (IPI) in patients with symptomatic melanoma brain metastases (CheckMate 204). J. Clin. Oncol. 37 (Suppl. 15), 9501 (2019).
Long, G. V. et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 19, 672–681 (2018).
Long, G. V. et al. Long-term outcomes from the randomized phase II study of nivolumab (nivo) or nivo+ipilimumab (ipi) in patients (pts) with melanoma brain metastases (mets): anti-PD1 brain collaboration (ABC). Ann. Oncol. 30, v533–v563 (2019).
Cooper, Z. A. et al. Response to BRAF inhibition in melanoma is enhanced when combined with immune checkpoint blockade. Cancer Immunol. Res. 2, 643–654 (2014).
Frederick, D. T. et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin. Cancer Res. 19, 1225–1231 (2013).
Taggart, D. et al. Anti-PD-1/anti-CTLA-4 efficacy in melanoma brain metastases depends on extracranial disease and augmentation of CD8+ T cell trafficking. Proc. Natl Acad. Sci. USA 115, E1540–E1549 (2018).
Harter, P. N. et al. Distribution and prognostic relevance of tumor-infiltrating lymphocytes (TILs) and PD-1/PDL-1 immune checkpoints in human brain metastases. Oncotarget 6, 40836–40849 (2015).
Cohen, J. V. et al. Melanoma brain metastasis pseudoprogression after pembrolizumab treatment. Cancer Immunol. Res. 4, 179–182 (2016).
Yi, M. et al. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol. Cancer 18, 60–64 (2019).
Manon, R. et al. Phase II trial of radiosurgery for one to three newly diagnosed brain metastases from renal cell carcinoma, melanoma, and sarcoma: an Eastern Cooperative Oncology Group study (E 6397). J. Clin. Oncol. 23, 8870–8876 (2005).
Sambade, M. J. et al. Melanoma cells show a heterogeneous range of sensitivity to ionizing radiation and are radiosensitized by inhibition of B-RAF with PLX-4032. Radiother. Oncol. 98, 394–399 (2011).
Narayana, A. et al. Vemurafenib and radiation therapy in melanoma brain metastases. J. Neurooncol. 113, 411–416 (2013).
Wolf, A. et al. Impact on overall survival of the combination of BRAF inhibitors and stereotactic radiosurgery in patients with melanoma brain metastases. J. Neurooncol. 127, 607–615 (2016).
Xu, Z. et al. BRAF V600E mutation and BRAF kinase inhibitors in conjunction with stereotactic radiosurgery for intracranial melanoma metastases. J. Neurosurg. 126, 726–734 (2017).
Kotecha, R. et al. Melanoma brain metastasis: the impact of stereotactic radiosurgery, BRAF mutational status, and targeted and/or immune-based therapies on treatment outcome. J. Neurosurg. 29, 50–59 (2018).
Mastorakos, P. et al. BRAF V600 mutation and BRAF kinase inhibitors in conjunction with stereotactic radiosurgery for intracranial melanoma metastases: a multicenter retrospective study. Neurosurgery 84, 868–880 (2019).
Lukas, R. V. Commentary: BRAF V600 mutation and BRAF kinase inhibitors in conjunction with stereotactic radiosurgery for intracranial melanoma metastases: a multicenter retrospective study. Neurosurgery 84, 881–882 (2019).
Anker, C. J. et al. Avoiding severe toxicity from combined BRAF inhibitor and radiation treatment: consensus guidelines from the eastern cooperative oncology group (ECOG). Int. J. Radiat. Oncol. Biol. Phys. 95, 632–646 (2016).
Patel, K. R. et al. BRAF inhibitor and stereotactic radiosurgery is associated with an increased risk of radiation necrosis. Melanoma Res. 26, 387–394 (2016).
Ly, D. et al. Local control after stereotactic radiosurgery for brain metastases in patients with melanoma with and without BRAF mutation and treatment. J. Neurosurg. 123, 395–401 (2015).
Walle, T. et al. Radiation effects on antitumor immune responses: current perspectives and challenges. Ther. Adv. Med. Oncol. 10, 1758834017742575 (2018).
Dewan, M. Z. et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin. Cancer Res. 15, 5379–5388 (2009).
Pfannenstiel, L. W. et al. Combination PD-1 blockade and irradiation of brain metastasis induces an effective abscopal effect in melanoma. Oncoimmunology 8, e1507669 (2018).
Knisely, J. P. et al. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J. Neurosurg. 117, 227–233 (2012).
Silk, A. W., Bassetti, M. F., West, B. T., Tsien, C. I. & Lao, C. D. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med. 2, 899–906 (2013).
Kiess, A. P. et al. Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: safety profile and efficacy of combined treatment. Int. J. Radiat. Oncol. Biol. Phys. 92, 368–375 (2015).
Sumimoto, H. et al. Effective inhibition of cell growth and invasion of melanoma by combined suppression of BRAF (V599E) and Skp2 with lentiviral RNAi. Int. J. Cancer 118, 472–476 (2006).
Colaco, R. J., Martin, P., Kluger, H. M., Yu, J. B. & Chiang, V. L. Does immunotherapy increase the rate of radiation necrosis after radiosurgical treatment of brain metastases? J. Neurosurg. 125, 17–23 (2016).
Patel, K. R. et al. Ipilimumab and stereotactic radiosurgery versus stereotactic radiosurgery alone for newly diagnosed melanoma brain metastases. Am. J. Clin. Oncol. 40, 444–450 (2017).
Cohen-Inbar, O., Shih, H. H., Xu, Z., Schlesinger, D. & Sheehan, J. P. The effect of timing of stereotactic radiosurgery treatment of melanoma brain metastases treated with ipilimumab. J. Neurosurg. 127, 1007–1014 (2017).
Pin, Y. et al. Brain metastasis formation and irradiation by stereotactic radiation therapycombined with immunotherapy: a systematic review. Crit. Rev. Oncol. Hematol. 149, 102923 (2020).
Forst, D. A. & Wen, P. Y. Neurological complications of targeted therapies in cancer neurology in clinical practice (eds D. Schiff, I. Arrillaga, P. Y. Wen) 311–334 (Springer, 2018).
Tran, T. T. et al. Complications associated with immunotherapy for brain metastases. Curr. Opin. Neurol. 32, 907–916 (2019).
Galldiks, N. et al. Imaging challenges of immunotherapy and targeted therapy in patients with brain metastases: response, progression, and pseudoprogression. Neuro Oncol. 22, 17–30 (2019).
Hendriks, L. E. L. et al. Outcome of patients with non-small cell lung cancer and brain metastases treated with checkpoint inhibitors. J. Thorac. Oncol. 14, 1244–1254 (2019).
Rahman, R. et al. The impact of timing of immunotherapy with cranial irradiation in melanoma patients with brain metastases: intracranial progression, survival and toxicity. J. Neurooncol. 138, 299–306 (2018).
Okada, H. et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. 16, 534–542 (2015).
Champiat, S. et al. Hyperprogressive disease: recognizing a novel pattern to improve patient management. Nat. Rev. Clin. Oncol. 15, 748–762 (2018).
Ferrara, R. et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PDL-1 inhibitors or with single-agent chemotherapy. JAMA Oncol. 4, 1543–1552 (2018).
Kanai, O., Fujita, K., Okamura, M., Nakatani, K. & Mio, T. Severe exacerbation or manifestation of primary disease related to nivolumab in non-small-cell lung cancer patients with poor performance status or brain metastases. Ann. Oncol. 27, 1354–1356 (2016).
Chuang, M. T., Liu, Y. S., Tsai, Y. S., Chen, Y. C. & Wang, C. K. Differentiating radiation-induced necrosis from recurrent brain tumor using MR perfusion and spectroscopy: a meta-analysis. PLoS ONE 11, e0141438 (2016).
Galldiks, N. et al. PET imaging in patients with brain metastasis-report of the RANO/PET group. Neuro Oncol. 21, 585–595 (2019).
Abdulla, D. S. Y. et al. Monitoring treatment response to erlotinib in EGFR-mutated non-small-cell lung cancer brain metastases using serial O-(2-[18F]fluoroethyl)-L-tyrosine PET. Clin. Lung Cancer 20, e148–e151 (2019).
Kebir, S. et al. Dynamic O-(2-[18F]fluoroethyl)-L-tyrosine PET imaging for the detection of checkpoint inhibitor-related pseudoprogression in melanoma brain metastases. Neuro Oncol. 18, 1462–1464 (2016).
Lohmann, P. et al. Radiation injury vs. recurrent brain metastasis: combining textural feature radiomics analysis and standard parameters may increase 18F-FET PET accuracy without dynamic scans. Eur. Radiol. 27, 2916–2927 (2017).
Lohmann, P. et al. Combined FET PET/MRI radiomics differentiates radiation injury from recurrent brain metastasis. Neuroimage Clin. 20, 537–542 (2018).
Lin, N. U. et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 16, e270–e278 (2015).
Camidge, D. R. et al. Clinical trial design for systemic agents in patients with brain metastases from solid tumours: a guideline by the response assessment in neuro-oncology brain metastases working group. Lancet Oncol. 19, e20–e32 (2018).
Vogelbaum, M. A. et al. Phase 0 and window of opportunity clinical trial design in neuro-oncology: a RANO review. Neuro Oncol. https://doi.org/10.1093/neuonc/noaa149 (2020).
Boire, A. et al. Liquid biopsy in central nervous system metastases: a RANO review and proposals for clinical applications. Neuro Oncol. 21, 571–584 (2019).
Priego, N. et al. STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis. Nat. Med. 24, 1024–1035 (2018).
Izraely, S. et al. The metastatic microenvironment: Melanoma-microglia cross-talk promotes the malignant phenotype of melanoma cells. Int. J. Cancer 144, 802–817 (2019).
Zeng, Q. et al. Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature 573, 526–531 (2019).
Soffietti, R., Pellerino, A. & Rudà, R. Strategies to prevent brain metastasis. Curr. Opin. Oncol. 31, 493–500 (2019).
Author information
Authors and Affiliations
Contributions
R.S. researched data for the article, made a substantial contribution to the discussion of content, and wrote and reviewed/edited the manuscript before submission. M.A., N.U.L. and R.R. researched data for the article, made a substantial contribution to the discussion of content and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Neurology thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ClinicalTrials.gov: https://clinicaltrials.gov
Supplementary information
Rights and permissions
About this article
Cite this article
Soffietti, R., Ahluwalia, M., Lin, N. et al. Management of brain metastases according to molecular subtypes. Nat Rev Neurol 16, 557–574 (2020). https://doi.org/10.1038/s41582-020-0391-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-020-0391-x
This article is cited by
-
Current status and perspectives of interventional clinical trials for brain metastases: analysis of ClinicalTrials.gov
Radiation Oncology (2023)
-
Neratinib for HER2-positive breast cancer with an overlooked option
Molecular Medicine (2023)
-
Outcome differences between PD-1/PD-L1 inhibitors-based monotherapy and combination treatments in NSCLC with brain metastases
Experimental Hematology & Oncology (2023)
-
Breast cancer brain metastasis: from etiology to state-of-the-art modeling
Journal of Biological Engineering (2023)
-
Harnessing immunotherapy for brain metastases: insights into tumor–brain microenvironment interactions and emerging treatment modalities
Journal of Hematology & Oncology (2023)