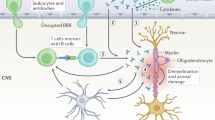
Abstract
Anti-myelin oligodendrocyte glycoprotein (MOG) antibodies (MOG-Abs) were first detected by immunoblot and enzyme-linked immunosorbent assay nearly 30 years ago, but their association with multiple sclerosis (MS) was not specific. Use of cell-based assays with native MOG as the substrate enabled identification of a group of MOG-Ab-positive patients with demyelinating phenotypes. Initially, MOG-Abs were reported in children with acute disseminated encephalomyelitis (ADEM). Further studies identified MOG-Abs in adults and children with ADEM, seizures, encephalitis, anti-aquaporin-4-antibody (AQP4-Ab)-seronegative neuromyelitis optica spectrum disorder (NMOSD) and related syndromes (optic neuritis, myelitis and brainstem encephalitis), but rarely in MS. This shift in our understanding of the diagnostic assays has re-invigorated the examination of MOG-Abs and their role in autoimmune and demyelinating disorders of the CNS. The clinical phenotypes, disease courses and responses to treatment that are associated with MOG-Abs are currently being defined. MOG-Ab-associated disease is different to AQP4-Ab-positive NMOSD and MS. This Review provides an overview of the current knowledge of MOG, the metrics of MOG-Ab assays and the clinical associations identified. We collate the data on antibody pathogenicity and the mechanisms that are thought to underlie this. We also highlight differences between MOG-Ab-associated disease, NMOSD and MS, and describe our current understanding on how best to treat MOG-Ab-associated disease.
Key points
-
Antibodies against myelin oligodendrocyte glycoprotein (MOG-Abs) that are detectable with cell-based assays are associated with non-MS acquired demyelinating syndromes of the CNS.
-
MOG-Ab-associated disorders account for a larger proportion of paediatric patients than that of adult patients who present with acquired demyelinating disease.
-
The clinical presentation of MOG-Ab-associated disorders changes with age: MOG-Abs are associated with an ADEM-like presentation in young children and an opticospinal presentation in children aged >9 years and adults.
-
Most patients with MOG-Ab-associated disorders have favourable outcomes, but a subset are left with permanent disability, usually as a result of the initial attack.
-
Many patients develop relapsing disease; relapses usually involve optic neuritis and often occur during steroid weaning or soon after steroid cessation, suggesting that a longer initial treatment duration is required.
-
Investigation of human MOG-Ab pathogenicity is hampered by their limited binding to rodent MOG; nevertheless, the place of MOG-Ab-associated disorders in the spectrum of inflammatory demyelinating diseases is becoming clearer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Lebar, R., Baudrimont, M. & Vincent, C. Chronic experimental autoimmune encephalomyelitis in the guinea pig. Presence of anti-M2 antibodies in central nervous system tissue and the possible role of M2 autoantigen in the induction of the disease. J. Autoimmun. 2, 115–132 (1989).
Linington, C., Bradl, M., Lassmann, H., Brunner, C. & Vass, K. Augmentation of demyelination in rat acute allergic encephalomyelitis by circulating mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. Am. J. Pathol. 130, 443–454 (1988). The first demonstration of the pathogenicity of MOG-Abs.
Brunner, C., Lassmann, H., Waehneldt, T. V., Matthieu, J. M. & Linington, C. Differential ultrastructural localization of myelin basic protein, myelin/oligodendroglial glycoprotein, and 2ʹ,3ʹ-cyclic nucleotide 3ʹ-phosphodiesterase in the CNS of adult rats. J. Neurochem. 52, 296–304 (1989).
Pham-Dinh, D. et al. Myelin/oligodendrocyte glycoprotein is a member of a subset of the immunoglobulin superfamily encoded within the major histocompatibility complex. Proc. Natl Acad. Sci. USA 90, 7990–7994 (1993).
Delarasse, C. et al. Complex alternative splicing of the myelin oligodendrocyte glycoprotein gene is unique to human and non-human primates. J. Neurochem. 98, 1707–1717 (2006).
Kang, H. J. et al. Spatio-temporal transcriptome of the human brain. Nature 478, 483–489 (2011).
Boyle, L. H., Traherne, J. A., Plotnek, G., Ward, R. & Trowsdale, J. Splice variation in the cytoplasmic domains of myelin oligodendrocyte glycoprotein affects its cellular localisation and transport. J. Neurochem. 102, 1853–1862 (2007).
Marta, C. B. et al. Signaling cascades activated upon antibody cross-linking of myelin oligodendrocyte glycoprotein: potential implications for multiple sclerosis. J. Biol. Chem. 280, 8985–8993 (2005).
Johns, T. G. & Bernard, C. C. The structure and function of myelin oligodendrocyte glycoprotein. J. Neurochem. 72, 1–9 (1999).
von Budingen, H. C. et al. The myelin oligodendrocyte glycoprotein directly binds nerve growth factor to modulate central axon circuitry. J. Cell Biol. 210, 891–898 (2015).
Cong, H., Jiang, Y. & Tien, P. Identification of the myelin oligodendrocyte glycoprotein as a cellular receptor for rubella virus. J. Virol. 85, 11038–11047 (2011).
Garcia-Vallejo, J. J. et al. CNS myelin induces regulatory functions of DC-SIGN-expressing, antigen-presenting cells via cognate interaction with MOG. J. Exp. Med. 211, 1465–1483 (2014).
Delarasse, C. et al. Myelin/oligodendrocyte glycoprotein-deficient (MOG-deficient) mice reveal lack of immune tolerance to MOG in wild-type mice. J. Clin. Invest. 112, 544–553 (2003).
Linares, D. et al. The magnitude and encephalogenic potential of autoimmune response to MOG is enhanced in MOG deficient mice. J. Autoimmun. 21, 339–351 (2003).
Iglesias, A., Bauer, J., Litzenburger, T., Schubart, A. & Linington, C. T- and B cell responses to myelin oligodendrocyte glycoprotein in experimental autoimmune encephalomyelitis and multiple sclerosis. Glia 36, 220–234 (2001).
Peschl, P., Bradl, M., Hoftberger, R., Berger, T. & Reindl, M. Myelin oligodendrocyte glycoprotein: deciphering a target in inflammatory demyelinating diseases. Front. Immunol. 8, 529 (2017).
Karni, A., Bakimer-Kleiner, R., Abramsky, O. & Ben-Nun, A. Elevated levels of antibody to myelin oligodendrocyte glycoprotein is not specific for patients with multiple sclerosis. Arch. Neurol. 56, 311–315 (1999).
Lindert, R. B. et al. Multiple sclerosis: B− and T cell responses to the extracellular domain of the myelin oligodendrocyte glycoprotein. Brain 122, 2089–2100 (1999).
Reindl, M. et al. Antibodies against the myelin oligodendrocyte glycoprotein and the myelin basic protein in multiple sclerosis and other neurological diseases: a comparative study. Brain 122, 2047–2056 (1999).
Egg, R., Reindl, M., Deisenhammer, F., Linington, C. & Berger, T. Anti-MOG and anti-MBP antibody subclasses in multiple sclerosis. Mult. Scler. 7, 285–289 (2001).
Schmidt, S. et al. Serum autoantibody responses to myelin oligodendrocyte glycoprotein and myelin basic protein in X-linked adrenoleukodystrophy and multiple sclerosis. J. Neuroimmunol. 119, 88–94 (2001).
Lutterotti, A. et al. Antibody response to myelin oligodendrocyte glycoprotein and myelin basic protein depend on familial background and are partially associated with human leukocyte antigen alleles in multiplex families and sporadic multiple sclerosis. J. Neuroimmunol. 131, 201–207 (2002).
Berger, T. et al. Antimyelin antibodies as a predictor of clinically definite multiple sclerosis after a first demyelinating event. N. Engl. J. Med. 349, 139–145 (2003).
Mantegazza, R. et al. Anti-MOG autoantibodies in Italian multiple sclerosis patients: specificity, sensitivity and clinical association. Int. Immunol. 16, 559–565 (2004).
O’Connor, K. C. et al. Antibodies from inflamed central nervous system tissue recognize myelin oligodendrocyte glycoprotein. J. Immunol. 175, 1974–1982 (2005).
Pittock, S. J. et al. Anti-myelin antibodies: frequency, stability and clinicopathologic associations in a biopsy MS cohort. Mult. Scler. 11, 109–109 (2005).
Kuhle, J. et al. Lack of association between antimyelin antibodies and progression to multiple sclerosis. N. Engl. J. Med. 356, 371–378 (2007).
O’Connor, K. C. et al. Self-antigen tetramers discriminate between myelin autoantibodies to native or denatured protein. Nat. Med. 13, 211–217 (2007).
Wang, H. et al. Myelin oligodendrocyte glycoprotein antibodies and multiple sclerosis in healthy young adults. Neurology 71, 1142–1146 (2008).
Di Pauli, F. et al. Temporal dynamics of anti-MOG antibodies in CNS demyelinating diseases. Clin. Immunol. 138, 247–254 (2011). The first study in which CBA-IF was used for the detection of MOG-Abs.
Lalive, P. H. et al. Highly reactive anti-myelin oligodendrocyte glycoprotein antibodies differentiate demyelinating diseases from viral encephalitis in children. Mult. Scler. 17, 297–302 (2011).
Menge, T., Lalive, P. H., von Budingen, H. C. & Genain, C. P. Conformational epitopes of myelin oligodendrocyte glycoprotein are targets of potentially pathogenic antibody responses in multiple sclerosis. J. Neuroinflamm. 8, 161 (2011).
Lackner, P. et al. Antibodies to myelin oligodendrocyte glycoprotein in HIV-1 associated neurocognitive disorder: a cross-sectional cohort study. J. Neuroinflamm. 7, 79 (2010).
Kezuka, T. et al. Relationship between NMO-antibody and anti-MOG antibody in optic neuritis. J. Neuroophthalmol. 32, 107–110 (2012).
Wingerchuk, D. M., Lennon, V. A., Pittock, S. J., Lucchinetti, C. F. & Weinshenker, B. G. Revised diagnostic criteria for neuromyelitis optica. Neurology 66, 1485–1489 (2006).
Lampasona, V. et al. Similar low frequency of anti-MOG IgG and IgM in MS patients and healthy subjects. Neurology 62, 2092–2094 (2004).
Waters, P., Pettingill, P. & Lang, B. Detection methods for neural autoantibodies. Handb. Clin. Neurol. 133, 147–163 (2016).
Rogers, S. W. et al. Autoantibodies to glutamate receptor GluR3 in Rasmussen’s encephalitis. Science 265, 648–651 (1994).
Lennon, V. A., Kryzer, T. J., Pittock, S. J., Verkman, A. S. & Hinson, S. R. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J. Exp. Med. 202, 473–477 (2005).
Haase, C. G. et al. The fine specificity of the myelin oligodendrocyte glycoprotein autoantibody response in patients with multiple sclerosis and normal healthy controls. J. Neuroimmunol. 114, 220–225 (2001).
Lalive, P. H. et al. Antibodies to native myelin oligodendrocyte glycoprotein are serologic markers of early inflammation in multiple sclerosis. Proc. Natl Acad. Sci. USA 103, 2280–2285 (2006).
Zhou, D. et al. Identification of a pathogenic antibody response to native myelin oligodendrocyte glycoprotein in multiple sclerosis. Proc. Natl Acad. Sci. USA 103, 19057–19062 (2006).
Brilot, F. et al. Antibodies to native myelin oligodendrocyte glycoprotein in children with inflammatory demyelinating central nervous system disease. Ann. Neurol. 66, 833–842 (2009). One of the studies in which a CBA-FACS assay for detection of MOG-Abs was established.
McLaughlin, K. A. et al. Age-dependent B cell autoimmunity to a myelin surface antigen in pediatric multiple sclerosis. J. Immunol. 183, 4067–4076 (2009). One of the studies in which a CBA-FACS assay for detection of MOG-Abs was established.
Waters, P. et al. MOG cell-based assay detects non-MS patients with inflammatory neurologic disease. Neurol. Neuroimmunol. Neuroinflamm. 2, e89 (2015). A study in which a novel assay for IgG1 MOG-Abs was established.
Dahm, L. et al. Seroprevalence of autoantibodies against brain antigens in health and disease. Ann. Neurol. 76, 82–94 (2014).
Ramberger, M. et al. Comparison of diagnostic accuracy of microscopy and flow cytometry in evaluating N-methyl-D-aspartate receptor antibodies in serum using a live cell-based assay. PLOS ONE 10, e0122037 (2015).
Probstel, A. K. et al. Anti-MOG antibodies are present in a subgroup of patients with a neuromyelitis optica phenotype. J. Neuroinflamm. 12, 46 (2015).
Yan, Y. et al. Autoantibody to MOG suggests two distinct clinical subtypes of NMOSD. Sci. China Life Sci. 59, 1270–1281 (2016).
van Pelt, E. D., Wong, Y. Y., Ketelslegers, I. A., Hamann, D. & Hintzen, R. Q. Neuromyelitis optica spectrum disorders: comparison of clinical and magnetic resonance imaging characteristics of AQP4-IgG versus MOG-IgG seropositive cases in the Netherlands. Eur. J. Neurol. 23, 580–587 (2016).
Jitprapaikulsan, J. et al. Aquaporin-4 and myelin oligodendrocyte glycoprotein autoantibody status predict outcome of recurrent optic neuritis. Ophthalmology 125, 1628–1637 (2018).
Jitprapaikulsan, J. et al. Novel glial targets and recurrent longitudinally extensive transverse myelitis. JAMA Neurol. 75, 892–895 (2018).
Mader, S. et al. Complement activating antibodies to myelin oligodendrocyte glycoprotein in neuromyelitis optica and related disorders. J. Neuroinflamm. 8, 184 (2011). The first study to demonstrate the presence of MOG-Abs in NMOSD.
Kitley, J. et al. Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology 79, 1273–1277 (2012).
Woodhall, M. et al. Glycine receptor and myelin oligodendrocyte glycoprotein antibodies in Turkish patients with neuromyelitis optica. J. Neurol. Sci. 335, 221–223 (2013).
Kitley, J. et al. Neuromyelitis optica spectrum disorders with aquaporin-4 and myelin-oligodendrocyte glycoprotein antibodies: a comparative study. JAMA Neurol. 71, 276–283 (2014). One of the initial studies of MOG-Abs in NMOSD.
Sato, D. K. et al. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology 82, 474–481 (2014). One of the initial studies of MOG-Abs in NMOSD.
Tanaka, M. & Tanaka, K. Anti-MOG antibodies in adult patients with demyelinating disorders of the central nervous system. J. Neuroimmunol. 270, 98–99 (2014).
Hacohen, Y. et al. Myelin oligodendrocyte glycoprotein antibodies are associated with a non-MS course in children. Neurol. Neuroimmunol. Neuroinflamm. 2, e81 (2015).
Hoftberger, R. et al. Antibodies to MOG and AQP4 in adults with neuromyelitis optica and suspected limited forms of the disease. Mult. Scler. 21, 866–874 (2015).
Kim, S. M. et al. Antibodies to MOG in adults with inflammatory demyelinating disease of the CNS. Neurol. Neuroimmunol. Neuroinflamm. 2, e163 (2015).
Martinez-Hernandez, E. et al. Antibodies to aquaporin 4, myelin-oligodendrocyte glycoprotein, and the glycine receptor alpha1 subunit in patients with isolated optic neuritis. JAMA Neurol. 72, 187–193 (2015).
Ramberger, M. et al. NMDA receptor antibodies: a rare association in inflammatory demyelinating diseases. Neurol. Neuroimmunol. Neuroinflamm. 2, e141 (2015).
Jarius, S. et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 1: frequency, syndrome specificity, influence of disease activity, long-term course, association with AQP4-IgG, and origin. J. Neuroinflamm. 13, 279 (2016). One of the largest analyses of MOG-Abs in NMOSD.
Piccolo, L. et al. Isolated new onset ‘atypical’ optic neuritis in the NMO clinic: serum antibodies, prognoses and diagnoses at follow-up. J. Neurol. 263, 370–379 (2016).
Sepulveda, M. et al. Neuromyelitis optica spectrum disorders: comparison according to the phenotype and serostatus. Neurol. Neuroimmunol. Neuroinflamm. 3, e225 (2016).
Siritho, S., Sato, D. K., Kaneko, K., Fujihara, K. & Prayoonwiwat, N. The clinical spectrum associated with myelin oligodendrocyte glycoprotein antibodies (anti-MOG-Ab) in Thai patients. Mult. Scler. 22, 964–968 (2016).
Hamid, S. H. M. et al. What proportion of AQP4-IgG-negative NMO spectrum disorder patients are MOG-IgG positive? A cross sectional study of 132 patients. J. Neurol. 264, 2088–2094 (2017).
Hyun, J. W. et al. Longitudinal analysis of myelin oligodendrocyte glycoprotein antibodies in CNS inflammatory diseases. J. Neurol. Neurosurg. Psychiatry 88, 811–817 (2017).
Sepulveda, M. et al. Epidemiology of NMOSD in Catalonia: influence of the new 2015 criteria in incidence and prevalence estimates. Mult. Scler. https://doi.org/10.1177/1352458517735191 (2017).
Duignan, S. et al. Myelin oligodendrocyte glycoprotein and aquaporin-4 antibodies are highly specific in children with acquired demyelinating syndromes. Dev. Med. Child Neurol. 60, 958–962 (2018).
Mader, S. et al. Understanding the antibody repertoire in neuropsychiatric systemic lupus erythematosus and neuromyelitis optica spectrum disorder: do they share common targets? Arthritis Rheumatol. 70, 277–286 (2018).
Titulaer, M. J. et al. Overlapping demyelinating syndromes and anti-N-methyl-D-aspartate receptor encephalitis. Ann. Neurol. 75, 411–428 (2014).
Zhou, L. et al. MOG-antibody associated demyelinating disease of the CNS: a clinical and pathological study in Chinese Han patients. J. Neuroimmunol. 305, 19–28 (2017).
Zhao, G. et al. Clinical characteristics of myelin oligodendrocyte glycoprotein seropositive optic neuritis: a cohort study in Shanghai, China. J. Neurol. 265, 33–40 (2018).
Rostasy, K. et al. Persisting myelin oligodendrocyte glycoprotein antibodies in aquaporin-4 antibody negative pediatric neuromyelitis optica. Mult. Scler. 19, 1052–1059 (2013).
Chalmoukou, K. et al. Anti-MOG antibodies are frequently associated with steroid-sensitive recurrent optic neuritis. Neurol. Neuroimmunol. Neuroinflamm. 2, e131 (2015).
Elong Ngono, A. et al. Decreased frequency of circulating myelin oligodendrocyte glycoprotein B lymphocytes in patients with relapsing-remitting multiple sclerosis. J. Immunol. Res. 2015, 673503 (2015).
Hacohen, Y. et al. Diagnostic algorithm for relapsing acquired demyelinating syndromes in children. Neurology 89, 269–278 (2017). One of the largest studies of MOG-Abs in children.
Hennes, E. M. et al. Prognostic relevance of MOG antibodies in children with an acquired demyelinating syndrome. Neurology 89, 900–908 (2017). One of the largest studies of MOG-Abs in children.
Mariotto, S. et al. Clinical spectrum and IgG subclass analysis of anti-myelin oligodendrocyte glycoprotein antibody-associated syndromes: a multicenter study. J. Neurol. 264, 2420–2430 (2017).
Soelberg, K. et al. A population-based prospective study of optic neuritis. Mult. Scler. 23, 1893–1901 (2017).
Jarius, S. et al. MOG-IgG in primary and secondary chronic progressive multiple sclerosis: a multicenter study of 200 patients and review of the literature. J. Neuroinflamm. 15, 88 (2018).
Rostasy, K. et al. Anti-myelin oligodendrocyte glycoprotein antibodies in pediatric patients with optic neuritis. Arch. Neurol. 69, 752–756 (2012).
Jarius, S. et al. Screening for MOG-IgG and 27 other anti-glial and anti-neuronal autoantibodies in ‘pattern II multiple sclerosis’ and brain biopsy findings in a MOG-IgG-positive case. Mult. Scler. 22, 1541–1549 (2016).
Cobo-Calvo, A. et al. MOG antibody-related disorders: common features and uncommon presentations. J. Neurol. 264, 1945–1955 (2017).
Thompson, A. J. et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 17, 162–173 (2018).
Chen, J. J. et al. Prevalence of myelin oligodendrocyte glycoprotein and aquaporin-4-IgG in patients in the Optic Neuritis Treatment Trial. JAMA Ophthalmol. 136, 419–422 (2018).
Dale, R. C. et al. Antibodies to MOG have a demyelination phenotype and affect oligodendrocyte cytoskeleton. Neurol. Neuroimmunol. Neuroinflamm. 1, e12 (2014).
de Mol, C. L. et al. Incidence and outcome of acquired demyelinating syndromes in Dutch children: update of a nationwide and prospective study. J. Neurol. 265, 1310–1319 (2018). One of the largest studies of MOG-Abs in children.
Fernandez-Carbonell, C. et al. Clinical and MRI phenotype of children with MOG antibodies. Mult. Scler. 22, 174–184 (2016).
Ketelslegers, I. A. et al. Anti-MOG antibodies plead against MS diagnosis in an acquired demyelinating syndromes cohort. Mult. Scler. 21, 1513–1520 (2015).
Probstel, A. K. et al. Antibodies to MOG are transient in childhood acute disseminated encephalomyelitis. Neurology 77, 580–588 (2011).
Ramanathan, S. et al. Antibodies to myelin oligodendrocyte glycoprotein in bilateral and recurrent optic neuritis. Neurol. Neuroimmunol. Neuroinflamm. 1, e40 (2014).
Selter, R. C. et al. Antibody responses to EBV and native MOG in pediatric inflammatory demyelinating CNS diseases. Neurology 74, 1711–1715 (2010).
Lopez-Chiriboga, A. S. et al. Association of MOG-IgG serostatus with relapse after acute disseminated encephalomyelitis and proposed diagnostic criteria for MOG-IgG-associated disorders. JAMA Neurol. https://doi.org/10.1001/jamaneurol.2018.1814 (2018). A large study on MOG-Abs in ADEM.
Hacohen, Y. et al. Autoantibody biomarkers in childhood-acquired demyelinating syndromes: results from a national surveillance cohort. J. Neurol. Neurosurg. Psychiatry 85, 456–461 (2014).
Cobo-Calvo, A. et al. Antibodies to myelin oligodendrocyte glycoprotein in aquaporin 4 antibody seronegative longitudinally extensive transverse myelitis: clinical and prognostic implications. Mult. Scler. 22, 312–319 (2016).
Bouzar, M. et al. Neuromyelitis optica spectrum disorders with antibodies to myelin oligodendrocyte glycoprotein or aquaporin-4: clinical and paraclinical characteristics in Algerian patients. J. Neurol. Sci. 381, 240–244 (2017).
Hacohen, Y. et al. Paediatric brainstem encephalitis associated with glial and neuronal autoantibodies. Dev. Med. Child Neurol. 58, 836–841 (2016).
Baumann, M. et al. Clinical and neuroradiological differences of paediatric acute disseminating encephalomyelitis with and without antibodies to the myelin oligodendrocyte glycoprotein. J. Neurol. Neurosurg. Psychiatry 86, 265–272 (2015). A large study of MOG-Abs in ADEM.
Lechner, C. et al. Antibodies to MOG and AQP4 in children with neuromyelitis optica and limited forms of the disease. J. Neurol. Neurosurg. Psychiatry 87, 897–905 (2016).
Pandit, L. et al. Relapsing optic neuritis and isolated transverse myelitis are the predominant clinical phenotypes for patients with antibodies to myelin oligodendrocyte glycoprotein in India. Mult. Scler. J. Exp. Transl Clin. https://doi.org/10.1177/2055217316675634 (2016).
Costa, B. K. D., Passos, G. R. D., Becker, J. & Sato, D. K. MOG-IgG associated optic neuritis is not multiple sclerosis. Arq. Neuropsiquiatr. 75, 687–691 (2017).
Ogawa, R. et al. MOG antibody-positive, benign, unilateral, cerebral cortical encephalitis with epilepsy. Neurol. Neuroimmunol. Neuroinflamm. 4, e322 (2017). The first description of the association of MOG-Abs with cortical encephalitis.
Hardy, T. A. et al. Atypical inflammatory demyelinating syndromes of the CNS. Lancet Neurol. 15, 967–981 (2016). An overview of atypical demyelinating disorders.
Waters, P. et al. Multicentre comparison of a diagnostic assay: aquaporin-4 antibodies in neuromyelitis optica. J. Neurol. Neurosurg. Psychiatry 87, 1005–1015 (2016). A large, multicentre evaluation of assays for the detection of AQP4-Abs.
Graus, F. et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 15, 391–404 (2016).
Jarius, S. et al. MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J. Neuroinflamm. 15, 134 (2018).
Wells, E. et al. Neuroimmune disorders of the central nervous system in children in the molecular era. Nat. Rev. Neurol. 14, 433–445 (2018).
Thompson, A. J., Baranzini, S. E., Geurts, J., Hemmer, B. & Ciccarelli, O. Multiple sclerosis. Lancet 391, 1622–1636 (2018).
Wingerchuk, D. M. et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 85, 177–189 (2015). Clinical criteria for autoimmune encephalitis.
Jurynczyk, M. et al. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain 140, 3128–3138 (2017). One of the largest studies of MOG-Abs.
Cobo-Calvo, A. et al. Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults: the MOGADOR study. Neurology 90, e1858–e1869 (2018). One of the largest studies of MOG-Abs.
Akaishi, T. et al. Different etiologies and prognoses of optic neuritis in demyelinating diseases. J. Neuroimmunol. 299, 152–157 (2016).
Jarius, S. et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J. Neuroinflamm. 13, 280 (2016).
Jurynczyk, M. et al. Distinct brain imaging characteristics of autoantibody-mediated CNS conditions and multiple sclerosis. Brain 140, 617–627 (2017).
Sepulveda, M. et al. Clinical spectrum associated with MOG autoimmunity in adults: significance of sharing rodent MOG epitopes. J. Neurol. 263, 1349–1360 (2016).
Baumann, M. et al. MRI of the first event in pediatric acquired demyelinating syndromes with antibodies to myelin oligodendrocyte glycoprotein. J. Neurol. 265, 845–855 (2018).
Hacohen, Y. et al. ‘Leukodystrophy-like’ phenotype in children with myelin oligodendrocyte glycoprotein antibody-associated disease. Dev. Med. Child Neurol. 60, 417–423 (2018).
Hacohen, Y. et al. Disease course and treatment responses in children with relapsing myelin oligodendrocyte glycoprotein antibody-associated disease. JAMA Neurol. 75, 478–487 (2018).
Ramanathan, S. et al. Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J. Neurol. Neurosurg. Psychiatry 89, 127–137 (2018).
Biotti, D. et al. Optic neuritis in patients with anti-MOG antibodies spectrum disorder: MRI and clinical features from a large multicentric cohort in France. J. Neurol. 264, 2173–2175 (2017).
Akaishi, T., Konno, M., Nakashima, I. & Aoki, M. Intractable hiccup in demyelinating disease with anti-myelin oligodendrocyte glycoprotein (MOG) antibody. Intern. Med. 55, 2905–2906 (2016).
Jarius, S. et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 3: brainstem involvement - frequency, presentation and outcome. J. Neuroinflamm. 13, 281 (2016).
Baumann, M. et al. Children with multiphasic disseminated encephalomyelitis and antibodies to the myelin oligodendrocyte glycoprotein (MOG): extending the spectrum of MOG antibody positive diseases. Mult. Scler. 22, 1821–1829 (2016).
Huppke, P. et al. Acute disseminated encephalomyelitis followed by recurrent or monophasic optic neuritis in pediatric patients. Mult. Scler. 19, 941–946 (2013).
Wong, Y. Y. M. et al. Paediatric acute disseminated encephalomyelitis followed by optic neuritis: disease course, treatment response and outcome. Eur. J. Neurol. 25, 782–786 (2018).
Fujimori, J. et al. Bilateral frontal cortex encephalitis and paraparesis in a patient with anti-MOG antibodies. J. Neurol. Neurosurg. Psychiatry 88, 534–536 (2017).
Hamid, S. H. M. et al. Seizures and encephalitis in myelin oligodendrocyte glycoprotein IgG disease versus aquaporin 4 IgG disease. JAMA Neurol. 75, 65–71 (2018).
Mariotto, S. et al. MOG antibody seropositivity in a patient with encephalitis: beyond the classical syndrome. BMC Neurol. 17, 190 (2017).
Havla, J. et al. Myelin-oligodendrocyte-glycoprotein (MOG) autoantibodies as potential markers of severe optic neuritis and subclinical retinal axonal degeneration. J. Neurol. 264, 139–151 (2017).
Pache, F. et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 4: afferent visual system damage after optic neuritis in MOG-IgG-seropositive versus AQP4-IgG-seropositive patients. J. Neuroinflamm. 13, 282 (2016).
Ramanathan, S. et al. Radiological differentiation of optic neuritis with myelin oligodendrocyte glycoprotein antibodies, aquaporin-4 antibodies, and multiple sclerosis. Mult. Scler. 22, 470–482 (2016).
Thompson, J. et al. The importance of early immunotherapy in patients with faciobrachial dystonic seizures. Brain 141, 348–356 (2018).
Bradl, M., Reindl, M. & Lassmann, H. Mechanisms for lesion localization in neuromyelitis optica spectrum disorders. Curr. Opin. Neurol. 31, 325–333 (2018). An up-to-date review of the pathophysiology of NMOSD.
Lassmann, H., Brunner, C., Bradl, M. & Linington, C. Experimental allergic encephalomyelitis: the balance between encephalitogenic T lymphocytes and demyelinating antibodies determines size and structure of demyelinated lesions. Acta Neuropathol. 75, 566–576 (1988).
Weissert, R. et al. MHC haplotype-dependent regulation of MOG-induced EAE in rats. J. Clin. Invest. 102, 1265–1273 (1998).
Weissert, R. et al. MHC class II-regulated central nervous system autoaggression and T cell responses in peripheral lymphoid tissues are dissociated in myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis. J. Immunol. 166, 7588–7599 (2001).
Storch, M. K. et al. Autoimmunity to myelin oligodendrocyte glycoprotein in rats mimics the spectrum of multiple sclerosis pathology. Brain Pathol. 8, 681–694 (1998).
Lassmann, H. & Bradl, M. Multiple sclerosis: experimental models and reality. Acta Neuropathol. 133, 223–244 (2017).
Bettelli, E., Baeten, D., Jager, A., Sobel, R. A. & Kuchroo, V. K. Myelin oligodendrocyte glycoprotein-specific T and B cells cooperate to induce a Devic-like disease in mice. J. Clin. Invest. 116, 2393–2402 (2006).
Krishnamoorthy, G., Lassmann, H., Wekerle, H. & Holz, A. Spontaneous opticospinal encephalomyelitis in a double-transgenic mouse model of autoimmune T cell/B cell cooperation. J. Clin. Invest. 116, 2385–2392 (2006).
Burrer, R. et al. Exacerbated pathology of viral encephalitis in mice with central nervous system-specific autoantibodies. Am. J. Pathol. 170, 557–566 (2007).
Peschl, P. et al. Human antibodies against the myelin oligodendrocyte glycoprotein can cause complement-dependent demyelination. J. Neuroinflamm. 14, 208 (2017). A study that demonstrated that human MOG-Abs mediate demyelination ex vivo.
Saadoun, S. et al. Neuromyelitis optica MOG-IgG causes reversible lesions in mouse brain. Acta Neuropathol. Commun. 2, 35 (2014).
Flach, A. C. et al. Autoantibody-boosted T cell reactivation in the target organ triggers manifestation of autoimmune CNS disease. Proc. Natl Acad. Sci. USA 113, 3323–3328 (2016).
Mayer, M. C. et al. Distinction and temporal stability of conformational epitopes on myelin oligodendrocyte glycoprotein recognized by patients with different inflammatory central nervous system diseases. J. Immunol. 191, 3594–3604 (2013).
Spadaro, M. et al. Histopathology and clinical course of MOG-antibody-associated encephalomyelitis. Ann. Clin. Transl Neurol. 2, 295–301 (2015).
Kinzel, S. et al. Myelin-reactive antibodies initiate T cell-mediated CNS autoimmune disease by opsonization of endogenous antigen. Acta Neuropathol. 132, 43–58 (2016).
Spadaro, M. et al. Pathogenicity of human antibodies against myelin oligodendrocyte glycoprotein. Ann. Neurol. 84, 315–328 (2018). A study that demonstrated that human MOG-Abs mediate demyelination in vivo.
Spadaro, M. et al. Autoantibodies to MOG in a distinct subgroup of adult multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm 3, e257 (2016).
Hyun, J. W. et al. Evaluation of brain lesion distribution criteria at disease onset in differentiating MS from NMOSD and MOG-IgG-associated encephalomyelitis. Mult. Scler. https://doi.org/10.1177/1352458518761186 (2018).
Jurynczyk, M. et al. Brain lesion distribution criteria distinguish MS from AQP4-antibody NMOSD and MOG-antibody disease. J. Neurol. Neurosurg. Psychiatry 88, 132–136 (2017).
Geraldes, R. et al. The current role of MRI in differentiating multiple sclerosis from its imaging mimics. Nat. Rev. Neurol. 14, 199–213 (2018).
Kortvelyessy, P. et al. ADEM-like presentation, anti-MOG antibodies, and MS pathology: TWO case reports. Neurol. Neuroimmunol. Neuroinflamm. 4, e335 (2017).
Konig, F. B. et al. Persistence of immunopathological and radiological traits in multiple sclerosis. Arch. Neurol. 65, 1527–1532 (2008).
Di Pauli, F. et al. Fulminant demyelinating encephalomyelitis: insights from antibody studies and neuropathology. Neurol. Neuroimmunol. Neuroinflamm. 2, e175 (2015).
Wang, J. J. et al. Inflammatory demyelination without astrocyte loss in MOG antibody-positive NMOSD. Neurology 87, 229–231 (2016).
Lucchinetti, C. F., Bruck, W., Rodriguez, M. & Lassmann, H. Distinct patterns of multiple sclerosis pathology indicates heterogeneity on pathogenesis. Brain Pathol. 6, 259–274 (1996).
Kaneko, K. et al. Myelin injury without astrocytopathy in neuroinflammatory disorders with MOG antibodies. J. Neurol. Neurosurg. Psychiatry 87, 1257–1259 (2016).
Bennett, J. L. et al. Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann. Neurol. 66, 617–629 (2009).
Jarius, S. et al. Cerebrospinal fluid antibodies to aquaporin-4 in neuromyelitis optica and related disorders: frequency, origin, and diagnostic relevance. J. Neuroinflamm. 7, 52 (2010).
Yanagida, A. et al. MOG-IgG-positive multifocal myelitis with intrathecal IgG synthesis as a spectrum associated with MOG autoimmunity: two case reports. J. Neurol. Sci. 382, 40–43 (2017).
Papadopoulos, M. C., Bennett, J. L. & Verkman, A. S. Treatment of neuromyelitis optica: state-of-the-art and emerging therapies. Nat. Rev. Neurol. 10, 493–506 (2014).
Acknowledgements
The research of M.R. is supported by research grants from the Austrian Federal Ministry of Science, Research and Education (grant BIG WIG MS, Markus Reindl), the Austrian Research promotion Agency (FFG, Bridge 1 project Nr. 853209 EDNA), the Austrian Science Funds (FWF, project W1206) and the Austrian Multiple Sclerosis Research Society. Research in the laboratory of P.W. is supported by the NHS National Specialised Commissioning Group for Neuromyelitis Optica, UK and the NIHR Oxford Biomedical Research Centre, UK.
Reviewer information
Nature Reviews Neurology thanks J. de Seze, M. Levy, S. Pittock and the other anonymous reviewers for their contribution to the peer review of this work.
Review criteria
We searched PubMed for original articles that were published between 1990 and 2018 and focused on anti-MOG antibodies in neurological diseases. We used the search terms “myelin oligodendrocyte glycoprotein”, “MOG”, “antibodies”, “autoantibodies”, “cell based assay”, “immunofluorescence”, “flow cytometry”, “multiple sclerosis”, “acute disseminated encephalomyelitis”, “neuromyelitis optica”, “optic neuritis” and “myelitis” alone and in combination. All articles identified were English-language, full-text papers. We also searched the reference lists of identified articles for further relevant papers. For the summaries of study results, only studies in which there were more than 10 patients per group were included.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to researching data for the article, discussion of the content, writing, and reviewing and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The University Hospital and Medical University of Innsbruck (Austria; M.R.) receives payments for antibody assays (MOG, AQP4, and other autoantibodies) and for MOG and AQP4 antibody validation experiments organized by Euroimmun (Lübeck, Germany). P.W. is a named inventor on patents for antibody assays and has received royalties. He has received honoraria and/or consulting fees from Biogen, Euroimmun, Mereo Biopharma and Retrogenix, and has received travel grants from the Guthy-Jackson Charitable Foundation. He has received research funding from Euroimmun.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Reindl, M., Waters, P. Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nat Rev Neurol 15, 89–102 (2019). https://doi.org/10.1038/s41582-018-0112-x
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-018-0112-x
This article is cited by
-
Bilaterale Myelin-Oligodendrozyten-Glykoprotein (MOG)-Antikörper-assoziierte Optikusneuritis
Die Ophthalmologie (2024)
-
Proteomic profiling of cerebrospinal fluid in pediatric myelin oligodendrocyte glycoprotein antibody-associated disease
World Journal of Pediatrics (2024)
-
Clinical features of adult patients with positive NMDAR-IgG coexisting with MOG-IgG
Neurological Sciences (2024)
-
Risk of central nervous system demyelinating attack or optic neuritis recurrence after pediatric optic neuritis in Korea
Neurological Sciences (2024)
-
Distinct features of B cell receptors in neuromyelitis optica spectrum disorder among CNS inflammatory demyelinating diseases
Journal of Neuroinflammation (2023)