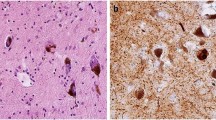
Abstract
Dementia with Lewy bodies (DLB) is the underlying aetiology of 10–15% of all cases of dementia and as such is a clinically important diagnosis. In the past few years, substantial advances have been made in understanding the genetics and pathology of this condition. For example, research has expanded our knowledge of the proteinaceous inclusions that characterize the disease, has provided an appreciation of the role of disease-associated processes such as inflammation and has revealed an association between DLB and genes such as GBA. These insights might have broader relevance to other neurodegenerative conditions and are beginning to be translated into clinical trials. In this Review, we provide clinical insights for the basic scientist and a basic science foundation for the clinician. We discuss the history of the condition; the definition of DLB; the relationship between DLB and other neurodegenerative conditions; current understanding of the pathology, genetics, clinical presentation and diagnosis of DLB; options for treatment; and potential future directions for research.
Key points
-
Dementia with Lewy bodies (DLB) is a common cause of dementia.
-
Although DLB is hard to diagnose clinically, accurate diagnosis is important as it influences management.
-
Awareness of the disorder and investigative techniques such as 123I-ioflupane single-photon emission CT can enhance diagnostic accuracy.
-
Our understanding of the genetics and pathology of the condition is growing.
-
This increased understanding is feeding through to new therapeutic strategies and clinical trials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Parkinson, J. An essay on the shaking palsy. 1817. J. Neuropsychiatry Clin. Neurosci. 14, 223–236; discussion 222 (2002).
Lewy, F. H. in Handbuch der Neurologie (ed. Lewandowsky, M. H.) 920–933 (J. Springer, 1912).
Tretiakoff, C. Contribution a l’etude de l’anatomie pathologique du Locus Niger de Soemmering: avec quelques deductions relatives a la pathogenie des troubles du tonus musculaire et de la maladie de Parkinson. (Univ. of Paris, Paris, 1919).
Kosaka, K., Oyanagi, S., Matsushita, M. & Hori, A. Presenile dementia with Alzheimer-, Pick- and Lewy-body changes. Acta Neuropathol. 36, 221–233 (1976).
Kosaka, K., Yoshimura, M., Ikeda, K. & Budka, H. Diffuse type of Lewy body disease: progressive dementia with abundant cortical Lewy bodies and senile changes of varying degree — a new disease? Clin. Neuropathol. 3, 185–192 (1984).
Gibb, W. R., Esiri, M. M. & Lees, A. J. Clinical and pathological features of diffuse cortical Lewy body disease (Lewy body dementia). Brain 110, 1131–1153 (1987).
McKeith, I. G., Perry, R. H., Fairbairn, A. F., Jabeen, S. & Perry, E. K. Operational criteria for senile dementia of Lewy body type (SDLT). Psychol. Med. 22, 911–922 (1992).
McKeith, I. G. et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology 47, 1113–1124 (1996).
Perry, R., McKeith, I. & Perry, E. (eds) Dementia with Lewy Bodies: Clinical, Pathological, and Treatment Issues (Cambridge Univ. Press, 1996).
Hansen, L. et al. The Lewy body variant of Alzheimer’s disease: a clinical and pathologic entity. Neurology 40, 1–8 (1990).
Byrne, E. J. et al. Dementia associated with cortical Lewy bodies: proposed clinical diagnostic criteria. Dement. Geriatr. Cogn. Disord. 2, 283–284 (1991).
McKeith, I., Fairbairn, A., Perry, R., Thompson, P. & Perry, E. Neuroleptic sensitivity in patients with senile dementia of Lewy body type. BMJ 305, 673–678 (1992).
Walker, Z., Costa, D. C., Ince, P., McKeith, I. G. & Katona, C. L. In-vivo demonstration of dopaminergic degeneration in dementia with Lewy bodies. Lancet 354, 646–647 (1999).
Polymeropoulos, M. H. et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276, 2045–2047 (1997).
Spillantini, M. G. et al. Alpha-synuclein in Lewy bodies. Nature 388, 839–940 (1997).
Boeve, B. F. et al. REM sleep behavior disorder and degenerative dementia: an association likely reflecting Lewy body disease. Neurology 51, 363–370 (1998).
Ince, P. G., Perry, E. K. & Morris, C. M. Dementia with Lewy bodies. A distinct non-Alzheimer dementia syndrome? Brain Pathol. 8, 299–324 (1998).
Watanabe, H. et al. Cardiac (123)I-meta-iodobenzylguanidine (MIBG) uptake in dementia with Lewy bodies: comparison with Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 70, 781–783 (2001).
Yoshita, M., Taki, J. & Yamada, M. A clinical role for [(123)I]MIBG myocardial scintigraphy in the distinction between dementia of the Alzheimer’s-type and dementia with Lewy bodies. J. Neurol. Neurosurg. Psychiatry 71, 583–588 (2001).
McKeith, I. et al. Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. Lancet Neurol. 6, 305–313 (2007).
Goedert, M., Spillantini, M. G., Del Tredici, K. & Braak, H. 100 years of Lewy pathology. Nat. Rev. Neurol. 9, 13–24 (2013).
McKeith, I. G. et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB consortium. Neurology 89, 88–100 (2017).
World Health Organisation. ICD-10 version 2015. International statistical classification of diseases and related health problems, 10th revision. WHO http://apps.who.int/classifications/icd10/browse/2015/en (2015).
Alzheimer’s Disease International. World Alzheimer report 2015. ADI https://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf (2015).
Vann Jones, S. A. & O’Brien, J. T. The prevalence and incidence of dementia with Lewy bodies: a systematic review of population and clinical studies. Psychol. Med. 44, 673–683 (2014).
Litvan, I. I. et al. Accuracy of the clinical diagnoses of lewy body disease, parkinson disease, and dementia with lewy bodies: a clinicopathologic study. Arch. Neurol. 55, 969–978 (1998).
Rizzo, G. et al. Accuracy of clinical diagnosis of dementia with Lewy bodies: a systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 89, 358–366 (2017).
Karantzoulis, S. & Galvin, J. E. Distinguishing Alzheimer’s disease from other major forms of dementia. Expert Rev. Neurother. 11, 1579–1591 (2011).
Merdes, A. R. et al. Influence of Alzheimer pathology on clinical diagnostic accuracy in dementia with Lewy bodies. Neurology 60, 1586–1590 (2003).
McKeith, I. G. et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB consortium. Neurology 65, 1863–1872 (2005).
Tiraboschi, P. et al. Clinicians’ ability to diagnose dementia with Lewy bodies is not affected by beta-amyloid load. Neurology 84, 496–499 (2015).
Jakes, R., Spillantini, M. G. & Goedert, M. Identification of two distinct synucleins from human brain. FEBS Lett. 345, 27–32 (1994).
Clayton, D. F. & George, J. M. The synucleins: a family of proteins involved in synaptic function, plasticity, neurodegeneration and disease. Trends Neurosci. 21, 249–254 (1998).
Ritchie, C. M. & Thomas, P. J. Alpha-synuclein truncation and disease. Health 4, 1167–1177 (2012).
Tofaris, G. K. & Spillantini, M. G. Physiological and pathological properties of alpha-synuclein. Cell. Mol. Life Sci. 64, 2194–2201 (2007).
Trojanowski, J. Q. & Lee, V. M. Aggregation of neurofilament and alpha-synuclein proteins in Lewy bodies: implications for the pathogenesis of Parkinson disease and Lewy body dementia. Arch. Neurol. 55, 151–152 (1998).
Wakabayashi, K. et al. The Lewy body in Parkinson’s disease and related neurodegenerative disorders. Mol. Neurobiol. 47, 495–508 (2013).
Wakabayashi, K., Tanji, K., Mori, F. & Takahashi, H. The Lewy body in Parkinson’s disease: molecules implicated in the formation and degradation of alpha-synuclein aggregates. Neuropathology 27, 494–506 (2007).
Baba, M. et al. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am. J. Pathol. 152, 879–884 (1998).
Wolozin, B. & Behl, C. Mechanisms of neurodegenerative disorders: part 1: protein aggregates. Arch. Neurol. 57, 793–796 (2000).
Tiraboschi, P. et al. Early and widespread cholinergic losses differentiate dementia with Lewy bodies from Alzheimer disease. Arch. Gen. Psychiatry 59, 946–951 (2002).
Ulmer, T. S., Bax, A., Cole, N. B. & Nussbaum, R. L. Structure and dynamics of micelle-bound human alpha-synuclein. J. Biol. Chem. 280, 9595–9603 (2005).
Lee, H. J., Bae, E. J. & Lee, S. J. Extracellular alpha—synuclein-a novel and crucial factor in Lewy body diseases. Nat. Rev. Neurol. 10, 92–98 (2014).
Louis, E. D. et al. Essential tremor associated with focal nonnigral Lewy bodies: a clinicopathologic study. Arch. Neurol. 62, 1004–1007 (2005).
Newell, K. L. et al. Alpha-synuclein immunoreactivity is present in axonal swellings in neuroaxonal dystrophy and acute traumatic brain injury. J. Neuropathol. Exp. Neurol. 58, 1263–1268 (1999).
Higashi, S. et al. Concurrence of TDP-43, tau and alpha-synuclein pathology in brains of Alzheimer’s disease and dementia with Lewy bodies. Brain Res. 1184, 284–294 (2007).
Mori, F. et al. Valosin-containing protein immunoreactivity in tauopathies, synucleinopathies, polyglutamine diseases and intranuclear inclusion body disease. Neuropathology 33, 637–644 (2013).
Morales, I., Jimenez, J. M., Mancilla, M. & Maccioni, R. B. Tau oligomers and fibrils induce activation of microglial cells. J. Alzheimers Dis. 37, 849–856 (2013).
Maphis, N. et al. Reactive microglia drive tau pathology and contribute to the spreading of pathological tau in the brain. Brain 138, 1738–1755 (2015).
King, E. et al. Peripheral inflammation in prodromal Alzheimer’s and Lewy body dementias. J. Neurol. Neurosurg. Psychiatry 89, 339–345 (2017).
Campbell, B. C. et al. The solubility of alpha-synuclein in multiple system atrophy differs from that of dementia with Lewy bodies and Parkinson’s disease. J. Neurochem. 76, 87–96 (2001).
Dickson, D. W. et al. Neuropathological assessment of Parkinson’s disease: refining the diagnostic criteria. Lancet Neurol. 8, 1150–1157 (2009).
Braak, H. & Del Tredici, K. Neuropathological staging of brain pathology in sporadic Parkinson’s disease: separating the wheat from the chaff. J. Parkinsons Dis. 7, S73–S87 (2017).
Braak, H. et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 24, 197–211 (2003).
Hyman, B. T. et al. National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 8, 1–13 (2012).
Dickson, D. W. & Fujishiro, H. In dementia with Lewy bodies, Braak stage determines phenotype, not Lewy body distribution. Neurology 70, 2087–2089 (2008).
Jellinger, K. A. A critical evaluation of current staging of alpha-synuclein pathology in Lewy body disorders. Biochim. Biophys. Acta 1792, 730–740 (2009).
Tsuang, D. W., DiGiacomo, L. & Bird, T. D. Familial occurrence of dementia with Lewy bodies. Am. J. Geriatr. Psychiatry 12, 179–188 (2004).
Wakabayashi, K. et al. Autosomal dominant diffuse Lewy body disease. Acta Neuropathol. 96, 207–210 (1998).
Tsuang, D. et al. APOE ε4 increases risk for dementia in pure synucleinopathies. JAMA Neurol. 70, 223 (2013).
Keogh, M. J. et al. Exome sequencing in dementia with Lewy bodies. Transl Psychiatry 6, e728 (2016).
Tsuang, D. et al. GBA mutations increase risk for Lewy body disease with and without Alzheimer disease pathology. Neurology 79, 1944–1950 (2012).
Shiner, T. et al. High frequency of GBA gene mutations in dementia with Lewy bodies among Ashkenazi Jews. JAMA Neurol. 73, 1448 (2016).
Nalls, M. A. et al. A multicenter study of glucocerebrosidase mutations in dementia with Lewy bodies. JAMA Neurol. 70, 727 (2013).
Guerreiro, R. et al. Investigating the genetic architecture of dementia with Lewy bodies: a two-stage genome-wide association study. Lancet Neurol. 17, 64–74 (2018).
Price, A. et al. Mortality in dementia with Lewy bodies compared with Alzheimer’s dementia: a retrospective naturalistic cohort study. BMJ Open 7, e017504 (2017).
Harada, C. N., Natelson Love, M. C. & Triebel, K. Normal cognitive aging. Clin. Geriatr. Med. 29, 737–752 (2013).
Gangisetty, O., Cabrera, M. A. & Murugan, S. Impact of epigenetics in aging and age related neurodegenerative diseases. Front. Biosci. 23, 1445–1464 (2018).
Petersen, R. C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 256, 183–194 (2004).
Mioshi, E., Dawson, K., Mitchell, J., Arnold, R. & Hodges, J. R. The Addenbrooke’s Cognitive Examination Revised (ACE-R): a brief cognitive test battery for dementia screening. Int. J. Geriatr. Psychiatry 21, 1078–1085 (2006).
Kelland, D. Z. & Lewis, R. F. The Digit Vigilance Test: reliability, validity, and sensitivity to diazepam. Arch. Clin. Neuropsychol 11, 339–344 (1996).
Palmqvist, S., Hansson, O., Minthon, L. & Londos, E. Practical suggestions on how to differentiate dementia with Lewy bodies from Alzheimer’s disease with common cognitive tests. Int. J. Geriatr. Psychiatry 24, 1405–1412 (2009).
Sadiq, D. et al. Prodromal dementia with Lewy bodies and prodromal Alzheimer’s disease: a comparison of the cognitive and clinical profiles. J. Alzheimers Dis. 58, 463–470 (2017).
Albert, M. S. et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 270–279 (2011).
Donaghy, P. C. et al. Neuropsychiatric symptoms and cognitive profile in mild cognitive impairment with Lewy bodies. Psychol. Med. 48, 2384–2390 (2018).
Donaghy, P. C., O’Brien, J. T. & Thomas, A. J. Prodromal dementia with Lewy bodies. Psychol. Med. 45, 259–268 (2015).
Harding, A. J., Broe, G. A. & Halliday, G. M. Visual hallucinations in Lewy body disease relate to Lewy bodies in the temporal lobe. Brain 125, 391–403 (2002).
Khundakar, A. A. et al. Analysis of primary visual cortex in dementia with Lewy bodies indicates GABAergic involvement associated with recurrent complex visual hallucinations. Acta Neuropathol. Commun. 4, 66 (2016).
Erskine, D. et al. Neuronal loss and alpha-synuclein pathology in the superior colliculus and its relationship to visual hallucinations in dementia with Lewy bodies. Am. J. Geriatr. Psychiatry 25, 595–604 (2017).
Onofrj, M. et al. Cohort study of prevalence and phenomenology of tremor in dementia with Lewy bodies. J. Neurol. 260, 1731–1742 (2013).
Donaghy, P. C. & McKeith, I. G. The clinical characteristics of dementia with Lewy bodies and a consideration of prodromal diagnosis. Alzheimers Res. Ther. 6, 46 (2014).
Chandra, R., Hiniker, A., Kuo, Y. M., Nussbaum, R. L. & Liddle, R. A. α-Synuclein in gut endocrine cells and its implications for Parkinson’s disease. JCI Insight 2, 92295 (2017).
Allan, L. M. et al. Autonomic dysfunction in dementia. J. Neurol. Neurosurg. Psychiatry 78, 671–677 (2007).
Low, P. A., Tomalia, V. A. & Park, K.-J. Autonomic function tests: some clinical applications. J. Clin. Neurol. 9, 1–8 (2013).
Sakakibara, R. et al. Lower urinary tract function in dementia of Lewy body type. J. Neurol. Neurosurg. Psychiatry 76, 729–732 (2005).
Tateno, F. et al. Lower urinary tract function in dementia with Lewy bodies (DLB). Mov. Disord. 30, 411–415 (2015).
Cummings, J. L. et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 44, 2308–2314 (1994).
Takahashi, S., Mizukami, K., Yasuno, F. & Asada, T. Depression associated with dementia with Lewy bodies (DLB) and the effect of somatotherapy. Psychogeriatrics 9, 56–61 (2009).
Chiu, P.-Y. et al. Depression in dementia with Lewy bodies: a comparison with Alzheimer’s disease. PLOS ONE 12, e0179399 (2017).
Barber, R., Ballard, C., McKeith, I. G., Gholkar, A. & O’Brien, J. T. MRI volumetric study of dementia with Lewy bodies: a comparison with AD and vascular dementia. Neurology 54, 1304–1309 (2000).
Burton, E. J. et al. Medial temporal lobe atrophy on MRI differentiates Alzheimer’s disease from dementia with Lewy bodies and vascular cognitive impairment: a prospective study with pathological verification of diagnosis. Brain 132, 195–203 (2009).
Mak, E. et al. Differential atrophy of hippocampal subfields: a comparative study of dementia with lewy bodies and Alzheimer disease. Am. J. Geriatr. Psychiatry 24, 136–143 (2016).
Roquet, D. et al. Insular atrophy at the prodromal stage of dementia with Lewy bodies: a VBM DARTEL study. Sci. Rep. 7, 9437 (2017).
Colloby, S. J., Elder, G. J., Rabee, R., O’Brien, J. T. & Taylor, J.-P. Structural grey matter changes in the substantia innominata in Alzheimer’s disease and dementia with Lewy bodies: a DARTEL-VBM study. Int. J. Geriatr. Psychiatry 32, 615–623 (2017).
Watson, R., O’Brien, J. T., Barber, R. & Blamire, A. M. Patterns of gray matter atrophy in dementia with Lewy bodies: a voxel-based morphometry study. Int. Psychogeriatr. 24, 532–540 (2012).
Burton, E. J. et al. Patterns of cerebral atrophy in dementia with Lewy bodies using voxel-based morphometry. Neuroimage 17, 618–630 (2002).
Su, L. et al. Tissue microstructural changes in dementia with Lewy bodies revealed by quantitative MRI. J. Neurol. 262, 165–172 (2015).
Taylor, J. P., Firbank, M. & O’Brien, J. T. Visual cortical excitability in dementia with Lewy bodies. Br. J. Psychiatry 208, 497–498 (2016).
Galvin, J. E., Price, J. L., Yan, Z., Morris, J. C. & Sheline, Y. I. Resting bold fMRI differentiates dementia with Lewy bodies versus Alzheimer disease. Neurology 76, 1797–1803 (2011).
Taylor, J.-P. et al. Visual cortex in dementia with Lewy bodies: magnetic resonance imaging study. Br. J. Psychiatry 200, 491–498 (2012).
Graff-Radford, J. et al. Dementia with Lewy bodies: basis of cingulate island sign. Neurology 83, 801–809 (2014).
Iizuka, T., Iizuka, R. & Kameyama, M. Cingulate island sign temporally changes in dementia with Lewy bodies. Sci. Rep. 7, 14745 (2017).
O’Brien, J. T. et al. 18F-FDG PET and perfusion SPECT in the diagnosis of Alzheimer and Lewy Body Dementias. J. Nucl. Med. 55, 1959–1965 (2014).
Lobotesis, K. et al. Occipital hypoperfusion on SPECT in dementia with Lewy bodies but not AD. Neurology 56, 643–649 (2001).
O’Brien, J. T. et al. Dopamine transporter loss visualized with FP-CIT SPECT in the differential diagnosis of dementia with Lewy bodies. Arch. Neurol. 61, 919 (2004).
O’Brien, J. T. et al. Diagnostic accuracy of 123I-FP-CIT SPECT in possible dementia with Lewy bodies. Br. J. Psychiatry 194, 34–39 (2009).
Mak, E., Su, L., Williams, G. B. & O’Brien, J. T. Neuroimaging characteristics of dementia with Lewy bodies. Alzheimers Res. Ther. 6, 18 (2014).
McCleery, J. et al. Dopamine transporter imaging for the diagnosis of dementia with Lewy bodies. Cochrane Database Syst. Rev. 1, CD010633 (2015).
Nuvoli, S. et al. 123I-ioflupane brain SPECT and 123I-MIBG cardiac planar scintigraphy combined use in uncertain parkinsonian disorders. Medicine 96, e6967 (2017).
Surendranathan, A. & O’Brien, J. T. Clinical imaging in dementia with Lewy bodies. Evid. Based Ment. Health 21, 61–65 (2018).
Bradley, F. B., Michael, H. S. & Tanis, J. F. REM sleep behavior disorder in Parkinson’s disease and dementia with Lewy bodies. J. Geriatr. Psychiatry Neurol. 17, 146–157 (2004).
Terzaghi, M. et al. Analysis of video-polysomnographic sleep findings in dementia with Lewy bodies. Mov. Disord. 28, 1416–1423 (2013).
Pao, W. C. et al. Polysomnographic findings in dementia with Lewy bodies. Neurologist 19, 1–6 (2013).
Bonanni, L. et al. EEG comparisons in early Alzheimer’s disease, dementia with Lewy bodies and Parkinson’s disease with dementia patients with a 2-year follow-up. Brain 131, 690–705 (2008).
Bonanni, L. et al. Quantitative electroencephalogram utility in predicting conversion of mild cognitive impairment to dementia with Lewy bodies. Neurobiol. Aging 36, 434–445 (2015).
Colloby, S. J. et al. Multimodal EEG-MRI in the differential diagnosis of Alzheimer’s disease and dementia with Lewy bodies. J. Psychiatr. Res. 78, 48–55 (2016).
Peraza, L. R. et al. Electroencephalographic derived network differences in Lewy body dementia compared to Alzheimer’s disease patients. Sci. Rep. 8, 4637 (2018).
Zupancic, M., Mahajan, A. & Handa, K. Dementia with Lewy bodies. Prim. Care Companion CNS Disord. 13, PCC.11r01190 (2011).
Kane, J. P. M. et al. Clinical prevalence of Lewy body dementia. Alzheimers Res. Ther. 10, 19 (2018).
Schneider-Thoma, J. et al. Second-generation antipsychotic drugs and short-term mortality: a systematic review and meta-analysis of placebo-controlled randomised controlled trials. Lancet Psychiatry 5, 653–663 (2018).
Bonanni, L. et al. Akinetic crisis in dementia with Lewy bodies. J. Neurol. Neurosurg. Psychiatry 87, 1123–1126 (2016).
Aarsland, D. et al. Neuroleptic sensitivity in Parkinson’s disease and parkinsonian dementias. J. Clin. Psychiatry 66, 633–637 (2005).
Tiraboschi, P. et al. Cholinergic dysfunction in diseases with Lewy bodies. Neurology 54, 407–411 (2000).
Shimada, H. et al. Dementia with Lewy bodies can be well-differentiated from Alzheimer’s disease by measurement of brain acetylcholinesterase activity-a [11C]MP4A PET study. Int. J. Geriatr. Psychiatry 30, 1105–1113 (2015).
McKeith, I. et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet 356, 2031–2036 (2000).
Rolinski, M., Fox, C., Maidment, I. & McShane, R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson’s disease dementia and cognitive impairment in Parkinson’s disease. Cochrane Database Syst. Rev. 3, CD006504 (2012).
Stinton, C. et al. Pharmacological management of Lewy body dementia: a systematic review and meta-analysis. Am. J. Psychiatry 172, 731–742 (2015).
Wang, H. F. et al. Efficacy and safety of cholinesterase inhibitors and memantine in cognitive impairment in Parkinson’s disease, Parkinson’s disease dementia, and dementia with Lewy bodies: systematic review with meta-analysis and trial sequential analysis. J. Neurol. Neurosurg. Psychiatry 86, 135–143 (2015).
Aarsland, D. et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet. Neurol. 8, 613–618 (2009).
Leroi, I., Overshott, R., Byrne, E. J., Daniel, E. & Burns, A. Randomized controlled trial of memantine in dementia associated with Parkinson’s disease. Mov. Disord. 24, 1217–1221 (2009).
Emre, M. et al. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 9, 969–977 (2010).
Brennan, L. et al. Memantine and cognition in Parkinson’s disease dementia/dementia with Lewy bodies: a meta-analysis. Mov. Disord. Clin. Pract. 3, 161–167 (2016).
Murata, M. et al. Adjunct zonisamide to levodopa for DLB parkinsonism: a randomized double-blind phase 2 study. Neurology 90, e664–e672 (2018).
Satoh, M. et al. Improved visual hallucination by donepezil and occipital glucose metabolism in dementia with lewy bodies: the Osaki-Tajiri project. Eur. Neurol. 64, 337–344 (2010).
Desmarais, P., Massoud, F., Filion, J., Nguyen, Q. D. & Bajsarowicz, P. Quetiapine for psychosis in Parkinson disease and neurodegenerative parkinsonian disorders. J. Geriatr. Psychiatry Neurol. 29, 227–236 (2016).
Standards of Practice Committee. Best practice guide for the treatment of REM sleep behavior disorder (RBD). J. Clin. Sleep Med. 6, 85–95 (2010).
Connors, M. H. et al. Non-pharmacological interventions for Lewy body dementia: a systematic review. Psychol. Med. 48, 1749–1758 (2017).
Halliday, M. et al. Repurposed drugs targeting eIF2α-P-mediated translational repression prevent neurodegeneration in mice. Brain 140, 1768–1783 (2017).
Ravikumar, B. et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat. Genet. 36, 585–595 (2004).
Bevan-Jones, W. R. et al. Neuroimaging of inflammation in memory and related other disorders (NIMROD) study protocol: a deep phenotyping cohort study of the role of brain inflammation in dementia, depression and other neurological illnesses. BMJ Open 7, e013187 (2017).
McKeith, I. G. et al. HEADWAY-DLB: a multinational study evaluating the safety and efficacy of intepirdine (rvt-101) in dementia with lewy bodies. Alzheimers Dement. 13, 936 (2017).
PyMOL Molecular Graphics System version 2.0 (Schrodinger LLC, New York, NY, USA).
Mackenzie, I. R. A. Activated microglia in dementia with Lewy bodies. Neurology 55, 132–134 (2000).
Slaets, S. et al. Diagnostic value of MIBG cardiac scintigraphy for differential dementia diagnosis. Int. J. Geriatr. Psychiatry 30, 864–869 (2015).
Nakajima, K., Yoshita, M., Matsuo, S., Taki, J. & Kinuya, S. Iodine-123-MIBG sympathetic imaging in Lewy-body diseases and related movement disorders. Q. J. Nucl. Med. Mol. Imaging 52, 378–387 (2008).
Iwasa, K. et al. Decreased myocardial 123I-MIBG uptake in Parkinson’s disease. Acta Neurol. Scand. 97, 303–306 (1998).
Dubois, B. & Albert, M. L. Amnestic MCI or prodromal Alzheimer’s disease? Lancet. Neurol. 3, 246–248 (2004).
Metzler-Baddeley, C. A review of cognitive impairments in dementia with Lewy bodies relative to Alzheimer’s disease and Parkinson’s disease with dementia. Cortex 43, 583–600 (2007).
Dubois, B. et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 13, 614–629 (2014).
Watson, G. S. & Leverenz, J. B. Profile of cognitive impairment in Parkinson’s disease. Brain Pathol. 20, 640–645 (2010).
Acknowledgements
The authors thank A. L. Kantsadi (Department of Biochemistry, University of Oxford, UK), for representing α-synuclein monomer as a cartoon. The authors thank E. Mak (Department of Psychiatry, University of Cambridge, UK), for providing the MRI and 123I-ioflupane single-photon emission CT images in figure 4. The authors express appreciation and gratitude to the Windsor Unit and all associated staff for supporting dementia with Lewy bodies (DLB) studies. The authors thank the Cambridgeshire and Peterborough NHS Foundation Trust for funding N.A.A.’s Fellowship. J.T.O. is supported by the UK National Institute for Health Research (NIHR) Cambridge Biomedical Research Centre. J.T.O. has acted as a consultant for GE Healthcare, Avid/Lilly, TauRx, Axon, Axovant and Eisai and has received grant income from Avid/Lilly and Alliance Medical. B.R.U. was a principal investigator in the recent trial of intepirdine in DLB.
Reviewer information
Nature Reviews Neurology thanks G. Halliday, L. Bonanni and E. Londos for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Lewy Body Dementia Association: https://www.lbda.org
International Psychogeriatric Association: http://www.ipa-online.org
Lewy Body Society: https://www.lewybody.org
Alzheimer’s Research UK: https://www.alzheimersresearchuk.org
Alzheimer’s Society: https://www.alzheimers.org.uk
Windsor Research Unit — NIHR: http://www.cpft.nhs.uk/RandD/windsor-research-unit-2.htm
The Michael J. Fox Foundation for Parkinson’s Research: http://www.michaeljfox.org
Rights and permissions
About this article
Cite this article
Arnaoutoglou, N.A., O’Brien, J.T. & Underwood, B.R. Dementia with Lewy bodies — from scientific knowledge to clinical insights. Nat Rev Neurol 15, 103–112 (2019). https://doi.org/10.1038/s41582-018-0107-7
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-018-0107-7
This article is cited by
-
Immunological aspects of central neurodegeneration
Cell Discovery (2024)
-
PIWI-interacting RNA expression regulates pathogenesis in a Caenorhabditis elegans model of Lewy body disease
Nature Communications (2023)
-
Functional brain networks in the evaluation of patients with neurodegenerative disorders
Nature Reviews Neurology (2023)
-
DOPA decarboxylase is an emerging biomarker for Parkinsonian disorders including preclinical Lewy body disease
Nature Aging (2023)
-
YTHDF2 facilitates aggresome formation via UPF1 in an m6A-independent manner
Nature Communications (2023)