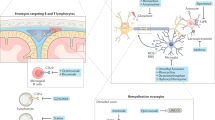
Abstract
The prevalence of multiple sclerosis (MS) and the age of affected patients are increasing owing to increased longevity of the general population and the availability of effective disease-modifying therapies. However, ageing presents unique challenges in patients with MS largely as a result of their increased frequency of age-related and MS-related comorbidities as well as transition of the disease course from an inflammatory to a neurodegenerative phenotype. Immunosenescence (the weakening of the immune system associated with natural ageing) might be at least partly responsible for this transition, which further complicates disease management. Currently approved therapies for MS are effective in preventing relapse but are not as effective in preventing the accumulation of disability associated with ageing and disease progression. Thus, ageing patients with MS represent a uniquely challenging population that is currently underserved by existing therapeutic regimens. This Review focuses on the epidemiology of MS in ageing patients. Unique considerations relevant to this population are discussed, including the immunology and pathobiology of the complex relationship between ageing and MS, the safety and efficacy of disease-modifying therapies, when discontinuation of treatment might be appropriate and the important role of approaches to support wellness and cognition.
Key points
-
The prevalence of ageing individuals with multiple sclerosis (MS) is increasing worldwide.
-
Ageing people with MS present with unique challenges, including a high burden of comorbidities and an altered immune system profile.
-
Data on the safety and efficacy of current disease-modifying therapy regimens in elderly patients with MS are lacking, indicating the need for further studies in this specific population.
-
A substantial proportion of elderly patients with stable MS will need to consider whether to discontinue disease-modifying therapy; data are currently insufficient to provide evidence-based recommendations on this topic.
-
Complementary lifestyle modifications that promote wellness and cognition can help ageing patients with MS to manage their comorbidities and improve their quality of life.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Marrie, R. A., Yu, N., Blanchard, J., Leung, S. & Elliott, L. The rising prevalence and changing age distribution of multiple sclerosis in Manitoba. Neurology 74, 465–471 (2010).
Finlayson, M. Concerns about the future among older adults with multiple sclerosis. Am. J. Occup. Ther. 58, 54–63 (2004).
Tutuncu, M. et al. Onset of progressive phase is an age-dependent clinical milestone in multiple sclerosis. Mult. Scler. 19, 188–198 (2013).
Scalfari, A., Neuhaus, A., Daumer, M., Muraro, P. A. & Ebers, G. C. Onset of secondary progressive phase and long-term evolution of multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 85, 67–75 (2014).
Koch-Henriksen, N., Laursen, B., Stenager, E. & Magyari, M. Excess mortality among patients with multiple sclerosis in Denmark has dropped significantly over the past six decades: a population based study. J. Neurol. Neurosurg. Psychiatry 88, 626–631 (2017).
Hirst, C., Swingler, R., Compston, D. A., Ben-Shlomo, Y. & Robertson, N. P. Survival and cause of death in multiple sclerosis: a prospective population-based study. J. Neurol. Neurosurg. Psychiatry 79, 1016–1021 (2008).
Hurwitz, B. J. Analysis of current multiple sclerosis registries. Neurology 76 (Suppl. 1), S7–S13 (2011).
Lunde, H. M. B., Assmus, J., Myhr, K. M., Bo, L. & Grytten, N. Survival and cause of death in multiple sclerosis: a 60-year longitudinal population study. J. Neurol. Neurosurg. Psychiatry 88, 621–625 (2017).
Kingwell, E. et al. Relative mortality and survival in multiple sclerosis: findings from British Columbia. J. Neurol. Neurosurg. Psychiatry 83, 61–66 (2012).
Minden, S. L., Frankel, D., Hadden, L. S., Srinath, K. P. & Perloff, J. N. Disability in elderly people with multiple sclerosis: an analysis of baseline data from the Sonya Slifka Longitudinal Multiple Sclerosis Study. Neurorehabilitation 19, 55–67 (2004).
Rotstein, D. L. et al. Temporal trends in multiple sclerosis prevalence and incidence in a large population. Neurology 90, e1435–e1441 (2018).
Grytten, N., Torkildsen, O. & Myhr, K. M. Time trends in the incidence and prevalence of multiple sclerosis in Norway during eight decades. Acta Neurol. Scand. 132, 29–36 (2015).
Alla, S., Pearson, J., Debernard, L., Miller, D. & Mason, D. The increasing prevalence of multiple sclerosis in New Zealand. Neuroepidemiology 42, 154–160 (2014).
Dilokthornsakul, P. et al. Multiple sclerosis prevalence in the United States commercially insured population. Neurology 86, 1014–1021 (2016).
Solaro, C. et al. The changing face of multiple sclerosis: prevalence and incidence in an aging population. Mult. Scler. 21, 1244–1250 (2015).
Barnett, M. H., Williams, D. B., Day, S., Macaskill, P. & McLeod, J. G. Progressive increase in incidence and prevalence of multiple sclerosis in Newcastle, Australia: a 35-year study. J. Neurol. Sci. 213, (1–6 (2003).
Simpson, S. Jr. et al. Trends in the epidemiology of multiple sclerosis in Greater Hobart, Tasmania: 1951 to 2009. J. Neurol. Neurosurg. Psychiatry 82, 180–187 (2011).
Sarasoja, T., Wikstrom, J., Paltamaa, J., Hakama, M. & Sumelahti, M. L. Occurrence of multiple sclerosis in central Finland: a regional and temporal comparison during 30 years. Acta Neurol. Scand. 110, 331–336 (2004).
Mayr, W. T. et al. Incidence and prevalence of multiple sclerosis in Olmsted County, Minnesota, 1985–2000. Neurology 61, 1373–1377 (2003).
Daltrozzo, T., Hapfelmeier, A., Donnachie, E., Schneider, A. & Hemmer, B. A systematic assessment of prevalence, incidence and regional distribution of multiple sclerosis in Bavaria from 2006 to 2015. Front. Neurol. 9, 871 (2018).
Grassivaro, F. et al. Multiple sclerosis incidence and prevalence trends in the province of Padua, northeast Italy, 1965–2018. Neuroepidemiology 52, 41–46 (2018).
Ribbons, K., Lea, R., Tiedeman, C., Mackenzie, L. & Lechner-Scott, J. Ongoing increase in incidence and prevalence of multiple sclerosis in Newcastle, Australia: a 50-year study. Mult. Scler. 23, (1063–1071 (2017).
Koch-Henriksen, N. & Sorensen, P. S. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 9, 520–532 (2010).
Bermel, R. A., Rae-Grant, A. D. & Fox, R. J. Diagnosing multiple sclerosis at a later age: more than just progressive myelopathy. Mult. Scler. 16, 1335–1340 (2010).
Poser, C. M. & Brinar, V. V. Diagnostic criteria for multiple sclerosis: an historical review. Clin. Neurol. Neurosurg. 106, 147–158 (2004).
Gafson, A., Giovannoni, G. & Hawkes, C. H. The diagnostic criteria for multiple sclerosis: from Charcot to McDonald. Mult. Scler. Relat. Disord. 1, 9–14 (2012).
Polliack, M. L., Barak, Y. & Achiron, A. Late-onset multiple sclerosis. J. Am. Geriatr. Soc. 49, 168–171 (2001).
Delalande, S., De Seze, J., Ferriby, D., Stojkovic, T. & Vermersch, P. Late onset multiple sclerosis [French]. Rev. Neurol. (Paris) 158, 1082–1087 (2002).
Tremlett, H. & Devonshire, V. Is late-onset multiple sclerosis associated with a worse outcome? Neurology 67, 954–959 (2006).
Hooge, J. P. & Redekop, W. K. Multiple sclerosis with very late onset. Neurology 42, 1907–1910 (1992).
Koch-Henriksen, N., Thygesen, L. C., Stenager, E., Laursen, B. & Magyari, M. Incidence of MS has increased markedly over six decades in Denmark particularly with late onset and in women. Neurology 90, e1954–e1963 (2018).
Marrie, R. A. et al. A systematic review of the incidence and prevalence of comorbidity in multiple sclerosis: overview. Mult. Scler. 21, 263–281 (2015).
Capkun, G. et al. Mortality and comorbidities in patients with multiple sclerosis compared with a population without multiple sclerosis: an observational study using the US Department of Defense administrative claims database. Mult. Scler. Relat. Disord. 4, 546–554 (2015).
Marrie, R. A. et al. Comorbidity delays diagnosis and increases disability at diagnosis in MS. Neurology 72, 117–124 (2009).
Manouchehrinia, A., Tanasescu, R., Tench, C. R. & Constantinescu, C. S. Mortality in multiple sclerosis: meta-analysis of standardised mortality ratios. J. Neurol. Neurosurg. Psychiatry 87, 324–331 (2016).
Scalfari, A. et al. Mortality in patients with multiple sclerosis. Neurology 81, 184–192 (2013).
Kaufman, D. W. et al. Survival in commercially insured multiple sclerosis patients and comparator subjects in the U.S. Mult. Scler. Relat. Disord. 3, 364–371 (2014).
Bronnum-Hansen, H., Koch-Henriksen, N. & Stenager, E. Trends in survival and cause of death in Danish patients with multiple sclerosis. Brain 127, 844–850 (2004).
Ragonese, P., Aridon, P., Salemi, G., D’Amelio, M. & Savettieri, G. Mortality in multiple sclerosis: a review. Eur. J. Neurol. 15, 123–127 (2008).
Warren, S. A., Janzen, W., Warren, K. G., Svenson, L. W. & Schopflocher, D. P. Multiple sclerosis mortality rates in Canada, 1975–2009. Can. J. Neurol. Sci. 43, 134–141 (2016).
Amezcua, L., Rivas, E., Joseph, S., Zhang, J. & Liu, L. Multiple sclerosis mortality by race/ethnicity, age, sex, and time period in the United States, 1999–2015. Neuroepidemiology 50, 35–40 (2018).
Hemmer, B., Kerschensteiner, M. & Korn, T. Role of the innate and adaptive immune responses in the course of multiple sclerosis. Lancet Neurol. 14, 406–419 (2015).
Weissert, R. The immune pathogenesis of multiple sclerosis. J. Neuroimmune Pharmacol. 8, 857–866 (2013).
Garg, N. & Smith, T. W. An update on immunopathogenesis, diagnosis, and treatment of multiple sclerosis. Brain Behav. 5, e00362 (2015).
Fletcher, J. M., Lalor, S. J., Sweeney, C. M., Tubridy, N. & Mills, K. H. G. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin. Exp. Immunol. 162, 1–11 (2010).
‘t Hart, B. A. et al. A B cell-driven autoimmune pathway leading to pathological hallmarks of progressive multiple sclerosis in the marmoset experimental autoimmune encephalomyelitis model. Front. Immunol. 8, 804 (2017).
Reich, D. S., Lucchinetti, C. F. & Calabresi, P. A. Multiple sclerosis. N. Engl. J. Med. 378, 169–180 (2018).
Gandhi, R., Laroni, A. & Weiner, H. L. Role of the innate immune system in the pathogenesis of multiple sclerosis. J. Neuroimmunol. 221, 7–14 (2010).
Kasper, L. H. & Shoemaker, J. Multiple sclerosis immunology: the healthy immune system versus the MS immune system. Neurology 74 (Suppl. 1), S2–S8 (2010).
Frischer, J. M. et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 132, 1175–1189 (2009).
Frischer, J. M. et al. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann. Neurol. 78, 710–721 (2015).
Lassmann, H., van Horssen, J. & Mahad, D. Progressive multiple sclerosis: pathology and pathogenesis. Nat. Rev. Neurol. 8, 647–656 (2012).
Stephenson, E., Nathoo, N., Mahjoub, Y., Dunn, J. F. & Yong, V. W. Iron in multiple sclerosis: roles in neurodegeneration and repair. Nat. Rev. Neurol. 10, 459–468 (2014).
Grebenciucova, E. & Berger, J. R. Immunosenescence: the role of aging in the predisposition to neuro-infectious complications arising from the treatment of multiple sclerosis. Curr. Neurol. Neurosci. Rep. 17, 61 (2017).
Lassmann, H. Pathology and disease mechanisms in different stages of multiple sclerosis. J. Neurol. Sci. 333, 1–4 (2013).
Sanai, S. A. et al. Aging and multiple sclerosis. Mult. Scler. 22, 717–725 (2016).
Plaza-Zabala, A., Sierra-Torre, V. & Sierra, A. Autophagy and microglia: novel partners in neurodegeneration and aging. Int. J. Mol. Sci. 18, E598 (2017).
Aguilera, M. O., Delgui, L. R., Romano, P. S. & Colombo, M. I. Chronic infections: a possible scenario for autophagy and senescence cross-talk. Cells 7, E162 (2018).
Alirezaei, M. et al. Elevated ATG5 expression in autoimmune demyelination and multiple sclerosis. Autophagy 5, 152–158 (2009).
Peterson, J. W. & Trapp, B. D. Neuropathobiology of multiple sclerosis. Neurol. Clin. 23, 107–129 (2005).
Dutta, R. & Trapp, B. D. Relapsing and progressive forms of multiple sclerosis: insights from pathology. Curr. Opin. Neurol. 27, 271–278 (2014).
Cevenini, E., Monti, D. & Franceschi, C. Inflamm-ageing. Curr. Opin. Clin. Nutr. Metab. Care 16, 14–20 (2013).
Franceschi, C. et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. NY Acad. Sci. 908, 244–254 (2000).
Thewissen, M. et al. Analyses of immunosenescent markers in patients with autoimmune disease. Clin. Immunol. 123, 209–218 (2007).
Childs, B. G. et al. Senescent cells: an emerging target for diseases of ageing. Nat. Rev. Drug Discov. 16, 718–735 (2017).
Aw, D. & Palmer, D. B. The origin and implication of thymic involution. Aging Dis. 2, 437–443 (2011).
Haegert, D. G. Multiple sclerosis: a disorder of altered T cell homeostasis. Mult. Scler. Int. 2011, 461304 (2011).
Musella, A. et al. Interplay between age and neuroinflammation in multiple sclerosis: effects on motor and cognitive functions. Front. Aging Neurosci. 10, 238 (2018).
Budni, J., Bellettini-Santos, T., Mina, F., Garcez, M. L. & Zugno, A. I. The involvement of BDNF, NGF and GDNF in aging and Alzheimer’s disease. Aging Dis. 6, 331–341 (2015).
Rist, J. M. & Franklin, R. J. Taking ageing into account in remyelination-based therapies for multiple sclerosis. J. Neurol. Sci. 274, 64–67 (2008).
Luchetti, S. et al. Progressive multiple sclerosis patients show substantial lesion activity that correlates with clinical disease severity and sex: a retrospective autopsy cohort analysis. Acta Neuropathol. 135, 511–528 (2018).
Correale, J., Gaitan, M. I., Ysrraelit, M. C. & Fiol, M. P. Progressive multiple sclerosis: from pathogenic mechanisms to treatment. Brain 140, 527–546 (2017).
Buck, D. & Hemmer, B. Treatment of multiple sclerosis: current concepts and future perspectives. J. Neurol. 258, 1747–1762 (2011).
Thompson, A. J. et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 17, 162–173 (2018).
Hammond, K. E. et al. Quantitative in vivo magnetic resonance imaging of multiple sclerosis at 7 Tesla with sensitivity to iron. Ann. Neurol 64, 707–713 (2008).
Bagnato, F. et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain 134, 3602–3615 (2011).
Absinta, M. et al. Identification of chronic active multiple sclerosis lesions on 3 T MRI. AJNR Am. J. Neuroradiol. 39, 1233–1238 (2018).
Vellinga, M. M. et al. Pluriformity of inflammation in multiple sclerosis shown by ultra-small iron oxide particle enhancement. Brain 131, 800–807 (2008).
Dwyer, M. G. et al. Atrophied brain lesion volume: a new imaging biomarker in multiple sclerosis. J. Neuroimaging 28, 490–495 (2018).
Calabrese, M. et al. Cortical lesion load associates with progression of disability in multiple sclerosis. Brain 135, 2952–2961 (2012).
Calabrese, M. et al. Exploring the origins of grey matter damage in multiple sclerosis. Nat. Rev. Neurosci. 16, 147–158 (2015).
Filippi, M. et al. Imaging cortical damage and dysfunction in multiple sclerosis. JAMA Neurol. 70, 556–564 (2013).
Absinta, M., Sati, P. & Reich, D. S. Advanced MRI and staging of multiple sclerosis lesions. Nat. Rev. Neurol. 12, 358–368 (2016).
Zurawski, J., Lassmann, H. & Bakshi, R. Use of magnetic resonance imaging to visualize leptomeningeal inflammation in patients with multiple sclerosis: a review. JAMA Neurol. 74, 100–109 (2016).
Zivadinov, R. et al. Leptomeningeal contrast enhancement is associated with progression of cortical atrophy in MS: a retrospective, pilot, observational longitudinal study. Mult. Scler. 23, 1336–1345 (2016).
Harrison, D. M. et al. Leptomeningeal enhancement at 7 T in multiple sclerosis: frequency, morphology, and relationship to cortical volume. J. Neuroimaging 27, 461–468 (2017).
Miller, D. H., Barkhof, F., Frank, J. A., Parker, G. J. & Thompson, A. J. Measurement of atrophy in multiple sclerosis: pathological basis, methodological aspects and clinical relevance. Brain 125, 1676–1695 (2002).
Zivadinov, R. et al. Clinical relevance of brain atrophy assessment in multiple sclerosis. Implications for its use in a clinical routine. Expert Rev. Neurother. 16, 777–793 (2016).
De Stefano, N. et al. Establishing pathological cut-offs of brain atrophy rates in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 87, 93–99 (2016).
Hedman, A. M., van Haren, N. E., Schnack, H. G., Kahn, R. S. & Hulshoff Pol, H. E. Human brain changes across the life span: a review of 56 longitudinal magnetic resonance imaging studies. Hum. Brain Mapp. 33, 1987–2002 (2012).
Fisher, E., Lee, J. C., Nakamura, K. & Rudick, R. A. Gray matter atrophy in multiple sclerosis: a longitudinal study. Ann. Neurol. 64, 255–265 (2008).
Fisniku, L. K. et al. Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann. Neurol. 64, 247–254 (2008).
Steenwijk, M. D. et al. Cortical atrophy patterns in multiple sclerosis are non-random and clinically relevant. Brain 139, 115–126 (2016).
Eshaghi, A. et al. Progression of regional grey matter atrophy in multiple sclerosis. Brain 141, 1665–1677 (2018).
Stankoff, B. & Louapre, C. Can we use regional grey matter atrophy sequence to stage neurodegeneration in multiple sclerosis? Brain 141, 1580–1583 (2018).
Cristofanilli, M. et al. Progressive multiple sclerosis cerebrospinal fluid induces inflammatory demyelination, axonal loss, and astrogliosis in mice. Exp. Neurol. 261, 620–632 (2014).
Vidaurre, O. G. et al. Cerebrospinal fluid ceramides from patients with multiple sclerosis impair neuronal bioenergetics. Brain 137, 2271–2286 (2014).
Haider, L. et al. The topograpy of demyelination and neurodegeneration in the multiple sclerosis brain. Brain 139, 807–815 (2016).
Eshaghi, A. et al. Deep grey matter volume loss drives disability worsening in multiple sclerosis. Ann. Neurol. 83, 210–222 (2018).
Azevedo, C. J. et al. Thalamic atrophy in MS: an MRI marker of neurodegeneration throughout disease. Ann. Neurol. 83, 223–234 (2018).
Cocozza, S. et al. Cerebellar lobule atrophy and disability in progressive MS. J. Neurol. Neurosurg. Psychiatry 88, 1065–1072 (2017).
Moroso, A. et al. Posterior lobules of the cerebellum and information processing speed at various stages of multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 88, 146–151 (2016).
Kearney, H., Miller, D. H. & Ciccarelli, O. Spinal cord MRI in multiple sclerosis — diagnostic, prognostic and clinical value. Nat. Rev. Neurol. 11, 327–338 (2015).
Abdel-Aziz, K. et al. Evidence for early neurodegeneration in the cervical cord of patients with primary progressive multiple sclerosis. Brain 138, 1568–1582 (2015).
Tsagkas, C. et al. Preferential spinal cord volume loss in primary progressive multiple sclerosis. Mult. Scler. https://doi.org/10.1177/1352458518775006 (2018).
Schlaeger, R. et al. Association between thoracic spinal cord gray matter atrophy and disability in multiple sclerosis. JAMA Neurol. 72, 897–904 (2015).
Tsagkas, C. et al. Spinal cord volume loss: a marker of disease progression in multiple sclerosis. Neurology 91, e349–e358 (2018).
Zeydan, B. et al. Cervical spinal cord atrophy: an early marker of progressive MS onset. Neurol. Neuroimmunol. Neuroinflamm. 5, e435 (2018).
Murtonen, A., Kurki, S., Hanninen, K., Soilu-Hanninen, M. & Sumelahti, M. L. Common comorbidities and survival in MS: risk for stroke, type 1 diabetes and infections. Mult. Scler. Relat. Disord. 19, 109–114 (2018).
Tettey, P., Simpson, S. Jr., Taylor, B. V. & van der Mei, I. A. Vascular comorbidities in the onset and progression of multiple sclerosis. J. Neurol. Sci. 347, 23–33 (2014).
Hussein, W. I. & Reddy, S. S. Prevalence of diabetes in patients with multiple sclerosis. Diabetes Care 29, 1984–1985 (2006).
Kang, J. H., Chen, Y. H. & Lin, H. C. Comorbidities amongst patients with multiple sclerosis: a population-based controlled study. Eur. J. Neurol. 17, 1215–1219 (2010).
Marrie, R. A. et al. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology 74, 1041–1047 (2010).
Christiansen, C. F. et al. Risk of arterial cardiovascular diseases in patients with multiple sclerosis: a population-based cohort study. Neuroepidemiology 35, 267–274 (2010).
Jadidi, E., Mohammadi, M. & Moradi, T. High risk of cardiovascular diseases after diagnosis of multiple sclerosis. Mult. Scler. 19, 1336–1340 (2013).
Marrie, R. A. et al. Rising prevalence of vascular comorbidities in multiple sclerosis: validation of administrative definitions for diabetes, hypertension, and hyperlipidemia. Mult. Scler. 18, 1310–1319 (2012).
Geraldes, R. et al. The current role of MRI in differentiating multiple sclerosis from its imaging mimics. Nat. Rev. Neurol. 14, 199–213 (2018).
Zivadinov, R. et al. Cerebral microbleeds in multiple sclerosis evaluated on susceptibility-weighted images and quantitative susceptibility maps: a case-control study. Radiology 281, 884–895 (2016).
Ter Telgte, A. et al. Cerebral small vessel disease: from a focal to a global perspective. Nat. Rev. Neurol. 14, 387–398 (2018).
Sati, P. et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat. Rev. Neurol. 12, 714–722 (2016).
Moon, S. Y. et al. Prospective associations between white matter hyperintensities and lower extremity function. Neurology 90, e1291–e1297 (2018).
Srinivasa, R. N. et al. Cardiovascular risk factors associated with smaller brain volumes in regions identified as early predictors of cognitive decline. Radiology 278, 198–204 (2016).
Kappus, N. et al. Cardiovascular risk factors are associated with increased lesion burden and brain atrophy in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 87, 181–187 (2016).
Jakimovski, D. et al. Hypertension and heart disease are associated with development of brain atrophy in multiple sclerosis: a 5-year longitudinal study. Eur. J. Neurol. 26, 87 (2019).
Kneebone, I. I., Dunmore, E. C. & Evans, E. Symptoms of depression in older adults with multiple sclerosis (MS): comparison with a matched sample of younger adults. Aging Ment. Health 7, 182–185 (2003).
Chwastiak, L. et al. Depressive symptoms and severity of illness in multiple sclerosis: epidemiologic study of a large community sample. Am. J. Psychiatry 159, 1862–1868 (2002).
Patten, S. B., Metz, L. M. & Reimer, M. A. Biopsychosocial correlates of lifetime major depression in a multiple sclerosis population. Mult. Scler. 6, 115–120 (2000).
Beal, C. C., Stuifbergen, A. K. & Brown, A. Depression in multiple sclerosis: a longitudinal analysis. Arch. Psychiatr. Nurs. 21, 181–191 (2007).
Roy, S. et al. Preliminary investigation of cognitive function in aged multiple sclerosis patients: challenges in detecting comorbid Alzheimer’s disease. Mult. Scler. Relat. Disord. 22, 52–56 (2018).
U.S. Food and Drug Administration. FDA News Release: FDA approves new oral treatment for multiple sclerosis. FDA https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm634837.htm (2019).
U.S. Food and Drug Administration. FDA News Release: FDA approves new oral drug to treat multiple sclerosis. FDA https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm634469.htm (2019).
National Multiple Sclerosis Society. Disease-modifying therapies for MS. NationalMSSociety https://www.nationalmssociety.org/NationalMSSociety/media/MSNationalFiles/Brochures/Brochure-MS-Disease-Modifying-Medications.pdf (2018).
Johnson, K. P. et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology 45, 1268–1276 (1995).
IFNβ Multiple Sclerosis Study Group. Interferon beta-lb is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. 1993 [classical article]. Neurology 57 (Suppl. 5), S3–S9 (2001).
Polman, C. H. et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 354, 899–910 (2006).
CAMMS223 Trial Investigators et al. Alemtuzumab versus interferon beta-1a in early multiple sclerosis. N. Engl. J. Med. 359, 1786–1801 (2008).
Cohen, J. A. et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 380, 1819–1828 (2012).
Jacobs, L. D. et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann. Neurol. 39, 285–294 (1996).
PRISMS (Prevention of Relapses and Disability by Interferon β-1a Subcutaneously in Multiple Sclerosis) Study Group. Randomised double-blind placebo-controlled study of interferon β-1a in relapsing/remitting multiple sclerosis. Lancet 352, 1498–1504 (1998).
Gold, R. et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N. Engl. J. Med. 367, 1098–1107 (2012).
Fox, R. J. et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N. Engl. J. Med. 367, 1087–1097 (2012).
Kappos, L. et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N. Engl. J. Med. 362, 387–401 (2010).
Cohen, J. A. et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N. Engl. J. Med. 362, 402–415 (2010).
O’Connor, P. et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N. Engl. J. Med. 365, 1293–1303 (2011).
Miller, A. E. et al. Oral teriflunomide for patients with a first clinical episode suggestive of multiple sclerosis (TOPIC): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 13, 977–986 (2014).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03523858 (2019).
Kappos, L. et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet 391, 1263–1273 (2018).
Zhang, T. et al. Examining the effects of comorbidities on disease-modifying therapy use in multiple sclerosis. Neurology 86, 1287–1295 (2016).
Devonshire, V. et al. Relapse and disability outcomes in patients with multiple sclerosis treated with fingolimod: subgroup analyses of the double-blind, randomised, placebo-controlled FREEDOMS study. Lancet Neurol. 11, 420–428 (2012).
Motl, R. W. et al. Validity of the timed 25-foot walk as an ambulatory performance outcome measure for multiple sclerosis. Mult. Scler. 23, 704–710 (2017).
Hervault, M., Balto, J. M., Hubbard, E. A. & Motl, R. W. Reliability, precision, and clinically important change of the Nine-Hole Peg Test in individuals with multiple sclerosis. Int. J. Rehabil. Res. 40, 91–93 (2017).
Kapoor, R. et al. Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): a phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension. Lancet Neurol. 17, 405–415 (2018).
Shirani, A. et al. Multiple sclerosis in older adults: the clinical profile and impact of interferon beta treatment. Biomed. Res. Int. 2015, 451912 (2015).
Giovannoni, G. et al. Is multiple sclerosis a length-dependent central axonopathy? The case for therapeutic lag and the asynchronous progressive MS hypotheses. Mult. Scler. Relat. Disord. 12, 70–78 (2017).
Montalban, X. et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N. Engl. J. Med. 376, 209–220 (2017).
Paz Soldan, M. M. et al. Relapses and disability accumulation in progressive multiple sclerosis. Neurology 84, 81–88 (2015).
Wolinsky, J. S. et al. Evaluation of no evidence of progression or active disease (NEPAD) in patients with primary progressive multiple sclerosis in the ORATORIO trial. Ann. Neurol. 84, 527–536 (2018).
Fox, E. J. et al. Ocrelizumab reduces progression of upper extremity impairment in patients with primary progressive multiple sclerosis: findings from the phase III randomized ORATORIO trial. Mult. Scler. 24, 1862–1870 (2018).
Manouchehrinia, A. et al. Age related multiple sclerosis severity score: disability ranked by age. Mult. Scler. 23, 1938–1946 (2017).
Kister, I. Disease-modifying therapies can be safely discontinued in an individual with stable relapsing-remitting MS — YES. Mult. Scler. 23, 1188–1190 (2017).
Tobin, W. O. & Weinshenker, B. G. Disease-modifying therapies can be safely discontinued in an individual with stable relapsing-remitting MS — NO. Mult. Scler. 23, 1190–1192 (2017).
Tremlett, H., Zhao, Y., Joseph, J. & Devonshire, V. Relapses in multiple sclerosis are age- and time-dependent. J. Neurol. Neurosurg. Psychiatry 79, 1368–1374 (2008).
Hua, L. H., Fan, T. H., Conway, D., Thompson, N. & Kinzy, T. G. Discontinuation of disease-modifying therapy in patients with multiple sclerosis over age 60. Mult. Scler. https://doi.org/10.1177/1352458518765656 (2018).
Kister, I. et al. Discontinuing disease-modifying therapy in MS after a prolonged relapse-free period: a propensity score-matched study. J. Neurol. Neurosurg. Psychiatry 87, 1133–1137 (2016).
Weideman, A. M., Tapia-Maltos, M. A., Johnson, K., Greenwood, M. & Bielekova, B. Meta-analysis of the age-dependent efficacy of multiple sclerosis treatments. Front. Neurol. 8, 577 (2017).
Sormani, M. P. et al. Autologous hematopoietic stem cell transplantation in multiple sclerosis: a meta-analysis. Neurology 88, 2115–2122 (2017).
Muraro, P. A. et al. Autologous haematopoietic stem cell transplantation for treatment of multiple sclerosis. Nat. Rev. Neurol. 13, 391–405 (2017).
Muraro, P. A. et al. Long-term outcomes after autologous hematopoietic stem cell transplantation for multiple sclerosis. JAMA Neurol. 74, 459–469 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03073603 (2019).
Moss, B. P., Rensel, M. R. & Hersh, C. M. Wellness and the role of comorbidities in multiple sclerosis. Neurotherapeutics 14, 999–1017 (2017).
Ramanujam, R. et al. Effect of smoking cessation on multiple sclerosis prognosis. JAMA Neurol. 72, 1117–1123 (2015).
D’Hooghe, M. B., Haentjens, P., Nagels, G. & De Keyser, J. Alcohol, coffee, fish, smoking and disease progression in multiple sclerosis. Eur. J. Neurol. 19, 616–624 (2012).
Manouchehrinia, A. et al. Tobacco smoking and disability progression in multiple sclerosis: United Kingdom cohort study. Brain 136, 2298–2304 (2013).
Sandroff, B. M., Motl, R. W., Scudder, M. R. & DeLuca, J. Systematic, evidence-based review of exercise, physical activity, and physical fitness effects on cognition in persons with multiple sclerosis. Neuropsychol. Rev. 26, 271–294 (2016).
Sandroff, B. M., Johnson, C. L. & Motl, R. W. Exercise training effects on memory and hippocampal viscoelasticity in multiple sclerosis: a novel application of magnetic resonance elastography. Neuroradiology 59, 61–67 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02282878 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03718247 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03808545 (2019).
Ploughman, M. et al. Factors influencing healthy aging with multiple sclerosis: a qualitative study. Disabil. Rehabil. 34, 26–33 (2012).
Harrison, T., Blozis, S. & Stuifbergen, A. Longitudinal predictors of attitudes toward aging among women with multiple sclerosis. Psychol. Aging 23, 823–832 (2008).
Julian, L. J. Cognitive functioning in multiple sclerosis. Neurol. Clin. 29, 507–525 (2011).
Bodling, A. M., Denney, D. R. & Lynch, S. G. Cognitive aging in patients with multiple sclerosis: a cross-sectional analysis of speeded processing. Arch. Clin. Neuropsychol. 24, 761–767 (2009).
Roy, S. et al. Differential effects of aging on motor and cognitive functioning in multiple sclerosis. Mult. Scler. 23, 1385–1393 (2017).
Sumowski, J. F., Chiaravalloti, N. & DeLuca, J. Cognitive reserve protects against cognitive dysfunction in multiple sclerosis. J. Clin. Exp. Neuropsychol. 31, 913–926 (2009).
Benedict, R. H., Morrow, S. A., Weinstock Guttman, B., Cookfair, D. & Schretlen, D. J. Cognitive reserve moderates decline in information processing speed in multiple sclerosis patients. J. Int. Neuropsychol. Soc. 16, 829–835 (2010).
Charvet, L. E., Shaw, M. T., Haider, L., Melville, P. & Krupp, L. B. Remotely-delivered cognitive remediation in multiple sclerosis (MS): protocol and results from a pilot study. Mult. Scler. J. Exp. Transl Clin. 1, 2055217315609629 (2015).
Perez-Martin, M. Y., Gonzalez-Platas, M., Eguia-Del Rio, P., Croissier-Elias, C. & Jimenez Sosa, A. Efficacy of a short cognitive training program in patients with multiple sclerosis. Neuropsychiatr. Dis. Treat. 13, 245–252 (2017).
Grasso, M. G. et al. Evaluation of the impact of cognitive training on quality of life in patients with multiple sclerosis. Eur. Neurol. 78, 111–117 (2017).
O’Carroll, C. B. et al. Is donepezil effective for multiple sclerosis-related cognitive dysfunction? A critically appraised topic. Neurologist 18, 51–54 (2012).
Villoslada, P., Arrondo, G., Sepulcre, J., Alegre, M. & Artieda, J. Memantine induces reversible neurologic impairment in patients with MS. Neurology 72, 1630–1633 (2009).
Peyro Saint Paul, L. et al. Efficacy and safety profile of memantine in patients with cognitive impairment in multiple sclerosis: a randomized, placebo-controlled study. J. Neurol. Sci. 363, 69–76 (2016).
Hartung, H. P. et al. Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet 360, 2018–2025 (2002).
Confavreux, C. et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 13, 247–256 (2014).
Hauser, S. L. et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N. Engl. J. Med. 376, 221–234 (2017).
Acknowledgements
The authors’ research is supported in part by grants from the National Multiple Sclerosis Society (HC 1411–02004) and Biogen Idec (US-MSG-15-10855) to B.W.-G. and from Advancing Research in Multiple Sclerosis (ARMS) to the Jacobs Multiple Sclerosis Center for Treatment and Research.
Reviewer information
Nature Reviews Neurology thanks M. Magyari and other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
C.B.V., D.J., K.S.K., R.Z. and B.W.-G. were involved in all aspects of article preparation. M.R. and R.H.B.B. contributed to discussions of the article content, writing and review or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
C.B.V. declares that she has received consultancy fees from Merck/EMD Serono. M.R. declares that he has received research funding from the US National Institute of Neurological Disorders and Stroke and the US National Science Foundation. R.H.B.B. declares that he has received research support from Accorda, Biogen, Genzyme, Mallinckrodt and Novartis; consultancy fees from Biogen, Genentech, Genzyme, Novartis, Roche, Sanofi and Teva; and compensation for activities relating to continuing medical education from EMD Serono. R.Z. declares that he has received speakers’ and consultancy fees from Celgene, Claret Medical, EMD Serono, Genzyme-Sanofi, IMS Health, Novartis and Roche-Genentech and financial research support from Claret Medical, IMS Health, Intekrin, Genzyme-Sanofi and Novartis. B.W.-G. declares that she has received fees for consultancy, acting as a speaker and serving on the scientific advisory boards of Biogen Idec, EMD Serono, Genzyme-Sanofi, Novartis, Questcor and Teva Neuroscience, and financial research support from Aspreva, Biogen Idec, EMD Serono, Genzyme, the Immune Tolerance Network Clinical Trials Group, the National Multiple Sclerosis Society, the NIH (not related to the present work), Novartis and Teva Neuroscience. D.J. and K.S.K. declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vaughn, C.B., Jakimovski, D., Kavak, K.S. et al. Epidemiology and treatment of multiple sclerosis in elderly populations. Nat Rev Neurol 15, 329–342 (2019). https://doi.org/10.1038/s41582-019-0183-3
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-019-0183-3
This article is cited by
-
Investigating the role of VDR gene variants in multiple sclerosis susceptibility: a case–control study in Egypt
The Egyptian Journal of Neurology, Psychiatry and Neurosurgery (2024)
-
Disease-modifying treatment, long-term outcomes and transition to progressive multiple sclerosis: data based on the New York State MS Consortium
Journal of Neurology (2024)
-
Frontal-striatal tract integrity and depression in older adults with and without multiple sclerosis
Neurological Sciences (2024)
-
Late-onset multiple sclerosis: disability trajectories in relapsing–remitting patients of the Italian MS Registry
Journal of Neurology (2024)
-
The brief repeatable battery of neuropsychological tests (BRB-N) version a: update of Italian normative data from the Italian Neuroimaging Network Initiative (INNI)
Journal of Neurology (2024)