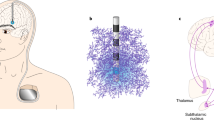
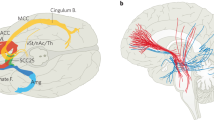
Abstract
Subthalamic deep brain stimulation (DBS) for Parkinson disease (PD) currently requires laborious open-loop programming, which can mitigate the benefits of this treatment. Experimental closed-loop DBS systems are emerging that can sense the electrophysiological surrogates of PD motor signs and respond with delivery of an automatically adapted stimulation. Such biomarker-based neural interfaces constitute a major advance towards improving the outcomes of patients treated with DBS and enhancing our understanding of the pathophysiological mechanisms underlying PD. In this Perspectives article, we argue that closed-loop DBS, in addition to offering advantages in patients with PD, might extend the current indications for DBS to include selected psychiatric disorders in which the symptoms are similarly driven by pathological brain circuit activity. The success of closed-loop DBS in such settings will depend on the identification of symptom-specific biomarkers, which ideally should reflect causal mechanisms of the underlying pathology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
16 April 2019
In the originally published article, one of the affiliations for Paul Krack was omitted — these should have included ‘Movement Disorders Center, Department of Neurology, University Hospital (Inselspital) and University of Bern, Bern, Switzerland.’ This error has been corrected in the HTML and PDF versions of the manuscript.
References
Limousin, P. et al. Electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N. Engl. J. Med. 339, 1105–1111 (1998).
Dayal, V., Limousin, P. & Foltynie, T. Subthalamic nucleus deep brain stimulation in Parkinson’s disease: the effect of varying stimulation parameters. J. Parkinsons Dis. 7, 235–245 (2017).
Castrioto, A., Lhommée, E., Moro, E. & Krack, P. Mood and behavioural effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurol. 13, 287–305 (2014).
Hatsopoulos, N. G. & Donoghue, J. P. The science of neural interface systems. Annu. Rev. Neurosci. 32, 249–266 (2009).
Arlotti, M. et al. Eight-hours adaptive deep brain stimulation in patients with Parkinson disease. Neurology 90, e971–e976 (2018).
Vansteensel, M. J. et al. Fully implanted brain–computer interface in a locked-in patient with ALS. N. Engl. J. Med. 375, 2060–2066 (2016).
Hoang, K. B., Cassar, I. R., Grill, W. M. & Turner, D. A. Biomarkers and stimulation algorithms for adaptive brain stimulation. Front. Neurosci. 11, 117–115 (2017).
Hebb, A. O. et al. Creating the feedback loop: closed-loop neurostimulation. Neurosurg. Clin. North Am. 25, 187–204 (2014).
Chaudhary, U., Birbaumer, N. & Ramos-Murguialday, A. Brain–computer interfaces for communication and rehabilitation. Nat. Rev. Neurol. 12, 513–525 (2016).
Ajiboye, A. B. et al. Restoration of reaching and grasping movements through brain-controlled muscle stimulation in a person with tetraplegia: a proof-of-concept demonstration. Lancet 389, 1821–1830 (2017).
Slutzky, M. W. & Flint, R. D. Physiological properties of brain–machine interface input signals. J. Neurophysiol. 118, 1329–1343 (2017).
Hariz, M. Twenty-five years of deep brain stimulation: celebrations and apprehensions. Mov. Disord. 27, 930–933 (2012).
Clausen, J. et al. Help, hope, and hype: ethical dimensions of neuroprosthetics. Science 356, 1338–1339 (2017).
Hariz, M., Blomstedt, P. & Zrinzo, L. Future of brain stimulation: new targets, new indications, new technology. Mov. Disord. 28, 1784–1792 (2013).
Yuste, R. et al. Four ethical priorities for neurotechnologies and AI. Nature 551, 159–163 (2017).
Lipsman, N., Mendelsohn, D., Taira, T. & Bernstein, M. The contemporary practice of psychiatric surgery: results from a survey of North American functional neurosurgeons. Stereotact. Funct. Neurosurg. 89, 103–110 (2011).
Baumeister, A. The Tulane electrical brain stimulation program: a historical case study in medical ethics. J. Hist. Neurosci. 9, 262–278 (2000).
Hariz, M. I., Blomstedt, P. & Zrinzo, L. Deep brain stimulation between 1947 and 1987: the untold story. Neurosurg. Focus 29, E1 (2010).
Lüscher, C. Dark past of deep-brain stimulation. Nature 555, 306–307 (2018).
Hochberg, L. R. et al. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature 485, 372–375 (2012).
Oswal, A., Brown, P. & Litvak, V. Synchronized neural oscillations and the pathophysiology of Parkinson’s disease. Curr. Opin. Neurol. 26, 662–670 (2013).
Birbaumer, N., Elbert, T., Canavan, A. G. M. & Rockstroh, B. Slow potentials of the cerebral cortex and behavior. Physiol. Rev. 70, 1–41 (1990).
Pfurtscheller, G. & da Silva, F. L. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin. Neurophysiol. 110, 1842–1857 (1999).
Cheyne, D. O. MEG studies of sensorimotor rhythms: a review. Exp. Neurol. 245, 27–39 (2013).
Hammond, C., Bergman, H. & Brown, P. Pathological synchronization in Parkinson’s disease: networks, models and treatments. Trends Neurosci. 30, 357–364 (2007).
Bergman, H. et al. Physiological aspects of information processing in the basal ganglia of normal and parkinsonian primates. Trends Neurosci. 21, 32–38 (1998).
Neumann, W.-J. et al. Long term correlation of subthalamic beta band activity with motor impairment in patients with Parkinson’s disease. Clin. Neurophysiol. 128, 2286–2291 (2017).
Whitmer, D. et al. High frequency deep brain stimulation attenuates subthalamic and cortical rhythms in Parkinson’s disease. Front. Hum. Neurosci. 6, 155 (2012).
Blumenfeld, Z. et al. Sixty-Hertz stimulation improves bradykinesia and amplifies subthalamic low-frequency oscillations. Mov. Disord. 32, 80–88 (2016).
Tinkhauser, G. et al. Directional local field potentials: a tool to optimize deep brain stimulation. Mov. Disord. 33, 159–164 (2017).
Brittain, J.-S. & Brown, P. Oscillations and the basal ganglia: motor control and beyond. Neuroimage 85, 637–647 (2014).
Miller, K. J. et al. Human motor cortical activity is selectively phase-entrained on underlying rhythms. PLOS Comput. Biol. 8, e1002655 (2012).
Rosin, B. et al. Closed-loop deep brain stimulation is superior in ameliorating parkinsonism. Neuron 72, 370–384 (2011).
Little, S. et al. Adaptive deep brain stimulation in advanced Parkinson disease. Ann. Neurol. 74, 449–457 (2013).
Little, S. et al. Bilateral adaptive deep brain stimulation is effective in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 87, 717–721 (2015).
Little, S. et al. Adaptive deep brain stimulation for Parkinson’s disease demonstrates reduced speech side effects compared to conventional stimulation in the acute setting. J. Neurol. Neurosurg. Psychiatry 87, 1388–1389 (2016).
Rosa, M. et al. Adaptive deep brain stimulation in a freely moving parkinsonian patient. Mov. Disord. 30, 1003–1005 (2015).
Rosa, M. et al. Adaptive deep brain stimulation controls levodopa-induced side effects in parkinsonian patients. Mov. Disord. 32, 628–629 (2017).
Daneshzand, M., Faezipour, M. & Barkana, B. D. Robust desynchronization of Parkinson’s disease pathological oscillations by frequency modulation of delayed feedback deep brain stimulation. PLOS ONE 13, e0207761 (2018).
Rappel, P. et al. Subthalamic theta activity: a novel human subcortical biomarker for obsessive compulsive disorder. Transl Psychiatry 8, 252 (2018).
Feingold, J., Gibson, D. J., DePasquale, B. & Graybiel, A. M. Bursts of beta oscillation differentiate postperformance activity in the striatum and motor cortex of monkeys performing movement tasks. Proc. Natl Acad. Sci. USA 112, 13687–13692 (2015).
Tinkhauser, G. et al. Beta burst dynamics in Parkinson’s disease OFF and ON dopaminergic medication. Brain 140, 2968–2981 (2017).
Tinkhauser, G. et al. The modulatory effect of adaptive deep brain stimulation on beta bursts in Parkinson’s disease. Brain 140, 1053–1067 (2017).
Torrecillos, F. et al. Modulation of beta bursts in the subthalamic nucleus predicts motor performance. J. Neurosci. 38, 8905–8917 (2018).
de Hemptinne, C. et al. Exaggerated phase–amplitude coupling in the primary motor cortex in Parkinson disease. Proc. Natl Acad. Sci. USA 10, 4780–4785 (2013).
Swann, N. C. et al. Elevated synchrony in Parkinson disease detected with electroencephalography. Ann. Neurol. 78, 742–750 (2015).
de Hemptinne, C. et al. Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson’s disease. Nat. Neurosci. 18, 779–786 (2015).
Swann, N. C. et al. Gamma oscillations in the hyperkinetic state detected with chronic human brain recordings in Parkinson’s disease. J. Neurosci. 36, 6445–6458 (2016).
Swann, N. C. et al. Adaptive deep brain stimulation for Parkinson’s disease using motor cortex sensing. J. Neural Eng. 15, 046006 (2018).
Cagnan, H., Kühn, A. A. & Brown, P. Co-modulation of finely tuned high-gamma band activity across hemispheres in Parkinson’s disease. Clin. Neurophysiol. 125, 777–785 (2014).
Cagnan, H. et al. Stimulating at the right time: phase-specific deep brain stimulation. Brain 140, 132–145 (2017).
Malekmohammadi, M. et al. Kinematic adaptive deep brain stimulation for resting tremor in Parkinson’s disease. Mov. Disord. 31, 426–428 (2016).
Houston, B. C., Thompson, M. C., Ojemann, J. G., Ko, A. L. & Chizeck, H. J. in 8th Int. IEEE/EMBS Conf. on Neural Engineering 316–320 (IEEE, 2017).
Shah, S. A., Tinkhauser, G., Chen, C. C., Little, S. & Brown, P. Parkinsonian tremor detection from subthalamic nucleus local field potentials for closed-loop deep brain stimulation. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2018, 2320–2324 (2018).
Krack, P. et al. Mirthful laughter induced by subthalamic nucleus stimulation. Mov. Disord. 16, 867–875 (2001).
Eusebio, A. et al. Subthalamic nucleus stimulation and compulsive use of dopaminergic medication in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 84, 868–874 (2013).
Lhommee, E. et al. Subthalamic stimulation in Parkinson’s disease: restoring the balance of motivated behaviours. Brain 135, 1463–1477 (2012).
Lhommée, E. et al. Behavioural outcomes of subthalamic stimulation and medical therapy versus medical therapy alone for Parkinson’s disease with early motor complications (EARLYSTIM trial): secondary analysis of an open-label randomised trial. Lancet Neurol. 17, 223–231 (2018).
Jahanshahi, M., Obeso, I., Baunez, C., Alegre, M. & Krack, P. Parkinson’s disease, the subthalamic nucleus, inhibition, and impulsivity. Mov. Disord. 30, 128–140 (2014).
Polosan, M. et al. Affective modulation of the associative-limbic subthalamic nucleus: deep brain stimulation in obsessive–compulsive disorder. Transl Psychiatry 9, 896 (2019).
Pagonabarraga, J., Kulisevsky, J., Strafella, A. & Krack, P. Apathy in Parkinson’s disease: clinical features, neural substrates, diagnosis, and treatment. Lancet Neurol. 14, 518–531 (2015).
Weintraub, D. & Burn, D. J. Parkinson’s disease: the quintessential neuropsychiatric disorder. Mov. Disord. 26, 1022–1031 (2011).
Martinez-Fernandez, R., Schmitt, E., Martinez-Martin, P. & Krack, P. The hidden sister of motor fluctuations in Parkinson’s disease: a review on nonmotor fluctuations. Mov. Disord. 31, 1080–1094 (2016).
Béreau, M. et al. Hyperdopaminergic behavioral spectrum in Parkinson’s disease: a review. Rev. Neurol. (Paris) 174, 653–663 (2018).
Eitan, R. et al. Asymmetric right/left encoding of emotions in the human subthalamic nucleus. Front. Syst. Neurosci. 7, 69 (2013).
Kuhn, A. A. et al. Activation of the subthalamic region during emotional processing in Parkinson disease. Neurology 65, 707–713 (2005).
Huebl, J. et al. Oscillatory subthalamic nucleus activity is modulated by dopamine during emotional processing in Parkinson’s disease. Cortex 60, 69–81 (2014).
Schmitt, E. et al. The neuropsychiatric fluctuations scale for Parkinson’s disease: a pilot study. Mov. Disord. Clin. Pract. 5, 265–272 (2018).
Rodriguez-Oroz, M. C. et al. Involvement of the subthalamic nucleus in impulse control disorders associated with Parkinson’s disease. Brain 134, 36–49 (2011).
Pelloux, Y. et al. Subthalamic nucleus high frequency stimulation prevents and reverses escalated cocaine use. Mol. Psychiatry 21, 1033 (2018).
Delpont, B. et al. Psychostimulant effect of dopaminergic treatment and addictions in Parkinson’s disease. Mov. Disord. 8, 464–468 (2017).
Canali, P. et al. Shared reduction of oscillatory natural frequencies in bipolar disorder, major depressive disorder and schizophrenia. J. Affect. Disord. 184, 111–115 (2015).
Avanzino, L., Pelosin, E., Martino, D. & Abbruzzese, G. Motor timing deficits in sequential movements in Parkinson disease are related to action planning: a motor imagery study. PLOS ONE 8, e75454 (2013).
Aron, A. R., Herz, D. M., Brown, P., Forstmann, B. U. & Zaghloul, K. Frontosubthalamic circuits for control of action and cognition. J. Neurosci. 36, 11489–11495 (2016).
Bour, L. J. et al. Directional recording of subthalamic spectral power densities in Parkinson’s disease and the effect of steering deep brain stimulation. Brain Stimul. 8, 730–741 (2015).
Trager, M. H. et al. Subthalamic beta oscillations are attenuated after withdrawal of chronic high frequency neurostimulation in Parkinson’s disease. Neurobiol. Dis. 96, 22–30 (2016).
Swann, N. C. et al. Chronic multisite brain recordings from a totally implantable bidirectional neural interface: experience in 5 patients with Parkinson’s disease. J. Neurosurg. 128, 605–616 (2018).
Meidahl, A. C. et al. Adaptive deep brain stimulation for movement disorders: the long road to clinical therapy. Mov. Disord. 32, 810–819 (2017).
Sani, O. G. et al. Mood variations decoded from multi-site intracranial human brain activity. Nat. Biotechnol. 156, 675 (2018).
Mallet, L. et al. Subthalamic nucleus stimulation in severe obsessive–compulsive disorder. N. Engl. J. Med. 359, 2121–2134 (2008).
Mallet, L. et al. Compulsions, Parkinson’s disease, and stimulation. Lancet 360, 1302–1304 (2002).
Schlaepfer, T. E. Deep brain stimulation for major depression — steps on a long and winding road. Biol. Psychiatry 78, 218–219 (2015).
Menzies, L. et al. Integrating evidence from neuroimaging and neuropsychological studies of obsessive–compulsive disorder: the orbitofronto-striatal model revisited. Neurosci. Biobehav. Rev. 32, 525–549 (2008).
Houeto, J. L. Tourette’s syndrome and deep brain stimulation. J. Neurol. Neurosurg. Psychiatry 76, 992–995 (2005).
Robertson, M. M. et al. Gilles de la Tourette syndrome. Nat. Rev. Dis. Primers 3, 16097 (2017).
Shahed, J., Poysky, J., Kenney, C., Simpson, R. & Jankovic, J. GPi deep brain stimulation for Tourette syndrome improves tics and psychiatric comorbidities. Neurology 68, 159–160 (2007).
Welter, M.-L. et al. Internal pallidal and thalamic stimulation in patients with Tourette syndrome. Arch. Neurol. 65, 952–957 (2008).
Zhang, C. et al. Pallidal deep brain stimulation combined with capsulotomy for Tourette’s syndrome with psychiatric comorbidity. J. Neurosurg. 4, 1–9 (2019).
Martinez-Ramirez, D. et al. Efficacy and safety of deep brain stimulation in Tourette syndrome. JAMA Neurol. 75, 353 (2018).
Neumann, W.-J. et al. Pallidal and thalamic neural oscillatory patterns in Tourette’s syndrome. Ann. Neurol. 84, 505–514 (2018).
Neumann, W.-J. et al. Toward electrophysiology-based intelligent adaptive deep brain stimulation for movement disorders. Neurotherapeutics 16, 105–118 (2019).
Molina, R. et al. Report of a patient undergoing chronic responsive deep brain stimulation for Tourette syndrome: proof of concept. J. Neurosurg. 129, 308–314 (2018).
Mayberg, H. S. et al. Deep brain stimulation for treatment-resistant depression. Neuron 45, 651–660 (2005).
Lozano, A. M. et al. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol. Psychiatry 64, 461–467 (2008).
Puigdemont, D. et al. A randomized double-blind crossover trial of deep brain stimulation of the subcallosal cingulate gyrus in patients with treatment-resistant depression: a pilot study of relapse prevention. J. Psychiatry Neurosci. 40, 224–231 (2015).
Holtzheimer, P. E. et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry 4, 839–849 (2017).
Neumann, W.-J. et al. Different patterns of local field potentials from limbic DBS targets in patients with major depressive and obsessive compulsive disorder. Mol. Psychiatry 19, 1186–1192 (2014).
Widge, A. S., Malone, D. A. Jr & Dougherty, D. D. Closing the loop on deep brain stimulation for treatment-resistant depression. Front. Neurosci. 12, 640 (2018).
Smart, O. et al. Initial unilateral exposure to deep brain stimulation in treatment-resistant depression patients alters spectral power in the subcallosal cingulate. Front. Comput. Neurosci. 12, 1764–1713 (2018).
Schlaepfer, T. E., Bewernick, B. H., Kayser, S., Hurlemann, R. & Coenen, V. A. Deep brain stimulation of the human reward system for major depression — rationale, outcomes and outlook. Neuropsychopharmacology 39, 1303–1314 (2014).
Coenen, V. A. et al. The medial forebrain bundle as a target for deep brain stimulation for obsessive-compulsive disorder. CNS Spectr. 22, 282–289 (2016).
Coenen, V. A. et al. Medial forebrain bundle stimulation as a pathophysiological mechanism for hypomania in subthalamic nucleus deep brain stimulation for Parkinson’s disease. Neurosurgery 64, 1106–1115 (2009).
Kulisevsky, J. et al. Mania following deep brain stimulation for Parkinson’s disease. Neurology 59, 1421–1424 (2002).
Romito, L. M. et al. Transient mania with hypersexuality after surgery for high frequency stimulation of the subthalamic nucleus in Parkinson’s disease. Mov. Disord. 17, 1371–1374 (2002).
Herzog, J. et al. Manic episode with psychotic symptoms induced by subthalamic nucleus stimulation in a patient with Parkinson’s disease. Mov. Disord. 18, 1382–1384 (2003).
Schlaepfer, T. E., Bewernick, B. H., Kayser, S., Mädler, B. & Coenen, V. A. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol. Psychiatry 73, 1204–1212 (2013).
Torres-Sanchez, S., Perez-Caballero, L. & Berrocoso, E. Cellular and molecular mechanisms triggered by deep brain stimulation in depression: a preclinical and clinical approach. Prog. Neuropsychopharmacol. Biol. Psychiatry 73, 1–10 (2017).
Schlaepfer, T. E. et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology 33, 368–377 (2007).
Coenen, V. A. et al. Tractography-assisted deep brain stimulation of the superolateral branch of the medial forebrain bundle (slMFB DBS) in major depression. Neuroimage Clin. 20, 580–593 (2018).
Mallet, L. et al. Stimulation of subterritories of the subthalamic nucleus reveals its role in the integration of the emotional and motor aspects of behavior. Proc. Natl Acad. Sci. USA 104, 10661–10666 (2007).
Bewernick, B. H. et al. Deep brain stimulation to the medial forebrain bundle for depression- long-term outcomes and a novel data analysis strategy. Brain Stimul. 10, 664–671 (2017).
Hamani, C. et al. Memory enhancement induced by hypothalamic/fornix deep brain stimulation. Ann. Neurol. 63, 119–123 (2008).
Mirzadeh, Z., Bari, A. & Lozano, A. M. The rationale for deep brain stimulation in Alzheimer’s disease. J. Neural Transm. 123, 775–783 (2015).
Leoutsakos, J.-M. S. et al. Deep brain stimulation targeting the fornix for mild Alzheimer dementia (the ADvance trial): a two year follow-up including results of delayed activation. J. Alzheimers Dis. 64, 597–606 (2018).
Kandel, E. R. The biology of memory: a forty-year perspective. J. Neurosci. 29, 12748–12756 (2009).
Rowland, N. C. et al. Task-related activity in sensorimotor cortex in Parkinson’s disease and essential tremor: changes in beta and gamma bands. Front. Hum. Neurosci. 9, 512 (2015).
Nelson, A. B. et al. Beta oscillatory changes and retention of motor skills during practice in healthy subjects and in patients with Parkinson’s disease. Front. Hum. Neurosci. 11, 104 (2017).
Heinrichs-Graham, E. et al. Neuromagnetic evidence of abnormal movement-related beta desynchronization in Parkinson’s disease. Cereb. Cortex 24, 2669–2678 (2013).
Beck, M. H. et al. Short- and long-term dopamine depletion causes enhanced beta oscillations in the cortico-basal ganglia loop of parkinsonian rats. Exp. Neurol. 286, 124–136 (2016).
Wichmann, T., Bergman, H. & DeLong, M. R. Basal ganglia, movement disorders and deep brain stimulation: advances made through non-human primate research. J. Neural Transm. 125, 419–430 (2018).
MacQueen, D. A., Young, J. W. & Cope, Z. A. in Biomarkers in Psychiatry (Current Topics in Behavioral Neurosciences) Vol. 40 (eds Pratt, J. & Hall, J.) 111–166 (Springer, 2018).
Ahmari, S. E. Using mice to model obsessive compulsive disorder: from genes to circuits. Neuroscience 321, 121–137 (2016).
Lüscher, C. The emergence of a circuit model for addiction. Annu. Rev. Neurosci. 39, 257–276 (2016).
Pascoli, V. et al. Stochastic synaptic plasticity underlying compulsion in a model of addiction. Nature 564, 366–371 (2018).
Kato, T., Kasahara, T., Kubota-Sakashita, M., Kato, T. M. & Nakajima, K. Animal models of recurrent or bipolar depression. Neuroscience 321, 189–196 (2016).
Ménard, C., Hodes, G. E. & Russo, S. J. Pathogenesis of depression: insights from human and rodent studies. Neuroscience 321, 138–162 (2016).
Engel, A. K., Moll, C. K. E., Fried, I. & Ojemann, G. A. Invasive recordings from the human brain: clinical insights and beyond. Nat. Rev. Neurosci. 6, 35–47 (2005).
Pandarinath, C. et al. High performance communication by people with paralysis using an intracortical brain-computer interface. eLife 6, e18554 (2017).
Winestone, J. S., Zaidel, A., Bergman, H. & Israel, Z. The use of macroelectrodes in recording cellular spiking activity. J. Neurosci. Methods 206, 34–39 (2012).
Benis, D. et al. Response inhibition rapidly increases single-neuron responses in the subthalamic nucleus of patients with Parkinson’s disease. Cortex 84, 111–123 (2016).
Pötter-Nerger, M. et al. Movement-related activity of human subthalamic neurons during a reach-to-grasp task. Front. Hum. Neurosci. 11, 436 (2017).
Burbaud, P. et al. Neuronal activity correlated with checking behaviour in the subthalamic nucleus of patients with obsessive–compulsive disorder. Brain 136, 304–317 (2013).
Creed, M., Pascoli, V. & Lüscher, C. Refining deep brain stimulation to emulate optogenetic treatment of synaptic pathology. Science 347, 659–664 (2015).
Wu, X. H., Song, J. J., Faull, R. L. M. & Waldvogel, H. J. GABAA and GABAB receptor subunit localization on neurochemically identified neurons of the human subthalamic nucleus. J. Comp. Neurol. 526, 803–823 (2018).
Arber, S. & Costa, R. M. Connecting neuronal circuits for movement. Science 360, 1403–1404 (2018).
Daneshzand, M., Faezipour, M. & Barkana, B. D. Computational stimulation of the basal ganglia neurons with cost effective delayed gaussian waveforms. Front. Comput. Neurosci. 11, 73 (2017).
Lüscher, C., Pascoli, V. & Creed, M. Optogenetic dissection of neural circuitry: from synaptic causalities to blue prints for novel treatments of behavioral diseases. Curr. Opin. Neurobiol. 35, 95–100 (2015).
Lo, M.-C. & Widge, A. S. Closed-loop neuromodulation systems: next-generation treatments for psychiatric illness. Int. Rev. Psychiatry 29, 191–204 (2017).
Buot, A. et al. Processing of emotional information in the human subthalamic nucleus. J. Neurol. Neurosurg. Psychiatry 84, 1331–1338 (2013).
Péron, J. et al. Vocal emotion decoding in the subthalamic nucleus: an intracranial ERP study in Parkinson’s disease. Brain Lang. 168, 1–11 (2017).
Bastin, J. et al. Changes of oscillatory activity in the subthalamic nucleus during obsessive-compulsive disorder symptoms: two case reports. Cortex 60, 145–150 (2014).
Smith, G. S. et al. Increased cerebral metabolism after 1 year of deep brain stimulation in Alzheimer disease. Arch. Neurol. 69, 1141–1148 (2012).
Acknowledgements
The views expressed in this article are the authors’ own and are not an official position of their institutions. W.B. is supported by Swiss National Science Foundation grant 323530–177577. P.M. is supported by Swiss National Science Foundation grant 167836. N.B. is supported by the Deutsche Forschungsgemeinschaft (DFG) and the Wyss Center for Bio and Neuroengineering. P.K. is supported by Swiss National Science Foundation grant 310030–170271.
Reviewer information
Nature Reviews Neurology thanks L. Zrinzo, R. Eitan and M. Faezipour for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
W.B., P.M. and P.K. researched the data for the manuscript, wrote the first draft of the article and prepared the display items. All authors contributed to the discussions of the article content, critically edited the article and approved its final version.
Corresponding author
Ethics declarations
Competing interests
W.B. declares that he received travel and accommodation funding from Boston Scientific (unrelated to the present work). J.D. declares that he is Director of the Wyss Center, a nonprofit foundation aimed at translating neurotechnology into human application. P.K. declares that he has received research grants and personal fees from Boston Scientific, Medtronic and St Jude (manufacturers of neurostimulators and electrodes for deep brain stimulation) and from the Annemarie Opprecht Foundation, Bertarelli Foundation, Centre National Recherche Scientifique, Lily E. Safra, France Parkinson, French Ministry of Health (PHRC), INSERM, Homeperf, Orkyn, Parkinson Schweiz, Roger De Spoelberch Foundation, Swiss National Science Foundation and UCB (unrelated to the present work). The other authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Event-related desynchronization
-
(ERD). Task-related transient power decreases observed in alpha (8–13 Hz) and beta (15–30 Hz) bands during preparation for and performance of physiological movement or movement imagery. A post-movement (rebound) increase in beta power is observed following movement-related decreases in beta power. In the context of scalp EEG recordings from sensorimotor regions, ERD denotes reduced amplitude of sensorimotor rhythms and thus decreased beta power.
- Event-related synchronization
-
(ERS). Task-related transient gamma (>30 Hz) power increases observed during self-paced and cued voluntary movements. In the context of scalp EEG recordings from sensorimotor regions, ERS denotes increased gamma power of sensorimotor rhythms.
- Extracellular recordings
-
Electrophysiological recordings from microelectrodes aid neuronavigation by identifying the borders of target brain nuclei during functional neurosurgery. Action potentials (spikes; detected using a high-pass filter) and local field potentials (detected using a low-pass filter) represent the activity of one or a cluster of neurons, respectively, near the recording tip.
- High-frequency DBS
-
Therapeutic deep brain stimulation (DBS) utilizes stimulation frequencies >100 Hz, the efficacy of which has been robustly reproduced. Low-frequency DBS (≤100 Hz) has been used in specific contexts but its therapeutic efficacy is debated.
- Local field potential
-
(LFP). A signal reflecting the sum of local extracellular electrical activity of a group of neurons. Low-impedance electrodes (recording surface areas ~3–10 mm2 or more) record LFPs from large collections of neurons; high-impedance microelectrodes record LFPs from small neuronal groups.
- Oscillations
-
Recurrent brain activity patterns generated by rhythmic, periodic or regular electrical activity synchronized across neurons. Focal spatiotemporal changes in oscillation signatures are linked to behaviours, mental tasks and environmental stimuli. Oscillations are divided into delta (1–4 Hz), theta (4–8 Hz), alpha (8–13 Hz), beta (13–30 Hz) and gamma (30–200 Hz) frequency bands. Oscillatory activity is often represented as changes in the power of different bands over time. Filter and signal processing characteristics can also alter the power and form of oscillations.
- Phase-amplitude coupling
-
Coupling is present when the oscillation amplitude in a high-frequency (typically gamma) band at a particular instant in time depends on the phase of oscillations in a low-frequency (typically beta) band at the same moment.
Rights and permissions
About this article
Cite this article
Bouthour, W., Mégevand, P., Donoghue, J. et al. Biomarkers for closed-loop deep brain stimulation in Parkinson disease and beyond. Nat Rev Neurol 15, 343–352 (2019). https://doi.org/10.1038/s41582-019-0166-4
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-019-0166-4
This article is cited by
-
Advanced therapies in Parkinson’s disease: an individualized approach to their indication
Journal of Neural Transmission (2024)
-
Nanoporous graphene-based thin-film microelectrodes for in vivo high-resolution neural recording and stimulation
Nature Nanotechnology (2024)
-
Neuronal and synaptic adaptations underlying the benefits of deep brain stimulation for Parkinson's disease
Translational Neurodegeneration (2023)
-
The effect of deep brain stimulation in Parkinson’s disease reflected in EEG microstates
npj Parkinson's Disease (2023)
-
Optimierte Therapie motorischer Spätkomplikationen
NeuroTransmitter (2023)