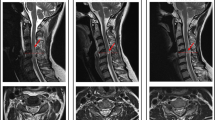
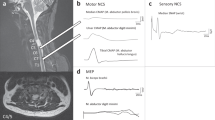
Abstract
Although lesion size is widely considered to be the most reliable predictor of outcome after CNS injury, lesions of comparable size can produce vastly different magnitudes of functional impairment and subsequent recovery. This neuroanatomical–functional paradox is likely to contribute to the many failed attempts to independently replicate findings from animal models of neurotrauma. In humans, the analogous clinical–radiological paradox could explain why individuals with similar injuries can respond differently to rehabilitation. We describe the neuroanatomical–functional paradox in the context of traumatic spinal cord injury (SCI) and discuss the underlying mechanisms of the paradox, including the concepts of lesion-affected and recovery-related networks. We also consider the various secondary complications that further limit the accuracy of outcome prediction in SCI and provide suggestions for how to increase the predictive, translational value of preclinical SCI models.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
08 August 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41582-023-00865-4
References
Marino, R. J., Ditunno, J. F. Jr., Donovan, W. H. & Maynard, F. Jr. Neurologic recovery after traumatic spinal cord injury: data from the Model Spinal Cord Injury Systems. Arch. Phys. Med. Rehabil. 80, 1391–1396 (1999).
Fawcett, J. W. et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord 45, 190–205 (2007).
Schucht, P., Raineteau, O., Schwab, M. E. & Fouad, K. Anatomical correlates of locomotor recovery following dorsal and ventral lesions of the rat spinal cord. Exp. Neurol. 176, 143–153 (2002).
Hurd, C., Weishaupt, N. & Fouad, K. Anatomical correlates of recovery in single pellet reaching in spinal cord injured rats. Exp. Neurol. 247, 605–614 (2013).
Fouad, K., Hurd, C. & Magnuson, D. S. Functional testing in animal models of spinal cord injury: not as straight forward as one would think. Front. Integr. Neurosci. 7, 85 (2013).
Steward, O., Popovich, P. G., Dietrich, W. D. & Kleitman, N. Replication and reproducibility in spinal cord injury research. Exp. Neurol. 233, 597–605 (2012).
Lam, C. J., Assinck, P., Liu, J., Tetzlaff, W. & Oxland, T. R. Impact depth and the interaction with impact speed affect the severity of contusion spinal cord injury in rats. J. Neurotrauma 31, 1985–1997 (2014).
Ballermann, M. & Fouad, K. Spontaneous locomotor recovery in spinal cord injured rats is accompanied by anatomical plasticity of reticulospinal fibers. Eur. J. Neurosci. 23, 1988–1996 (2006).
Martinez, M., Delivet-Mongrain, H., Leblond, H. & Rossignol, S. Recovery of hindlimb locomotion after incomplete spinal cord injury in the cat involves spontaneous compensatory changes within the spinal locomotor circuitry. J. Neurophysiol. 106, 1969–1984 (2011).
Loy, D. N. et al. Functional redundancy of ventral spinal locomotor pathways. J. Neurosci. 22, 315–323 (2002).
Loy, D. N. et al. Both dorsal and ventral spinal cord pathways contribute to overground locomotion in the adult rat. Exp. Neurol. 177, 575–580 (2002).
Brustein, E. & Rossignol, S. Recovery of locomotion after ventral and ventrolateral spinal lesions in the cat. I. Deficits and adaptive mechanisms. J. Neurophysiol. 80, 1245–1267 (1998).
Filli, L. et al. Bridging the gap: a reticulo-propriospinal detour bypassing an incomplete spinal cord injury. J. Neurosci. 34, 13399–13410 (2014).
Aboul-Enein, F., Weiser, P., Höftberger, R., Lassmann, H. & Bradl, M. Transient axonal injury in the absence of demyelination: a correlate of clinical disease in acute experimental autoimmune encephalomyelitis. Acta Neuropathol. 111, 539–547 (2006).
Kerschensteiner, M., Schwab, M. E., Lichtman, J. W. & Misgeld, T. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat. Med. 11, 572–577 (2005).
Duncan, G. J. et al. Locomotor recovery following contusive spinal cord injury does not require oligodendrocyte remyelination. Nat. Commun. 9, 3066 (2018).
Bartus, K. et al. Neuregulin-1 controls an endogenous repair mechanism after spinal cord injury. Brain 139, 1394–1416 (2016).
Pukos, N., Goodus, M. T., Sahinkaya, F. R. & McTigue, D. M. Myelin status and oligodendrocyte lineage cells over time after spinal cord injury: what do we know and what still needs to be unwrapped? Glia 67, 2178–2202 (2019).
Chen, H. S., Holmes, N., Liu, J., Tetzlaff, W. & Kozlowski, P. Validating myelin water imaging with transmission electron microscopy in a rat spinal cord injury model. Neuroimage 153, 122–130 (2017).
Goldstein, B., Hammond, M. C., Stiens, S. A. & Little, J. W. Posttraumatic syringomyelia: profound neuronal loss, yet preserved function. Arch. Phys. Med. Rehabil. 79, 107–112 (1998).
Dreizin, D. et al. Will the real SCIWORA please stand up? exploring clinicoradiologic mismatch in closed spinal cord injuries. AJR Am. J. Roentgenol. 205, 853–860 (2015).
Curt, A. The translational dialogue in spinal cord injury research. Spinal Cord 50, 352–357 (2012).
Popovich, P. G. et al. The neuropathological and behavioral consequences of intraspinal microglial/macrophage activation. J. Neuropathol. Exp. Neurol. 61, 623–633 (2002).
Pouratian, N. & Bookheimer, S. Y. The reliability of neuroanatomy as a predictor of eloquence: a review. Neurosurg. Focus. 28, E3 (2002).
Levine, A. J. et al. Identification of a cellular node for motor control pathways. Nat. Neurosci. 17, 586–593 (2014).
Stepien, A. E., Tripodi, M. & Arber, S. Monosynaptic rabies virus reveals premotor network organization and synaptic specificity of cholinergic partition cells. Neuron 68, 456–472 (2010).
Conta, A. C. & Stelzner, D. J. Differential vulnerability of propriospinal tract neurons to spinal cord contusion injury. J. Comp. Neurol. 479, 347–359 (2004).
Filli, L. & Schwab, M. E. Structural and functional reorganization of propriospinal connections promotes functional recovery after spinal cord injury. Neural Regen. Res. 10, 509–513 (2015).
Miles, G. B., Hartley, R., Todd, A. J. & Brownstone, R. M. Spinal cholinergic interneurons regulate the excitability of motoneurons during locomotion. Proc. Natl Acad. Sci. USA 104, 2448–2453 (2007).
Liu, Y. et al. Touch and tactile neuropathic pain sensitivity are set by corticospinal projections. Nature 561, 547–550 (2018).
Kirshblum, S. et al. The impact of sacral sensory sparing in motor complete spinal cord injury. Arch. Phys. Med. Rehabil. 92, 376–383 (2011).
Waters, R. L., Adkins, R. H. & Yakura, J. S. Definition of complete spinal cord injury. Paraplegia 29, 573–581 (1991).
Zdunczyk, A. et al. The corticospinal reserve capacity: reorganization of motor area and excitability as a novel pathophysiological concept in cervical myelopathy. Neurosurgery 83, 810–818 (2018).
Torres-Espín, A. et al. Eliciting inflammation enables successful rehabilitative training in chronic spinal cord injury. Brain 141, 1946–1962 (2018).
Kanagal, S. G. & Muir, G. D. Task-dependent compensation after pyramidal tract and dorsolateral spinal lesions in rats. Exp. Neurol. 216, 193–206 (2009).
Whishaw, I. Q., Gorny, B. & Sarna, J. Paw and limb use in skilled and spontaneous reaching after pyramidal tract, red nucleus and combined lesions in the rat: behavioral and anatomical dissociations. Behav. Brain Res. 93, 167–183 (1998).
Curt, A., Van Hedel, H. J. A., Klaus, D. & Dietz, V., EM-SCI Study Group. Recovery from a spinal cord injury: significance of compensation, neural plasticity, and repair. J. Neurotrauma. 25, 677–685 (2008).
Fouad, K., Pedersen, V., Schwab, M. E. & Brösamle, C. Cervical sprouting of corticospinal fibers after thoracic spinal cord injury accompanies shifts in evoked motor responses. Curr. Biol. 11, 1766–1770 (2001).
Bareyre, F. M. et al. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat. Neurosci. 7, 269–277 (2004).
Krajacic, A., Weishaupt, N., Girgis, J., Tetzlaff, W. & Fouad, K. Training-induced plasticity in rats with cervical spinal cord injury: effects and side effects. Behav. Brain Res. 214, 323–331 (2010).
van den Brand, R. et al. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science 336, 1182–1185 (2012).
Liu, K. et al. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat. Neurosci. 13, 1075–1081 (2010).
Wang, Z., Reynolds, A., Kirry, A., Nienhaus, C. & Blackmore, M. G. Overexpression of Sox11 promotes corticospinal tract regeneration after spinal injury while interfering with functional recovery. J. Neurosci. 35, 3139–3145 (2015).
Jayaprakash, N. et al. Optogenetic interrogation of functional synapse formation by corticospinal tract axons in the injured spinal cord. J. Neurosci. 36, 5877–5890 (2016).
Fouad, K., Ng, C. & Basso, D. M. Behavioral testing in animal models of spinal cord injury. Exp. Neurol. 333, 113410 (2020).
Popovich, P. G., Lemeshow, S., Gensel, J. C. & Tovar, C. A. Independent evaluation of the effects of glibenclamide on reducing progressive hemorrhagic necrosis after cervical spinal cord injury. Exp. Neurol. 233, 615–622 (2002).
Simard, J. M., Popovich, P. G., Tsymbalyuk, O. & Gerzanich, V. Spinal cord injury with unilateral versus bilateral primary hemorrhage-effects of glibenclamide. Exp. Neurol. 233, 829–835 (2012).
Watzlawick, R. et al. Outcome heterogeneity and bias in acute experimental spinal cord injury: a meta-analysis. Neurology 93, e40–e51 (2019).
Begley, C. G. & Ioannidis, J. P. Reproducibility in science: improving the standard for basic and preclinical research. Circ. Res. 116, 116–126 (2015).
Callahan, A. et al. Developing a data sharing community for spinal cord injury research. Exp. Neurol. 295, 135–143 (2017).
Fouad, K. et al. FAIR SCI ahead: the evolution of the open data commons for pre-clinical spinal cord injury research. J. Neurotrauma 37, 831–838 (2020).
Hotamisligil, G. S. Foundations of immunometabolism and implications for metabolic health and disease. Immunity 47, 406–420 (2017).
Hotamisligil, G. S. Inflammation, metaflammation and immunometabolic disorders. Nature 542, 177–185 (2017).
Failli, V. et al. Functional neurological recovery after spinal cord injury is impaired in patients with infections. Brain 135, 3238–3250 (2012).
Kopp, M. A. et al. Long-term functional outcome in patients with acquired infections after acute spinal cord injury. Neurology 88, 892–900 (2017).
Jaja, B. N. R. et al. Association of pneumonia, wound infection, and sepsis with clinical outcomes after acute traumatic spinal cord injury. J. Neurotrauma 36, 3044–3050 (2019).
Gallagher, M. J. et al. Markedly deranged injury site metabolism and impaired functional recovery in acute spinal cord injury patients with fever. Crit. Care Med. 46, 1150–1157 (2018).
Marik, P. E. & Bellomo, R. Stress hyperglycemia: an essential survival response! Crit. Care 17, 305 (2013).
Kobayakawa, K. et al. Acute hyperglycemia impairs functional improvement after spinal cord injury in mice and humans. Sci. Transl Med. 6, 256ra137 (2014).
Ryken, T. C. et al. The acute cardiopulmonary management of patients with cervical spinal cord injuries. Neurosurgery 72 (Suppl. 2), 84–92 (2013).
Ehsanian, R. et al. Exploration of surgical blood pressure management and expected motor recovery in individuals with traumatic spinal cord injury. Spinal Cord 58, 377–386 (2019).
Gallagher, M. J., Hogg, F. R. A., Zoumprouli, A., Papadopoulos, M. C. & Saadoun, S. Spinal cord blood flow in patients with acute spinal cord injuries. J. Neurotrauma 36, 919–929 (2019).
Kigerl, K. A. et al. Gut dysbiosis impairs recovery after spinal cord injury. J. Exp. Med. 213, 2603–2620 (2016).
Schmidt, E. K. A. et al. Fecal transplant prevents gut dysbiosis and anxiety-like behaviour after spinal cord injury in rats. PLoS ONE 15, e0226128 (2020).
Ankeny, D. P., Guan, Z. & Popovich, P. G. B cells produce pathogenic antibodies and impair recovery after spinal cord injury in mice. J. Clin. Invest. 119, 2990–2999 (2009).
Schwab, J. M., Zhang, Y., Kopp, M. A., Brommer, B. & Popovich, P. G. The paradox of chronic neuroinflammation, systemic immune suppression, autoimmunity after traumatic chronic spinal cord injury. Exp. Neurol. 258, 121–129 (2014).
Shibata, D., Cain, K., Tanzi, P., Zierath, D. & Becker, K. Myelin basic protein autoantibodies, white matter disease and stroke outcome. J. Neuroimmunol. 252, 106–112 (2012).
Doyle, K. P. et al. B-lymphocyte-mediated delayed cognitive impairment following stroke. J. Neurosci. 35, 2133–2145 (2015).
Diamond, B., Huerta, P. T., Mina-Osorio, P., Kowal, C. & Volpe, B. T. Losing your nerves? Maybe it’s the antibodies. Nat. Rev. Immunol. 9, 449–456 (2009).
Freund, P. et al. MRI investigation of the sensorimotor cortex and the corticospinal tract after acute spinal cord injury: a prospective longitudinal study. Lancet Neurol. 12, 873–881 (2013).
James, N. D. et al. Conduction failure following spinal cord injury: functional and anatomical changes from acute to chronic stages. J. Neurosci. 31, 18543–18555 (2011).
Catalano, S. M. & Shatz, C. J. Activity-dependent cortical target selection by thalamic axons. Science 281, 559–562 (1998).
Zhang, L. I. & Poo, M. M. Electrical activity and development of neural circuits. Nat. Neurosci. 4 (Suppl.), 1207–1214 (2001).
Ditunno, J. F. Jr & Formal, C. S. Chronic spinal cord injury. N. Engl. J. Med. 330, 550–556 (1994).
Kirshblum, S., Millis, S., McKinley, W. & Tulsky, D. Late neurologic recovery after traumatic spinal cord injury. Arch. Phys. Med. Rehabil. 85, 1811–1817 (2004).
el Masry, W. S. Physiological instability of the spinal cord following injury. Paraplegia 31, 273–275 (1993).
Chen, Q., Smith, G. M. & Shine, H. D. Immune activation is required for NT-3-induced axonal plasticity in chronic spinal cord injury. Exp. Neurol. 209, 497–509 (2008).
Beauparlant, J. et al. Undirected compensatory plasticity contributes to neuronal dysfunction after severe spinal cord injury. Brain 136, 3347–3361 (2013).
Li, Y. et al. Pericytes impair capillary blood flow and motor function after chronic spinal cord injury. Nat. Med. 23, 733–741 (2017).
von Monakow, C. Lokalisation im Gehirn und funktionelle Stoerungen induziert durch kortikale Laesionen. (Bergmann JF, 1914).
Carrera, E. & Tononi, G. Diaschisis: past, present, future. Brain 137, 2408–2422 (2014).
Seitz, R. J. et al. The role of diaschisis in stroke recovery. Stroke 30, 1844–1850 (1999).
Baldassarre, A. et al. Dissociated functional connectivity profiles for motor and attention deficits in acute right-hemisphere stroke. Brain 139, 2024–2038 (2016).
Min, Y. S. et al. Alteration of resting-state brain sensorimotor connectivity following spinal cord injury: a resting-state functional magnetic resonance imaging study. J. Neurotrauma 32, 1422–1427 (2015).
Dietz, V. Behavior of spinal neurons deprived of supraspinal input. Nat. Rev. Neurol. 6, 167–174 (2010).
Vallotton, K. et al. Width and neurophysiologic properties of tissue bridges predict recovery after cervical injury. Neurology 92, e2793–e2802 (2019).
Huber, E. et al. Dorsal and ventral horn atrophy is associated with clinical outcome after spinal cord injury. Neurology 90, e1510–e1522 (2018).
Pfyffer, D., Huber, E., Sutter, R., Curt, A. & Freund, P. Tissue bridges predict recovery after traumatic and ischemic thoracic spinal cord injury. Neurology 93, e1550–e1560 (2019).
Talbott, J. F. et al. The Brain and Spinal Injury Center score: a novel, simple, and reproducible method for assessing the severity of acute cervical spinal cord injury with axial T2-weighted MRI findings. J. Neurosurg. Spine 23, 495–504 (2015).
Wheeler-Kingshott, C. A. et al. The current state-of-the-art of spinal cord imaging: applications. Neuroimage 84, 1082–1093 (2014).
Stroman, P. W. et al. The current state-of-the-art of spinal cord imaging: methods. Neuroimage 84, 1070–1081 (2014).
Lang, B. T. et al. Modulation of the proteoglycan receptor PTPσ promotes recovery after spinal cord injury. Nature 518, 404–408 (2015).
Blesch, A. & Tuszynski, M. H. Spinal cord injury: plasticity, regeneration and the challenge of translational drug development. Trends Neurosci. 32, 41–47 (2009).
Filli, L. & Schwab, M. E. The rocky road to translation in spinal cord repair. Ann. Neurol. 72, 491–501 (2012).
Freria, C. M. et al. Deletion of the fractalkine receptor, CX3CR1, improves endogenous repair, axon sprouting, and synaptogenesis after spinal cord injury in mice. J. Neurosci. 37, 3568–3587 (2017).
Church, J. S., Kigerl, K. A., Lerch, J. K., Popovich, P. G. & McTigue, D. M. TLR4 deficiency impairs oligodendrocyte formation in the injured spinal cord. J. Neurosci. 36, 6352–6364 (2016).
Hansen, C. N. et al. Elevated MMP-9 in the lumbar cord early after thoracic spinal cord injury impedes motor relearning in mice. J. Neurosci. 33, 13101–13111 (2013).
Tanadini, L. G. et al. Identifying homogeneous subgroups in neurological disorders: unbiased recursive partitioning in cervical complete spinal cord injury. Neurorehabil. Neural Repair 28, 507–515 (2014).
Ghasemlou, N., Kerr, B. J. & David, S. Tissue displacement and impact force are important contributors to outcome after spinal cord contusion injury. Exp. Neurol. 196, 9–17 (2005).
Santos-Benito, F. F., Muñoz-Quiles, C. & Ramón-Cueto, A. Long-term care of paraplegic laboratory mammals. J. Neurotrauma 23, 521–536 (2006).
Kigerl, K. A., Mostacada, K. & Popovich, P. G. Gut microbiota are disease-modifying factors after traumatic spinal cord injury. Neurotherapeutics 15, 60–67 (2018).
Kigerl, K. A., Zane, K., Adams, K., Sullivan, M. B. & Popovich, P. G. The spinal cord-gut-immune axis as a master regulator of health and neurological function after spinal cord injury. Exp. Neurol. 323, 113085 (2020).
Levy, M., Kolodziejczyk, A. A., Thaiss, C. A. & Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 17, 219–232 (2017).
Berer, K. et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479, 538–541 (2011).
Wiesel, T. N. & Hubel, D. H. Extent of recovery from the effects of visual deprivation in kittens. J. Neurophysiol. 28, 1060–1072 (1965).
Raineteau, O. & Schwab, M. E. Plasticity of motor systems after incomplete spinal cord injury. Nat. Rev. Neurosci. 2, 263–273 (2001).
May, Z., Fouad, K., Shum-Siu, A. & Magnuson, D. S. Challenges of animal models in SCI research: Effects of pre-injury task-specific training in adult rats before lesion. Behav. Brain Res. 291, 26–35 (2015).
Caudle, K. L. et al. Hindlimb immobilization in a wheelchair alters functional recovery following contusive spinal cord injury in the adult rat. Neurorehabil. Neural Repair 25, 729–739 (2011).
Basso, D. M., Beattie, M. S. & Bresnahan, J. C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 12, 1–21 (1995).
Kirshblum, S. C. et al. Patterns of sacral sparing components on neurologic recovery in newly injured persons with traumatic spinal cord injury. Arch. Phys. Med. Rehabil. 97, 1647–1655 (2016).
Courtine, G. et al. Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans? Nat. Med. 13, 561–566 (2007).
Farhadi, H. F. et al. Impact of admission imaging findings on neurological outcomes in acute cervical traumatic spinal cord injury. J. Neurotrauma 35, 1398–1406 (2018).
Bradbury, E. J. & McMahon, S. B. Spinal cord repair strategies: why do they work? Nat. Rev. Neurosci. 7, 644–653 (2006).
Kapur, N. Paradoxes in rehabilitation. Disabil. Rehabil. 42, 1495–1502 (2020).
Page, S. J., Gauthier, L. V. & White, S. Size doesn’t matter: cortical stroke lesion volume is not associated with upper extremity motor impairment and function in mild, chronic hemiparesis. Arch. Phys. Med. Rehabil. 94, 817–821 (2013).
Price, C. J., Hope, T. M. & Seghier, M. L. Ten problems and solutions when predicting individual outcome from lesion site after stroke. Neuroimage 145, 200–208 (2017).
Rorden, C. & Karnath, H. O. Using human brain lesions to infer function: a relic from a past era in the fMRI age? Nat. Rev. Neurosci. 5, 813–819 (2004).
Inoue, K., Madhyastha, T., Rudrauf, D., Mehta, S. & Grabowski, T. What affects detectability of lesion-deficit relationships in lesion studies? Neuroimage Clin. 6, 388–397 (2014).
Barkhof, F. The clinico-radiological paradox in multiple sclerosis revisited. Curr. Opin. Neurol. 15, 239–245 (2002).
Barkhof, F. MRI in multiple sclerosis: correlation with expanded disability status scale (EDSS). Mult. Scler. 5, 283–286 (1999).
Okuda, D. T. et al. Incidental MRI anomalies suggestive of multiple sclerosis: the radiologically isolated syndrome. Neurology 72, 800–805 (2009).
Wuerfel, J. et al. Mouse model mimics multiple sclerosis in the clinico-radiological paradox. Eur. J. Neurosci. 26, 190–198 (2007).
Acknowledgements
The work of K.F. is supported by grants from the Canadian Health Research Council (CIHR), the Wings for Life Spinal Cord Research Foundation, the Craig H. Neilsen Foundation, the Canadian Research Chair Program. The work of P.G.P. is supported by the National Institutes of Neurological Disorders-NIH (Grants R01 NS083942, R01 NS099532 and R35 NS111582) and the Ray W. Poppleton Endowment. The work of J.M.S. is supported by the National Institute of Disability, Independent Living and Rehabilitation Research (NIDILRR Grant 90SI5020), the National Institutes of Neurological Disorders – NIH (Grant R01 NS118200), the European Union (EU Era Net – Neuron Program, SILENCE Grant 01EW170A), the Craig H. Neilsen Foundation (Grant 596764), the Wings for Life Spinal Cord Research Foundation and the William E. Hunt and Charlotte M. Curtis Endowment. J.M.S. is a Discovery Theme Initiative Scholar (Chronic Brain Injury) of Ohio State University.
Author information
Authors and Affiliations
Contributions
J.M.S., K.F. and P.G.P. researched data for the article, made a substantial contribution to the discussion of article content, wrote the article, and reviewed and edited the manuscript before submission. M.A.K. made a substantial contribution to the discussion of article content, wrote parts of the article, and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Neurology thanks E. Bradbury, P. Freund, P. Reier and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Corticospinal tract
-
(CST). Comprises axons of the descending pyramidal tract, which controls motor function.
- External validity
-
The extent to which one can generalize the findings of an experimental study to reflect the situation in humans.
- Propriospinal projections
-
Axons that relay information on deep sensitivity and joint position. Constitute an essential pathway for the recovery of neurological function after spinal cord injury.
- Reticulospinal axons
-
Descending axons that relay information for extrapyramidal motor control.
- Syngeneic
-
Genetically similar or identical and immunologically compatible.
- Syringomyelia
-
Generic term referring to a fluid-formed cavity in the spinal cord that develops at the site of a lesion and can be present for a long time after the initial injury.
- Unbiased recursive partitioning
-
A regression analysis method that enables the binary analysis of non-parametric data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fouad, K., Popovich, P.G., Kopp, M.A. et al. The neuroanatomical–functional paradox in spinal cord injury. Nat Rev Neurol 17, 53–62 (2021). https://doi.org/10.1038/s41582-020-00436-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-020-00436-x
This article is cited by
-
Activating MC4R Promotes Functional Recovery by Repressing Oxidative Stress-Mediated AIM2 Activation Post-spinal Cord Injury
Molecular Neurobiology (2024)
-
Surveying people with spinal cord injuries in Brazil to ascertain research priorities
Scientific Reports (2023)
-
Natural and targeted circuit reorganization after spinal cord injury
Nature Neuroscience (2022)
-
SiRNA in MSC-derived exosomes silences CTGF gene for locomotor recovery in spinal cord injury rats
Stem Cell Research & Therapy (2021)
-
Improving Diagnostic Workup Following Traumatic Spinal Cord Injury: Advances in Biomarkers
Current Neurology and Neuroscience Reports (2021)