Abstract
Intraventricular haemorrhage (IVH) continues to be a major complication of prematurity that can result in cerebral palsy and cognitive impairment in survivors. No optimal therapy exists to prevent IVH or to treat its consequences. IVH varies in severity and can present as a bleed confined to the germinal matrix, small-to-large IVH or periventricular haemorrhagic infarction. Moderate-to-severe haemorrhage dilates the ventricle and damages the periventricular white matter. This white matter injury results from a constellation of blood-induced pathological reactions, including oxidative stress, glutamate excitotoxicity, inflammation, perturbed signalling pathways and remodelling of the extracellular matrix. Potential therapies for IVH are currently undergoing investigation in preclinical models and evidence from clinical trials suggests that stem cell treatment and/or endoscopic removal of clots from the cerebral ventricles could transform the outcome of infants with IVH. This Review presents an integrated view of new insights into the mechanisms underlying white matter injury in premature infants with IVH and highlights the importance of early detection of disability and immediate intervention in optimizing the outcomes of IVH survivors.
Key points
-
Intraventricular haemorrhage (IVH) results in periventricular white matter injury (WMI) in premature infants of 23–32 weeks gestation; survivors can develop neurodevelopmental sequelae, including cerebral palsy, cognitive deficits and hydrocephalus.
-
IVH triggers robust inflammation around the cerebral ventricles, damages axons and induces both apoptosis and maturational arrest of oligodendrocyte progenitors, leading to reduced myelination of white matter.
-
A constellation of blood-induced reactions, including oxidative stress, glutamate excitotoxicity, inflammation, deranged signalling pathways and alteration of the extracellular matrix, contribute to WMI in infants with IVH.
-
The early diagnosis of neurobehavioural impairments, timely referral to deficit-specific early intervention and family-centred care are crucial to optimizing the neurodevelopmental outcomes of these infants.
-
Neuroimaging studies are important for the early diagnosis of neurodevelopmental impairments, predicting outcomes and evaluating the efficacy of therapeutic interventions in both individual patients and clinical trials.
-
New therapies being tested in preclinical models might reduce cerebral inflammation and promote myelination; ongoing clinical trials are investigating stem cell treatment and the endoscopic removal of clots.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Courtney, S. E. et al. High-frequency oscillatory ventilation versus conventional mechanical ventilation for very-low-birth-weight infants. N. Engl. J. Med. 347, 643–652 (2002).
Horbar, J. D. et al. Trends in mortality and morbidity for very low birth weight infants, 1991-1999. Pediatrics 110, 143–151 (2002).
McGowan, E. C. & Vohr, B. R. Neurodevelopmental follow-up of preterm infants: what is new? Pediatr. Clin. North Am. 66, 509–523 (2019).
Vohr, B. R. Neurodevelopmental outcomes of extremely preterm infants. Clin. Perinatol. 41, 241–255 (2014).
Stoll, B. J. et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 126, 443–456 (2010).
Volpe, J. J. Intraventricular hemorrhage in the premature infant — current concepts. Part II. Ann. Neurol. 25, 109–116 (1989).
Papile, L. A., Burstein, J., Burstein, R. & Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 g. J. Pediatr. 92, 529–534 (1978).
Perlman, J. M. & Volpe, J. J. Cerebral blood flow velocity in relation to intraventricular hemorrhage in the premature newborn infant. J. Pediatr. 100, 956–959 (1982).
Valdez Sandoval, P., Hernandez Rosales, P., Quinones Hernandez, D. G., Chavana Naranjo, E. A. & Garcia Navarro, V. Intraventricular hemorrhage and posthemorrhagic hydrocephalus in preterm infants: diagnosis, classification, and treatment options. Childs Nerv. Syst. 35, 917–927 (2019).
Armstrong, D. L., Sauls, C. D. & Goddard-Finegold, J. Neuropathologic findings in short-term survivors of intraventricular hemorrhage. Am. J. Dis. Child. 141, 617–621 (1987).
Rushton, D. I., Preston, P. R. & Durbin, G. M. Structure and evolution of echo dense lesions in the neonatal brain. A combined ultrasound and necropsy study. Arch. Dis. Child. 60, 798–808 (1985).
Skullerud, K. & Westre, B. Frequency and prognostic significance of germinal matrix hemorrhage, periventricular leukomalacia, and pontosubicular necrosis in preterm neonates. Acta Neuropathol. 70, 257–261 (1986).
Bolisetty, S. et al. Intraventricular hemorrhage and neurodevelopmental outcomes in extreme preterm infants. Pediatrics 133, 55–62 (2014).
Davis, A. S. et al. Outcomes of extremely preterm infants following severe intracranial hemorrhage. J. Perinatol. 34, 203–208 (2014).
Vohr, B. R. et al. School-age outcomes of very low birth weight infants in the indomethacin intraventricular hemorrhage prevention trial. Pediatrics 111, e340–6 (2003).
Nosarti, C. et al. Impaired executive functioning in young adults born very preterm. J. Int. Neuropsychol. Soc. 13, 571–581 (2007).
Indredavik, M. S. et al. Low-birth-weight adolescents: psychiatric symptoms and cerebral MRI abnormalities. Pediatr. Neurol. 33, 259–266 (2005).
Whitaker, A. H. et al. Neonatal head ultrasound abnormalities in preterm infants and adolescent psychiatric disorders. Arch. Gen. Psychiatry 68, 742–752 (2011).
Taylor, H. G., Minich, N., Bangert, B., Filipek, P. A. & Hack, M. Long-term neuropsychological outcomes of very low birth weight: associations with early risks for periventricular brain insults. J. Int. Neuropsychol. Soc. 10, 987–1004 (2004).
Volpe, J. J. Edward B. Neuhauser lecture. Current concepts of brain injury in the premature infant. AJR Am. J. Roentgenol. 153, 243–251 (1989).
Larroche, J. C. Post-haemorrhagic hydrocephalus in infancy. Anatomical study. Biol. Neonate 20, 287–299 (1972).
Buser, J. R. et al. Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann. Neurol. 71, 93–109 (2012).
Bassan, H. et al. Ultrasonographic features and severity scoring of periventricular hemorrhagic infarction in relation to risk factors and outcome. Pediatrics 117, 2111–2118 (2006).
Takashima, S., Mito, T. & Ando, Y. Pathogenesis of periventricular white matter hemorrhages in preterm infants. Brain Dev. 8, 25–30 (1986).
Volpe, J. J. Intraventricular hemorrhage in the premature infant–current concepts. Part I. Ann. Neurol. 25, 3–11 (1989).
Dummula, K. et al. Bone morphogenetic protein inhibition promotes neurological recovery after intraventricular hemorrhage. J. Neurosci. 31, 12068–12082 (2011).
Ou, X. et al. Impaired white matter development in extremely low-birth-weight infants with previous brain hemorrhage. Am. J. Neuroradiol. 35, 1983–1989 (2014).
van de Bor, M., Guit, G. L., Schreuder, A. M., Wondergem, J. & Vielvoye, G. J. Early detection of delayed myelination in preterm infants. Pediatrics 84, 407–411 (1989).
Haynes, R. L., Billiards, S. S., Borenstein, N. S., Volpe, J. J. & Kinney, H. C. Diffuse axonal injury in periventricular leukomalacia as determined by apoptotic marker fractin. Pediatr. Res. 63, 656–661 (2008).
Georgiadis, P. et al. Characterization of acute brain injuries and neurobehavioral profiles in a rabbit model of germinal matrix hemorrhage. Stroke 39, 3378–3388 (2008).
Chua, C. O. et al. Consequences of intraventricular hemorrhage in a rabbit pup model. Stroke 40, 3369–3377 (2009).
Goulding, D. S. et al. Acute brain inflammation, white matter oxidative stress, and myelin deficiency in a model of neonatal intraventricular hemorrhage. J. Neurosurg. Pediatr. https://doi.org/10.3171/2020.5.PEDS20124 (2020).
Lin, S. et al. IGF-1 protects oligodendrocyte progenitor cells and improves neurological functions following cerebral hypoxia-ischemia in the neonatal rat. Brain Res. 1063, 15–26 (2005).
Keep, R. F. et al. Brain endothelial cell junctions after cerebral hemorrhage: Changes, mechanisms and therapeutic targets. J. Cereb. Blood Flow. Metab. 38, 1255–1275 (2018).
Aronowski, J. & Zhao, X. Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke 42, 1781–1786 (2011).
Xue, M. et al. Does thrombin play a role in the pathogenesis of brain damage after periventricular hemorrhage? Brain Pathol. 15, 241–249 (2005).
Xi, G., Keep, R. F. & Hoff, J. T. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 5, 53–63 (2006).
Coughlin, S. R. Thrombin signalling and protease-activated receptors. Nature 407, 258–264 (2000).
Gao, F. et al. Hydrocephalus after intraventricular hemorrhage: the role of thrombin. J. Cereb. Blood Flow. Metab. 34, 489–494 (2014).
Lekic, T. et al. PAR-1, -4, and the mTOR pathway following germinal matrix hemorrhage. Acta Neurochir. Suppl. 121, 213–216 (2016).
Yoon, H., Radulovic, M., Drucker, K. L., Wu, J. & Scarisbrick, I. A. The thrombin receptor is a critical extracellular switch controlling myelination. Glia 63, 846–859 (2015).
Hao, X. D. et al. Thrombin disrupts vascular endothelial-cadherin and leads to hydrocephalus via protease-activated receptors-1 pathway. CNS Neurosci. Ther. 25, 1142–1150 (2019).
Ley, D. et al. High presence of extracellular hemoglobin in the periventricular white matter following preterm intraventricular hemorrhage. Front. Physiol. 7, 330 (2016).
Romantsik, O. et al. The heme and radical scavenger α1-microglobulin (A1M) confers early protection of the immature brain following preterm intraventricular hemorrhage. J. Neuroinflammation 16, 122 (2019).
Huang, F. P. et al. Brain edema after experimental intracerebral hemorrhage: role of hemoglobin degradation products. J. Neurosurg. 96, 287–293 (2002).
Wu, H., Wu, T., Xu, X., Wang, J. & Wang, J. Iron toxicity in mice with collagenase-induced intracerebral hemorrhage. J. Cereb. Blood Flow. Metab. 31, 1243–1250 (2011).
Masuda, T. et al. Oral administration of metal chelator ameliorates motor dysfunction after a small hemorrhage near the internal capsule in rat. J. Neurosci. Res. 85, 213–222 (2007).
Guo, J. et al. Minocycline-induced attenuation of iron overload and brain injury after experimental germinal matrix hemorrhage. Brain Res. 1594, 115–124 (2015).
Meng, H. et al. Deferoxamine alleviates chronic hydrocephalus after intraventricular hemorrhage through iron chelation and Wnt1/Wnt3a inhibition. Brain Res. 1602, 44–52 (2015).
Wang, M. et al. Complement inhibition attenuates early erythrolysis in the hematoma and brain injury in aged rats. Stroke 50, 1859–1868 (2019).
Ahmad, S., Bhatia, K., Kindelin, A. & Ducruet, A. F. The role of complement C3a receptor in stroke. Neuromolecular Med. 21, 467–473 (2019).
Ducruet, A. F. et al. C3a receptor modulation of granulocyte infiltration after murine focal cerebral ischemia is reperfusion dependent. J. Cereb. Blood Flow. Metab. 28, 1048–1058 (2008).
Jarlestedt, K. et al. Receptor for complement peptide C3a: a therapeutic target for neonatal hypoxic-ischemic brain injury. FASEB J. 27, 3797–3804 (2013).
Moran, J. et al. Intranasal C3a treatment ameliorates cognitive impairment in a mouse model of neonatal hypoxic-ischemic brain injury. Exp. Neurol. 290, 74–84 (2017).
Stokowska, A. et al. Complement peptide C3a stimulates neural plasticity after experimental brain ischaemia. Brain 140, 353–369 (2017).
Yung, Y. C., Stoddard, N. C., Mirendil, H. & Chun, J. Lysophosphatidic acid signaling in the nervous system. Neuron 85, 669–682 (2015).
Yung, Y. C. et al. Lysophosphatidic acid signaling may initiate fetal hydrocephalus. Sci. Transl. Med. 3, 99ra87 (2011).
Yu-Taeger, L. et al. Intranasal administration of mesenchymal stem cells ameliorates the abnormal dopamine transmission system and inflammatory reaction in the R6/2 mouse model of Huntington disease. Cells 8, 595 (2019).
Lopez-Serrano, C. et al. Lysophosphatidic acid receptor type 2 activation contributes to secondary damage after spinal cord injury in mice. Brain Behav. Immun. 76, 258–267 (2019).
Back, S. A. et al. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J. Neurosci. 21, 1302–1312 (2001).
Back, S. A., Riddle, A. & McClure, M. M. Maturation-dependent vulnerability of perinatal white matter in premature birth. Stroke 38, 724–730 (2007).
Rakic, S. & Zecevic, N. Early oligodendrocyte progenitor cells in the human fetal telencephalon. Glia 41, 117–127 (2003).
Vinukonda, G. et al. Neuroprotection in a rabbit model of intraventricular haemorrhage by cyclooxygenase-2, prostanoid receptor-1 or tumour necrosis factor-α inhibition. Brain 133, 2264–2280 (2010).
Nicolay, D. J., Doucette, J. R. & Nazarali, A. J. Transcriptional control of oligodendrogenesis. Glia 55, 1287–1299 (2007).
Dohare, P. et al. AMPA-kainate receptor inhibition promotes neurologic recovery in premature rabbits with intraventricular hemorrhage. J. Neurosci. 36, 3363–3377 (2016).
Zia, M. T. et al. Oxidative-nitrosative stress in a rabbit pup model of germinal matrix hemorrhage: role of NAD(P)H oxidase. Stroke 40, 2191–2198 (2009).
Yang, Q., Huang, Q., Hu, Z. & Tang, X. Potential neuroprotective treatment of stroke: targeting excitotoxicity, oxidative stress, and inflammation. Front. Neurosci. 13, 1036 (2019).
Tilleux, S. & Hermans, E. Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J. Neurosci. Res. 85, 2059–2070 (2007).
Panfoli, I. et al. Oxidative stress as a primary risk factor for brain damage in preterm newborns. Front. Pediatr. 6, 369 (2018).
Hagberg, H. et al. The role of inflammation in perinatal brain injury. Nat. Rev. Neurol. 11, 192–208 (2015).
Xue, M., Balasubramaniam, J., Buist, R. J., Peeling, J. & Del Bigio, M. R. Periventricular/intraventricular hemorrhage in neonatal mouse cerebrum. J. Neuropathol. Exp. Neurol. 62, 1154–1165 (2003).
Follett, P. L. et al. Glutamate receptor-mediated oligodendrocyte toxicity in periventricular leukomalacia: a protective role for topiramate. J. Neurosci. 24, 4412–4420 (2004).
Follett, P. L., Rosenberg, P. A., Volpe, J. J. & Jensen, F. E. NBQX attenuates excitotoxic injury in developing white matter. J. Neurosci. 20, 9235–9241 (2000).
Perrone, S., Negro, S., Tataranno, M. L. & Buonocore, G. Oxidative stress and antioxidant strategies in newborns. J. Matern. Fetal Neonatal Med. 23, 63–65 (2010).
Ozsurekci, Y. & Aykac, K. Oxidative stress related diseases in newborns. Oxid. Med. Cell Longev. 2016, 2768365 (2016).
Perrone, S. et al. Early identification of the risk for free radical-related diseases in preterm newborns. Early Hum. Dev. 86, 241–244 (2010).
Ma, M. W. et al. NADPH oxidase in brain injury and neurodegenerative disorders. Mol. Neurodegener. 12, 7 (2017).
Chen, H., Song, Y. S. & Chan, P. H. Inhibition of NADPH oxidase is neuroprotective after ischemia-reperfusion. J. Cereb. Blood Flow. Metab. 29, 1262–1272 (2009).
Doverhag, C. et al. Pharmacological and genetic inhibition of NADPH oxidase does not reduce brain damage in different models of perinatal brain injury in newborn mice. Neurobiol. Dis. 31, 133–144 (2008).
Choi, B. Y. et al. Inhibition of NADPH oxidase activation reduces EAE-induced white matter damage in mice. J. Neuroinflammation 12, 104 (2015).
Dohare, P. et al. Glycogen synthase kinase-3β inhibition enhances myelination in preterm newborns with intraventricular hemorrhage, but not recombinant Wnt3A. Neurobiol. Dis. 118, 22–39 (2018).
Bergles, D. E. & Richardson, W. D. Oligodendrocyte development and plasticity. Cold Spring Harb. Perspect. Biol. 8, a020453 (2015).
Vinukonda, G. et al. Hyaluronidase and hyaluronan oligosaccharides promote neurological recovery after intraventricular hemorrhage. J. Neurosci. 36, 872–889 (2016).
Vose, L. R. et al. Treatment with thyroxine restores myelination and clinical recovery after intraventricular hemorrhage. J. Neurosci. 33, 17232–17246 (2013).
Dyer, A. H., Vahdatpour, C., Sanfeliu, A. & Tropea, D. The role of insulin-like growth factor 1 (IGF-1) in brain development, maturation and neuroplasticity. Neuroscience 325, 89–99 (2016).
Grinspan, J. B. et al. Stage-specific effects of bone morphogenetic proteins on the oligodendrocyte lineage. J. Neurobiol. 43, 1–17 (2000).
Gomes, W. A., Mehler, M. F. & Kessler, J. A. Transgenic overexpression of BMP4 increases astroglial and decreases oligodendroglial lineage commitment. Dev. Biol. 255, 164–177 (2003).
Samanta, J. & Kessler, J. A. Interactions between ID and OLIG proteins mediate the inhibitory effects of BMP4 on oligodendroglial differentiation. Development 131, 4131–4142 (2004).
Chang, J., Dettman, R. W. & Dizon, M. L. V. Bone morphogenetic protein signaling: a promising target for white matter protection in perinatal brain injury. Neural Regen. Res. 13, 1183–1184 (2018).
Palazuelos, J., Klingener, M. & Aguirre, A. TGFβ signaling regulates the timing of CNS myelination by modulating oligodendrocyte progenitor cell cycle exit through SMAD3/4/FoxO1/Sp1. J. Neurosci. 34, 7917–7930 (2014).
Cherian, S., Thoresen, M., Silver, I. A., Whitelaw, A. & Love, S. Transforming growth factor-βs in a rat model of neonatal posthaemorrhagic hydrocephalus. Neuropathol. Appl. Neurobiol. 30, 585–600 (2004).
Hoque, N., Thoresen, M., Aquilina, K., Hogan, S. & Whitelaw, A. Decorin and colchicine as potential treatments for post-haemorrhagic ventricular dilatation in a neonatal rat model. Neonatology 100, 271–276 (2011).
Shimizu, T. et al. Wnt signaling controls the timing of oligodendrocyte development in the spinal cord. Dev. Biol. 282, 397–410 (2005).
Langseth, A. J. et al. Wnts influence the timing and efficiency of oligodendrocyte precursor cell generation in the telencephalon. J. Neurosci. 30, 13367–13372 (2010).
Ye, F. et al. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the β-catenin-TCF interaction. Nat. Neurosci. 12, 829–838 (2009).
Azim, K. & Butt, A. M. GSK3β negatively regulates oligodendrocyte differentiation and myelination in vivo. Glia 59, 540–553 (2011).
Ortega, F. et al. Oligodendrogliogenic and neurogenic adult subependymal zone neural stem cells constitute distinct lineages and exhibit differential responsiveness to Wnt signalling. Nat. Cell Biol. 15, 602–613 (2013).
Genoud, S. et al. Notch1 control of oligodendrocyte differentiation in the spinal cord. J. Cell Biol. 158, 709–718 (2002).
Wang, S. et al. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron 21, 63–75 (1998).
Van Steenwinckel, J. et al. Decreased microglial Wnt/β-catenin signalling drives microglial pro-inflammatory activation in the developing brain. Brain 142, 3806–3833 (2019).
Lu, Q. R. et al. Common developmental requirement for OLIG function indicates a motor neuron/oligodendrocyte connection. Cell 109, 75–86 (2002).
Kessaris, N. et al. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat. Neurosci. 9, 173–179 (2006).
Carre, J. L. et al. Thyroid hormone receptor isoforms are sequentially expressed in oligodendrocyte lineage cells during rat cerebral development. J. Neurosci. Res. 54, 584–594 (1998).
Rodriguez-Pena, A. Oligodendrocyte development and thyroid hormone. J. Neurobiol. 40, 497–512 (1999).
Fernandez, M. et al. Thyroid hormone administration enhances remyelination in chronic demyelinating inflammatory disease. Proc. Natl Acad. Sci. USA 101, 16363–16368 (2004).
Harsan, L. A. et al. Recovery from chronic demyelination by thyroid hormone therapy: myelinogenesis induction and assessment by diffusion tensor magnetic resonance imaging. J. Neurosci. 28, 14189–14201 (2008).
Courtin, F. et al. Thyroid hormone deiodinases in the central and peripheral nervous system. Thyroid 15, 931–942 (2005).
Gereben, B. et al. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr. Rev. 29, 898–938 (2008).
Gonzalez-Perez, O. & Alvarez-Buylla, A. Oligodendrogenesis in the subventricular zone and the role of epidermal growth factor. Brain Res. Rev. 67, 147–156 (2011).
Vinukonda, G. et al. Epidermal growth factor preserves myelin and promotes astrogliosis after intraventricular hemorrhage. Glia 64, 1987–2004 (2016).
Hlavica, M. et al. Intrathecal insulin-like growth factor 1 but not insulin enhances myelin repair in young and aged rats. Neurosci. Lett. 648, 41–46 (2017).
Ley, D. et al. rhIGF-1/rhIGFBP-3 in preterm infants: a phase 2 randomized controlled trial. J. Pediatr. 206, 56–65.e8 (2019).
Wood, T. L. et al. Delayed IGF-1 administration rescues oligodendrocyte progenitors from glutamate-induced cell death and hypoxic-ischemic brain damage. Dev. Neurosci. 29, 302–310 (2007).
Lin, S., Fan, L. W., Rhodes, P. G. & Cai, Z. Intranasal administration of IGF-1 attenuates hypoxic-ischemic brain injury in neonatal rats. Exp. Neurol. 217, 361–370 (2009).
Kiechl-Kohlendorfer, U., Ralser, E., Pupp Peglow, U., Pehboeck-Walser, N. & Fussenegger, B. Early risk predictors for impaired numerical skills in 5-year-old children born before 32 weeks of gestation. Acta Paediatr. 102, 66–71 (2013).
Klebermass-Schrehof, K. et al. Impact of low-grade intraventricular hemorrhage on long-term neurodevelopmental outcome in preterm infants. Childs Nerv. Syst. 28, 2085–2092 (2012).
Sherlock, R. L., Anderson, P. J., Doyle, L. W. & Victorian Infant Collaborative Study Group. Neurodevelopmental sequelae of intraventricular haemorrhage at 8 years of age in a regional cohort of ELBW/very preterm infants. Early Hum. Dev. 81, 909–916 (2005).
Movsas, T. Z. et al. Autism spectrum disorder is associated with ventricular enlargement in a low birth weight population. J. Pediatr. 163, 73–78 (2013).
Ment, L. R. et al. Practice parameter: neuroimaging of the neonate: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 58, 1726–1738 (2002).
Spittle, A. J. et al. Neonatal white matter abnormality predicts childhood motor impairment in very preterm children. Dev. Med. Child. Neurol. 53, 1000–1006 (2011).
Woodward, L. J., Anderson, P. J., Austin, N. C., Howard, K. & Inder, T. E. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N. Engl. J. Med. 355, 685–694 (2006).
Leijser, L. M. et al. Posthemorrhagic ventricular dilatation in preterm infants: when best to intervene? Neurology 90, e698–e706 (2018).
Cayam-Rand, D. et al. Predicting developmental outcomes in preterm infants: a simple white matter injury imaging rule. Neurology 93, e1231–e1240 (2019).
Cizmeci, M. N. et al. Randomized controlled Early versus Late Ventricular Intervention Study (ELVIS) in posthemorrhagic ventricular dilatation: outcome at 2 years. J. Pediatr. https://doi.org/10.1016/j.jpeds.2020.08.014 (2020).
Lean, R. E. et al. Altered neonatal white and gray matter microstructure is associated with neurodevelopmental impairments in very preterm infants with high-grade brain injury. Pediatr. Res. 86, 365–374 (2019).
Morita, T. et al. Low-grade intraventricular hemorrhage disrupts cerebellar white matter in preterm infants: evidence from diffusion tensor imaging. Neuroradiology 57, 507–514 (2015).
Tortora, D. et al. The effects of mild germinal matrix-intraventricular haemorrhage on the developmental white matter microstructure of preterm neonates: a DTI study. Eur. Radiol. 28, 1157–1166 (2018).
Roze, E. et al. Neonatal DTI early after birth predicts motor outcome in preterm infants with periventricular hemorrhagic infarction. Pediatr. Res. 78, 298–303 (2015).
Ancel, P. Y. et al. Cerebral palsy among very preterm children in relation to gestational age and neonatal ultrasound abnormalities: the EPIPAGE cohort study. Pediatrics 117, 828–835 (2006).
Patra, K., Wilson-Costello, D., Taylor, H. G., Mercuri-Minich, N. & Hack, M. Grades I-II intraventricular hemorrhage in extremely low birth weight infants: effects on neurodevelopment. J. Pediatr. 149, 169–173 (2006).
Vavasseur, C., Slevin, M., Donoghue, V. & Murphy, J. F. Effect of low grade intraventricular hemorrhage on developmental outcome of preterm infants. J. Pediatr. 151, e6 (2007).
Mukerji, A., Shah, V. & Shah, P. S. Periventricular/intraventricular hemorrhage and neurodevelopmental outcomes: a meta-analysis. Pediatrics 136, 1132–1143 (2015).
Reubsaet, P. et al. The impact of low-grade germinal matrix-intraventricular hemorrhage on neurodevelopmental outcome of very preterm infants. Neonatology 112, 203–210 (2017).
Morgan, C. et al. The pooled diagnostic accuracy of neuroimaging, general movements, and neurological examination for diagnosing cerebral palsy early in high-risk infants: a case control study. J. Clin. Med. 8, 1879 (2019).
Soleimani, F. et al. Do NICU developmental care improve cognitive and motor outcomes for preterm infants? A systematic review and meta-analysis. BMC Pediatr. 20, 67 (2020).
Spittle, A., Orton, J., Anderson, P. J., Boyd, R. & Doyle, L. W. Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairment in preterm infants. Cochrane Database Syst. Rev. 11, CD005495 (2015).
Spittle, A. & Treyvaud, K. The role of early developmental intervention to influence neurobehavioral outcomes of children born preterm. Semin. Perinatol. 40, 542–548 (2016).
Council on Children With Disabilities, Section on Developmental Behavioral Pediatrics, Bright Futures Steering Committee and Medical Home Initiatives for Children With Special Needs Project Advisory Committee. Identifying infants and young children with developmental disorders in the medical home: an algorithm for developmental surveillance and screening. Pediatrics 118, 405–420 (2006).
Bassan, H. et al. Timing of external ventricular drainage and neurodevelopmental outcome in preterm infants with posthemorrhagic hydrocephalus. Eur. J. Paediatr. Neurol. 16, 662–670 (2012).
Levene, M. I. Measurement of the growth of the lateral ventricles in preterm infants with real-time ultrasound. Arch. Dis. Child. 56, 900–904 (1981).
de Vries, L. S. et al. Treatment thresholds for intervention in posthaemorrhagic ventricular dilation: a randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 104, F70–F75 (2019).
Agajany, N. et al. The impact of neonatal posthemorrhagic hydrocephalus of prematurity on family function at preschool age. Early Hum. Dev. 137, 104827 (2019).
Burnett, A. C., Cheong, J. L. Y. & Doyle, L. W. Biological and social influences on the neurodevelopmental outcomes of preterm infants. Clin. Perinatol. 45, 485–500 (2018).
Benavente-Fernandez, I. et al. Association of socioeconomic status and brain injury with neurodevelopmental outcomes of very preterm children. JAMA Netw. Open 2, e192914 (2019).
Taveggia, C., Feltri, M. L. & Wrabetz, L. Signals to promote myelin formation and repair. Nat. Rev. Neurol. 6, 276–287 (2010).
Wilkins, A., Chandran, S. & Compston, A. A role for oligodendrocyte-derived IGF-1 in trophic support of cortical neurons. Glia 36, 48–57 (2001).
Whitelaw, A. et al. Randomized trial of drainage, irrigation and fibrinolytic therapy for premature infants with posthemorrhagic ventricular dilatation: developmental outcome at 2 years. Pediatrics 125, e852–858 (2010).
Luyt, K. et al. Drainage, irrigation and fibrinolytic therapy (DRIFT) for posthaemorrhagic ventricular dilatation: 10-year follow-up of a randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 105, 466–473 (2020).
Etus, V., Kahilogullari, G., Karabagli, H. & Unlu, A. Early endoscopic ventricular irrigation for the treatment of neonatal posthemorrhagic hydrocephalus: a feasible treatment option or not? a multicenter study. Turk. Neurosurg. 28, 137–141 (2018).
Schulz, M., Buhrer, C., Pohl-Schickinger, A., Haberl, H. & Thomale, U. W. Neuroendoscopic lavage for the treatment of intraventricular hemorrhage and hydrocephalus in neonates. J. Neurosurg. Pediatr. 13, 626–35 (2014).
d’Arcangues, C. et al. Extended experience with neuroendoscopic lavage for posthemorrhagic hydrocephalus in neonates. World Neurosurg. 116, e217–e224 (2018).
Mahoney, L., Luyt, K., Harding, D. & Odd, D. Treatment for post-hemorrhagic ventricular dilatation: a multiple-treatment meta-analysis. Front. Pediatr. 8, 238 (2020).
El-Dib, M. et al. Management of post-hemorrhagic ventricular dilatation in the infant born preterm. J. Pediatr. https://doi.org/10.1016/j.jpeds.2020.07.079 (2020).
Klebe, D. et al. Dabigatran ameliorates post-haemorrhagic hydrocephalus development after germinal matrix haemorrhage in neonatal rat pups. J. Cereb. Blood Flow. Metab. 37, 3135–3149 (2017).
Liu, D. Z. et al. Blood–brain barrier breakdown and repair by Src after thrombin-induced injury. Ann. Neurol. 67, 526–533 (2010).
Ramos-Mandujano, G., Vazquez-Juarez, E., Hernandez-Benitez, R. & Pasantes-Morales, H. Thrombin potently enhances swelling-sensitive glutamate efflux from cultured astrocytes. Glia 55, 917–925 (2007).
Lekic, T. et al. Cyclooxygenase-2 inhibition provides lasting protection following germinal matrix hemorrhage in premature infant rats. Acta Neurochir. Suppl. 121, 203–207 (2016).
Garrido-Mesa, N., Zarzuelo, A. & Galvez, J. Minocycline: far beyond an antibiotic. Br. J. Pharmacol. 169, 337–352 (2013).
Malhotra, K. et al. Minocycline for acute stroke treatment: a systematic review and meta-analysis of randomized clinical trials. J. Neurol. 265, 1871–1879 (2018).
Hanada, T. et al. Perampanel: a novel, orally active, noncompetitive AMPA-receptor antagonist that reduces seizure activity in rodent models of epilepsy. Epilepsia 52, 1331–1340 (2011).
Rogawski, M. A. Revisiting AMPA receptors as an antiepileptic drug target. Epilepsy Curr. 11, 56–63 (2011).
Gidal, B. E. et al. Perampanel efficacy and tolerability with enzyme-inducing AEDs in patients with epilepsy. Neurology 84, 1972–1980 (2015).
Vazquez, B., Yang, H., Williams, B., Zhou, S. & Laurenza, A. Perampanel efficacy and safety by gender: subanalysis of phase III randomized clinical studies in subjects with partial seizures. Epilepsia 56, e90–4 (2015).
La Gamma, E. F. et al. Phase 1 trial of 4 thyroid hormone regimens for transient hypothyroxinemia in neonates of <28 weeks’ gestation. Pediatrics 124, e258–e268 (2009).
van Wassenaer, A. G. et al. Effects of thyroxine supplementation on neurologic development in infants born at less than 30 weeks’ gestation. N. Engl. J. Med. 336, 21–26 (1997).
van Wassenaer, A. G., Westera, J., Houtzager, B. A. & Kok, J. H. Ten-year follow-up of children born at <30 weeks’ gestational age supplemented with thyroxine in the neonatal period in a randomized, controlled trial. Pediatrics 116, e613–e618 (2005).
Aguirre, A., Rizvi, T. A., Ratner, N. & Gallo, V. Overexpression of the epidermal growth factor receptor confers migratory properties to nonmigratory postnatal neural progenitors. J. Neurosci. 25, 11092–11106 (2005).
Scafidi, J. et al. Intranasal epidermal growth factor treatment rescues neonatal brain injury. Nature 506, 230–234 (2014).
Hognason, T. et al. Epidermal growth factor receptor induced apoptosis: potentiation by inhibition of Ras signaling. FEBS Lett. 491, 9–15 (2001).
van Velthoven, C. T., Gonzalez, F., Vexler, Z. S. & Ferriero, D. M. Stem cells for neonatal stroke — the future is here. Front. Cell Neurosci. 8, 207 (2014).
Chang, Y. S., Ahn, S. Y., Sung, S. & Park, W. S. Stem cell therapy for neonatal disorders: prospects and challenges. Yonsei Med. J. 58, 266–271 (2017).
Ahn, S. Y., Chang, Y. S. & Park, W. S. Mesenchymal stem cells transplantation for neuroprotection in preterm infants with severe intraventricular hemorrhage. Korean J. Pediatr. 57, 251–256 (2014).
Ahn, S. Y. et al. Mesenchymal stem cells prevent hydrocephalus after severe intraventricular hemorrhage. Stroke 44, 497–504 (2013).
Kim, E. S. et al. Human umbilical cord blood-derived mesenchymal stem cell transplantation attenuates severe brain injury by permanent middle cerebral artery occlusion in newborn rats. Pediatr. Res. 72, 277–284 (2012).
Ahn, S. Y., Chang, Y. S., Sung, S. I. & Park, W. S. Mesenchymal stem cells for severe intraventricular hemorrhage in preterm infants: phase I dose-escalation clinical trial. Stem Cell Transl. Med. 7, 847–856 (2018).
Keller, T. et al. Intranasal breast milk for premature infants with severe intraventricular hemorrhage-an observation. Eur. J. Pediatr. 178, 199–206 (2019).
Acknowledgements
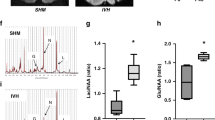
The authors sincerely thank Robert Hevner, University of California San Diego, CA, USA, for the images of brain slices from a human premature infant with IVH in Figs 1a and 1b. The authors also sincerely thank George Kleinman, New York Medical College, Valhalla, NY, USA, and Peter Nikkels, University Medical Center, Utrecht, the Netherlands, for the images of brain slices from a human premature infant with IVH in Figs 1c and 1d, respectively. The authors are grateful to Joseph Volpe, Harvard University, Boston, MA, USA, for a critical review of the manuscript. The authors’ research work is supported by NIH grants RO1 NS110760 and R21NS102897 (both to P.B.).
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Neurology thanks H. Hagberg, A. Whitelaw and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ballabh, P., de Vries, L.S. White matter injury in infants with intraventricular haemorrhage: mechanisms and therapies. Nat Rev Neurol 17, 199–214 (2021). https://doi.org/10.1038/s41582-020-00447-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-020-00447-8
This article is cited by
-
Evaluation of the effect of intraventricular haemorrhage on cerebral perfusion in preterm neonates using three-dimensional pseudo-continuous arterial spin labelling
Pediatric Radiology (2024)
-
Glycogen Synthase Kinase-3β Inhibitor VP3.15 Ameliorates Neurogenesis, Neuronal Loss and Cognitive Impairment in a Model of Germinal Matrix-intraventricular Hemorrhage of the Preterm Newborn
Translational Stroke Research (2024)
-
Ratios of head circumference to ventricular size vary over time and predict eventual need for CSF diversion in intraventricular hemorrhage of prematurity
Child's Nervous System (2024)
-
Recombinant Slit2 suppresses neuroinflammation and Cdc42-mediated brain infiltration of peripheral immune cells via Robo1–srGAP1 pathway in a rat model of germinal matrix hemorrhage
Journal of Neuroinflammation (2023)
-
Spontaneous resolution of post-hemorrhagic ventricular dilatation in preterm newborns and neurodevelopment
Pediatric Research (2023)