Abstract
The vast increase in Alzheimer disease (AD) worldwide has grave implications for individuals, family support systems and the health-care systems that will attempt to cope with the disease. Early markers of the disease are essential for efficient selection of clinical trial participants for drug development and for timely treatment once an intervention becomes available. There is avid interest in noninvasive, inexpensive markers that have the potential to identify prodromal AD. This Review considers sensory impairments that have the potential to serve as early indicators of AD, with a focus on olfaction, hearing and vision. Current evidence regarding the potential markers of AD in each modality is examined, with a particular emphasis on olfaction and current findings that olfactory function is associated with prodromal AD. Research suggests that olfactory impairment is associated with other markers that signal the emergence of prodromal AD. Auditory impairment is associated with dementia in epidemiological studies and visual system deficits have been reported in AD; however, the emergence of these deficits in prodromal AD is unclear. Further research is necessary to address the relative sensitivity and specificity of olfactory, auditory and visual measures for the detection of prodromal AD.
Key points
-
Brain areas involved in olfactory processing — such as the olfactory bulb and entorhinal cortex — show very early neuropathology in Alzheimer disease (AD), suggesting that olfactory function is a potential biomarker.
-
Odour memory and odour identification are profoundly affected in individuals with AD and in those at risk of AD, suggesting that several measures of olfactory function signal preclinical AD.
-
Olfaction currently shows the greatest promise of all sensory biomarkers of AD; odour identification impairment predicts conversion to mild cognitive impairment (MCI) in cognitively normal individuals and conversion to AD in patients with amnestic MCI, and shows substantial relationships with other biomarkers of AD.
-
Epidemiological research indicates that hearing loss at baseline is associated with cognitive impairment and incident dementia over a 10-year period.
-
Visual changes in prodromal and clinical AD include retinal thinning in AD, impaired contrast sensitivity in AD and mild MCI, and abnormal pupillary responses that reflect cognitive load in AD and amnestic MCI.
-
Numerous sensory measures in olfaction, hearing and vision are emerging as potential markers of AD; further research is necessary to determine their relationship to other biomarkers and to assess their sensitivity and specificity for early prediction of AD.
Similar content being viewed by others
Introduction
Alzheimer disease (AD) is a devastating neurodegenerative disorder that affects 46.8 million people globally1 and is rising at an alarming rate as the population ages. The AD epidemic presents a major public health issue and has profound economic consequences for the individual and for society. In the USA alone, AD costs >US$200 billion per year in medical expenses, caregiving and lost income2. AD erodes memory and cognition and leaves patients unable to care for themselves. Despite intense efforts to develop effective pharmacological interventions, AD is currently incurable. As drugs for AD become available, determining the individuals who are at risk of AD, predicting disease onset and progression, and characterizing the effectiveness of interventions will be crucial if treatment is to be applied before substantial neurological compromise. Sensory impairments have the potential to serve as very early disease indicators.
The central focus of this Review is the emerging evidence that olfactory impairments might reflect the onset of AD, amnestic mild cognitive impairment (MCI) and the presence of amyloid-β (Aβ) and tau pathology in cognitively normal adults. Evidence is discussed from laboratory studies, Alzheimer Disease Research Center (ADRC) populations, epidemiological studies and community samples that supports the scientific premise that olfactory dysfunction occurs before cognitive impairment and the development of dementia in patients with AD and in those with a genetic risk of AD or with amnestic MCI and, thus, could be an inexpensive, noninvasive biomarker.
Although a complete understanding of the roles of Aβ and tau, the hallmarks of AD, is still developing, accumulation of tau in the presence of Aβ presages development of cognitive symptoms in AD3. No single biomarker or risk factor has been identified that completely predicts disease onset or progression of AD; however, the levels of Aβ and tau in cerebrospinal fluid (CSF) and reduced volume of the hippocampus on structural MRI are used as biomarkers in research and drug trials4. This Review considers emerging findings that suggest that olfactory impairment shows an association with CSF biomarkers of AD — such as the ratio of total levels of tau (t-tau) or levels of tau phosphorylated at threonine 181 (P181-tau) to levels of Aβ1–42 — in individuals with a heightened risk of AD5 and that olfactory impairment is associated with hippocampal atrophy in AD6,7 and entorhinal cortical thinning in preclinical disease7. Several studies from the past few years have established that hearing loss is associated with the development of cognitive impairment and dementia8,9,10. Furthermore, retinal thinning, decreased contrast sensitivity and reduced pupillary response have been demonstrated in the visual system in people with AD.
Thus, multiple potential markers exist in sensory domains, and this Review discusses emerging evidence and important unanswered questions. The need for further research to determine the relative sensitivity and specificity of potential novel, noninvasive biomarkers for AD is also considered.
Populations at risk of AD
Populations at an increased risk of AD include those with genetic risk, those with MCI and those with Aβ deposition in the brain. Altered sensory function in individuals without dementia in these populations has the potential to contribute to early diagnosis of disease.
The apolipoprotein E (APOE)*ε4 allele is the most powerful genetic predictor of sporadic AD11. At least one APOE*ε4 allele is present in 80% of patients with AD. APOE*ε4 carriers are at an increased risk of AD, develop the disease earlier than non-carriers11 and have lower levels of the AD biomarker Aβ1–42. As many as 47% of individuals who carry one allele of APOE*ε4 and 90% of those who carry two alleles will develop AD11. This increased risk of AD makes APOE*ε4 carriers an especially relevant population in which to study the development of the disease in the silent preclinical stage. Clinical trials can be enriched with participants who are carriers of the APOE*ε4 allele because they have an increased likelihood of undergoing transitions in cognitive function over the course of a trial.
Individuals with MCI exhibit mild impairment in memory and cognition, but have intact global cognition and are able to perform activities of daily living12. These individuals are expected to develop AD at a rate of ~15% per year. Among individuals with MCI, those with primary memory impairment are classified as amnestic MCI and have an increased likelihood of converting to AD13.
Cognitively normal individuals who are amyloid positive have an increased risk of developing AD14. CSF levels of Aβ1–42 fall as amyloid accumulates in the brain and thus serve as a useful biomarker. Similarly, amyloid burden assessed by PET is a potential biomarker. However, the presence of amyloid is a risk factor rather than an indicator of disease, as a substantial proportion of patients with amyloid positive PET scans are cognitively normal. Patients who are amyloid positive and APOE*ε4 carriers are at an increased risk of transition to MCI and AD.
Olfaction
Post-mortem studies indicate that pathological changes in AD, particularly neurofibrillary tangles, occur very early in the disease in entorhinal and transentorhinal areas, the anterior olfactory nucleus and the olfactory bulb — regions involved in olfactory information processing. Importantly, AD neuropathology emerges in these areas before the onset of clinical symptomatology15,16,17,18,19,20,21,22,23. Braak staging16,17,18,24,25,26 describes very early AD pathology in entorhinal and transentorhinal areas. As disease progresses, tangles develop in a characteristic pattern that shows heaviest involvement of the entorhinal cortex, perirhinal cortex, CA1 and subicular area of the hippocampus, and amygdala, before tangle pathology is observed in the cortices, which suggests that areas that are key to olfactory information processing have heavy tau pathology15,27. The Braak and Tredici hypothesis suggests very early involvement of the locus coeruleus26, a structure that projects to the olfactory bulb, in AD. The pattern of early and consistent involvement of these areas in AD neuropathology suggests that olfactory function might be particularly vulnerable in AD and might reflect the disease process (Figs 1–3).
Areas crucial to olfactory processing are well-established to show early neurodegenerative changes in Alzheimer disease, including the locus coeruleus, olfactory bulb, prepiriform cortex, entorhinal cortex and hippocampus. Adapted with permission from ref.15, Wiley-VCH.
Tau pathology (orange) occurs in the entorhinal cortex very early in Alzheimer disease. Lesions in the entorhinal cortex disrupt the flow of incoming olfactory information to the hippocampus and would be expected to affect performance on odour memory and odour identification tasks. Reproduced with permission from ref.24, Springer Nature Limited.
Odour identification
Odour identification involves detection and recognition of a previously smelled odour and recall of objects, sources, and/or names associated with the odour. A number of widely used tests of odour identification have been employed in the investigation of individuals with AD and those at risk of AD (Box 1).
Alzheimer disease
Following initial observations by Waldton28, identification of odours is well-established to be profoundly impaired in patients with AD. The results are robust across a large number of studies that have used a variety of odorants and odour identification test formats28,29,30,31,32,33,34,35,36. Studies indicate that ~85–90% of patients with AD have impaired odour identification34. The sensitivity and specificity for discrimination of patients with AD from healthy controls varies somewhat across measures but the correct classification rate has been reported to be ~85%32,34. Impairment is increased in patients with AD who have the APOE*ε4 allele and dramatically so in those with two APOE*ε4 alleles33. Two meta-analyses comparing relative effect sizes of a number of existing olfactory tests found robust mean effect sizes for odour identification both for AD (d = 2.05)36 and for MCI (d = −0.86)37, suggesting that olfactory deficits have promise as potential biomarkers; however, these analyses are limited by the low number of studies that have used measures other than odour identification. Sensory, cognitive and semantic abilities are required for accurate odour identification; thus, compromised function of individual abilities or of a combination of abilities might underlie the profound impairment of odour identification in AD.
Populations at risk of AD
Odour identification is impaired in cognitively normal elderly adults with the APOE*ε4 allele38,39,40. This impairment has been confirmed in large population-based studies39,40. Notably, individuals with anosmia (that is, a complete loss of olfactory function) with the APOE*ε4 allele show an increased rate of development of AD over a 2 year period compared with individuals with normal olfaction39. Importantly, odour identification impairment exists in APOE*ε4 carriers before impairment on standard measures of dementia such as the Dementia Rating Scale (DRS41)42. In one study of cognitively normal APOE*ε4 carriers, odour identification declined substantially over a 4-year period whereas DRS scores remained stable42, suggesting that odour identification could be more sensitive than DRS scores in detecting very early decline.
Homozygous APOE*ε4/ε4 individuals diagnosed with AD have a higher amyloid burden and a more rapid rate of cognitive decline than heterozygous APOE*ε3/ε4 carriers. Accordingly, homozygous APOE*ε4/ε4 carriers also show greater impairments than heterozygous APOE*ε3/ε4 carriers in odour identification that could surpass impairments in other domains, such as vision. When comparing the San Diego Odor Identification Test with the Boston Naming Test (a visual confrontational naming task that requires identification of line drawings and is used in many neuropsychology batteries for assessing AD) in patients with AD, homozygous APOE*ε4/ε4 individuals showed significantly more impairment of odour identification than heterozygous APOE*ε3/ε4 individuals and homozygous APOE*ε3/ε3 individuals, but no statistically significant group differences were detected in the Boston Naming Test33. In the Betula Study, a large population study in Sweden, decline in odour identification was observed in middle-aged individuals and the rate of this decline was twice as rapid in homozygous APOE*ε4/ε4 individuals as in those who did not carry an APOE*ε4 allele43.
Impairment of odour identification has also been well established in MCI14,42,43,44,45,46,47,48. Among cognitively normal individuals, those with poor odour identification scores at baseline are more likely to develop MCI than those with good odour identification scores45,46,48, and individuals with MCI who have odour identification impairment are more likely to show progressive cognitive decline35,37 and convert to AD35 than those without such impairment. Several studies have found that patients with the amnestic subtype of MCI show increased impairment of odour identification compared with non-amnestic MCI14,47,48, although one study did not detect such a difference49. Patients with multiple domain amnestic MCI have also been reported to show poorer olfactory function than patients with other subtypes47,48, which suggests that those at highest risk of conversion from MCI to AD show the greatest impairment on olfactory testing14,47,48. One study compared the use of several versions of an odour identification test for clinical trials and reported that the sensitivity and specificity of such a test to detect conversion from amnestic MCI to AD (receiver operating characteristic (ROC) area under the curve (AUC) 0.61–0.65) was similar to that of more expensive and invasive markers — that is, somewhat inferior to structural MRI (ROC AUC of 0.69–0.73) but similar to CSF biomarkers (ROC AUC of 0.63–0.67)35. Research from the University of California San Diego ADRC comparing a number of measures of olfactory function suggests that ROC AUC for odour familiarity, a form of remote odour memory, predicts conversion to AD from MCI in those who are APOE*ε4 carriers better than odour identification but that a combination of the two measures provides the best prediction (P. Wheeler and C.M., unpublished observations).
Elderly adults without dementia
Detection of preclinical AD in the general population is crucial for the identification of individuals who could benefit most from new disease-modifying drugs and treatments as these become available. Population studies of typically ageing adults have reported that olfactory dysfunction increases with age50. Within these populations are individuals with undetected preclinical disease, whose decline in olfactory dysfunction probably contributes to the population estimates of olfactory impairment. Evidence of olfactory dysfunction in clinically normal elderly adults who go on to develop cognitive impairment, dementia or AD has emerged in a number of large-scale studies. Impairment of odour identification at baseline predicted cognitive impairment after 5 years in the Beaver Dam epidemiological study51. Predictions from olfactory impairment were more accurate than those from either hearing or vision impairment52. Impairment of odour identification predicted the development of MCI in individuals with intact cognitive function in the Rush Memory and Ageing Project, despite controlling for age, sex, education, APOE*ε4 status, and episodic memory function46. Similarly, in a cohort of elderly adults in a multi-ethnic community in Northern Manhattan53, impairment of odour identification predicted transition to dementia in cognitively normal individuals. Interestingly, odour identification predicted transition to AD better than a measure of immediate recall. In the Health, Ageing and Body Composition (Health ABC) study, individuals with performance in the poor or moderate tertiles of odour identification showed increased risk of dementia relative to those in the good tertile of odour identification48.
Thus, a large number of studies, a substantial number of which were published in the past 2 years, demonstrate that odour identification discriminates between cognitively normal individuals and those at risk of AD, and between individuals with dementia or AD and those at risk of these conditions. Some of these studies have considered the relationship between odour identification and other biomarkers of dementia and some have tested the predictive power of odour identification for conversion to MCI or dementia. Table 1 summarizes the results of key studies that have characterized odour identification in populations at risk of dementia and/or have investigated the ability of odour identification as a marker to discriminate among groups and to predict conversion to MCI and dementia.
Odour memory
Odour memory requires the detection of an odour, the encoding of that event and subsequent retrieval of the event either by recalling the name of the odour or recognizing that it was previously presented. This odour memory sequence does not require the participant to name the odour, although individuals often report that they attempt to name the odour and use the name in their memory retrieval effort. Existing tests of odour memory require greater testing time than odour identification tests do, and this difference probably accounts for the relative paucity of studies of odour memory in people with AD and in individuals at risk of AD. A number of tests of odour memory have been developed that assess multiple aspects of memory processing mediated by mesial temporal lobe (MTL) structures that are vulnerable in early AD. For example, the California Odor Learning Test54, an olfactory analogue of the California Verbal Learning Test55, produces measures of odour working memory, recall and recognition at short and long delays as well as measures of perseveration (that is, response repetition), serial and semantic clustering (organizational strategies that can enhance recall performance), and errors.
Episodic odour recognition memory has been investigated in patients with AD as well as in those at risk of AD due to MCI, genetic risk of AD or family history. In a series of studies, participants were presented with 20 odours and rated their familiarity. Subsequently, ten of these odours were presented in addition to ten distractor odours and participants attempted to indicate which odours had previously been presented. Episodic odour recognition memory was profoundly impaired in so-called questionable AD56, a term previously used to refer to individuals who would presently be classified as MCI, and in individuals with AD57, particularly in APOE*ε4 carriers57,58. In addition, elderly men who were APOE*ε4 carriers showed greater memory impairment than elderly women regardless of carrier status59.
A similar test developed within the past few years — the Percepts of Odour Episodic Memory (POEM) test — has been administered to participants who were cognitively normal, or who had subjective cognitive concerns, MCI or AD60. Imaging data were available for a subset of the participants. In this subset, impairments in odour recognition memory were associated with the APOE*ε4 allele, thinning of the entorhinal cortex and deterioration in Logical Memory scores in participants who were cognitively normal or who had memory concerns. Interestingly, healthy controls with POEM scores below those predicted by their identification and discrimination scores showed greater association with APOE*ε4 status, entorhinal thinning and trajectory on the memory task than did individuals in the lowest quartile of the odour identification scores, suggesting the potential of odour memory to signal risk of AD.
Studies that have focused on memory in people with AD and/or in APOE*ε4 carriers have established the presence of a profound loss of odour memory in APOE*ε4 carriers on the basis of behavioural tests57,58,59,61. Deficits in odour recognition memory develop early during the progression of dementia in APOE*ε4 carriers. One report demonstrated differences in neuronal networks between elderly APOE*ε4 carriers and non-carriers during a cross-modal odour recognition memory task62. The results were profound: APOE*ε4 carriers and APOE*ε4 non-carriers showed differential connectivity both when odour items were correctly identified and during false alarms, with frontal-temporal disconnection indicated by models of the APOE*ε4 carriers. These findings suggest that during odour memory processing, APOE*ε4 carriers might recruit from other neural networks as a means of compensation for inefficient processing.
Odour threshold
Odour identification involves numerous aspects of cognitive processing, whereas odour threshold — that is, the lowest concentration at which a person can detect odour — is considered a more purely olfactory task. Odour threshold tests include trials with odour and no odour and thus participants probably make a comparison between the current trial and previous trials to help them to determine the presence of an odour, a process that involves aspects of working memory. Odour threshold testing requires many trials with appropriate inter-trial intervals to prevent adaptation, and thus would be difficult to incorporate into a study with limited testing time. Few studies have used this technique and fewer still have compared threshold impairment with other measures of olfactory dysfunction.
Nevertheless, odour threshold sensitivity has been demonstrated to be profoundly affected in AD30,45,63 and the degree of threshold impairment is associated with the degree of dementia63. APOE*ε4 carriers who are cognitively normal and subsequently go on to develop AD show odour threshold impairment in the year before converting to an AD diagnosis64. Poorer thresholds are seen in AD than in MCI and in MCI than in cognitively normal individuals43. The combination of odour identification and odour threshold testing increases the correct classification rate of healthy control individuals from individuals with AD or individuals with MCI over odour identification alone43. Altered odour thresholds in patients with AD or MCI suggest that the peripheral level of the olfactory system is substantially affected in the disease process.
Brain response to olfactory stimuli
Brain activity can be measured from the surface of the scalp via EEG and, specifically, via detection of the event-related potential (ERP), a measure that is exquisitely sensitive to the timing of the brain’s response to a stimulus. Olfactometers that deliver stimuli of short, controlled duration without somatosensory artefacts are required for accurate stimulus delivery65. Olfactory ERPs (OERPs) recorded in relation to olfactory stimulation have demonstrated sensitivity to subtle changes in olfactory functioning associated with ageing, disease and APOE status66,67. The latency of brain response, quantified by OERPs, is substantially delayed in patients with AD66. The markedly increased difference in latency in the OERPs compared with auditory ERPs reflects the vulnerability of the olfactory system to AD. Furthermore, APOE*ε4 allele carriers show increased latencies and differential topographical distribution of OERP response compared with non-carriers68.
Few functional MRI studies of olfactory function have been conducted in patients with AD or in those at risk of AD; however, studies are beginning to indicate brain areas that are affected during key olfactory tasks. The primary olfactory cortex, amygdala and insula show decreased activation in patients with AD compared with healthy individuals in a passive odour task69,70 and a detection task71, and the piriform cortex and entorhinal cortex showed altered activity in patients with AD compared with healthy individuals when participants judged the quality of an olfactory stimulus72. Decreased activation in the primary olfactory cortex has also been reported in MCI71, and functional connectivity between mesial temporal areas and frontal areas is substantially altered in APOE*ε4 allele carriers compared with non-carriers in a recognition memory task62. Thus, functional MRI demonstrates neural correlates of altered performance in a number of olfactory tasks. Further research investigating brain response during tasks that engage memory areas will be of particular interest.
Underlying mechanisms
The necessity for involvement of the olfactory cortex, orbital frontal cortex and mesial temporal structures for good performance on an odour identification task probably makes such tasks particularly sensitive to the neuropathology of AD. Processing of tasks that combine odour naming with odour memory has been reported to be lateralized in the left hemisphere. Left hippocampal volume is associated with both verbal memory tasks and odour identification6.
Very early neuropathological changes in MTL structures, particularly the entorhinal cortex, might disrupt connections between the hippocampus and the isocortex that are necessary for memory formation16,18. Degeneration of the entorhinal cortex affects activity in the hippocampus that is required for odour-related tasks dependent on memory processes. Left hippocampal volume is highly correlated with performance on odour identification in patients with AD6. Indeed, low hippocampal volume and entorhinal cortex thickness are associated with poor odour identification in cognitively normal elderly adults7. Thus, structural measures of MTL volume and thickness are reflected in performance on odour identification. Research is needed to determine the sensitivity and specificity of these measures for predicting cognitive decline and AD. Additional functional neuroimaging studies that specifically engage networks involved in odour identification and odour memory are also needed to better understand the mechanisms underlying impairment of odour identification and odour memory in prodromal AD.
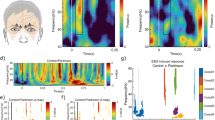
Accumulating research suggests that increased brain activation (hyperactivation) during effortful cognitive tasks is associated with Aβ deposition in individuals at risk of AD73. Greater activation on functional MRI during memory tasks is observed in patients with MCI and in individuals at risk of AD than in cognitively normal individuals, demonstrating functional compensation in brain areas important for memory, such as the MTL, temporoparietal junction, and posterior cingulate and precuneus. FigURE 4 illustrates activation in the precuneus in individuals at risk of AD owing to the APOE*ε4 allele who were performing an odour recognition memory task74. FigURE 5 presents a potential model for the central mechanisms underlying olfactory impairment in AD: individuals at risk of AD who have olfactory dysfunction would be expected to require greater effort to perform well in olfactory tasks, resulting in hyperactivation in olfactory sensory and cognitive processing areas. Hyperactivation causes degenerative changes that, over the lifespan, will result in decreased entorhinal thickness and hippocampal volume. Over time, performance on odour identification and odour memory tasks will decline further as a result of compromised brain integrity, reflecting the disease process.
Functional MRI shows activation in the precuneus in individuals carrying the apolipoprotein (APOE)*ε4 allele during an odour recognition memory task. Increased activation in APOE*ε4 carriers (APOE*ε4+) versus non-carriers (APOE*ε4–) suggests that increased effortful processing is required to perform the task. Changes in activation in the precuneus occur early in mild cognitive impairment. Warm colours indicate increased activation; cool colours indicate reduced activation.
When an individual with olfactory impairment who is at risk of Alzheimer disease (owing to amyloid burden, apolipoprotein (APOE)*ε4 or amnestic mild cognitive impairment (MCI)) attempts to detect, identify or remember an odour, hyperactivation in olfactory sensory and cognitive processing areas — such as the piriform cortex, entorhinal cortex and hippocampus — occurs. Over the lifespan, degenerative changes occur as a result and these changes are associated with thinning in the entorhinal cortex and decreased hippocampal volume.
Olfactory function and CSF biomarkers
CSF biomarkers of AD include Aβ1–40, Aβ1–42, the ratio of Aβ1–40:Aβ1–42, t-tau, P181-tau and the ratio of Aβ:tau3. Over the course of AD progression, levels of Aβ decrease peripherally as the peptide is deposited in the brain. If olfactory function (for example, as assessed by odour identification) is to function as a useful biomarker that can substitute for markers obtained with invasive measures, an understanding of the relationship between olfactory function and CSF biomarkers in prodromal AD is crucial. One study has reported meaningful relationships between CSF biomarkers and a capacity for odour identification in individuals with heightened risk of AD. In first degree relatives of patients with AD, odour identification ability was reported to be related to the ratio of t-tau:Aβ1-42 (ref.5). CSF t-tau:Aβ1-42, P181-tau:Aβ1-42, and t-tau levels correlated with capacity for odour identification in the overall sample, whereas a relationship with Aβ alone was present only in the lowest quartile in which almost half of patients were APOE*ε4 carriers. These data suggest that the emergence of tau pathology, whose interaction with amyloid is crucial to disease symptomatology, is related to emerging olfactory dysfunction (Fig. 6). The robust relationship between odour identification and tau parallels the Braak staging of the disease, in which tangles appear in the entorhinal and transentorhinal areas early in the disease process, whereas amyloid appears first in frontal areas18. As with CSF measures of amyloid, the relationship between odour identification and positive results on amyloid PET scans is not remarkable. One study found a statistically significant association between increased odds of anosmia and increased amyloid accumulation in cognitively normal participants75. In another study, amyloid positive and amyloid negative participants with amnestic MCI did not differ on odour identification76 and, in another report, amyloid burden was marginally associated with impaired odour identification in univariate analyses7.
Structural measures of brain integrity
Hippocampal volume and MTL thickness
Reduced hippocampal volume, as assessed by structural MRI, has been proposed as a marker of early pathology and of disease progression in AD77,78. Hippocampal volume is highly correlated with odour identification performance in patients with AD6. By contrast, the Boston Naming Test showed an appreciably lower correlation with hippocampal volume than odour identification, suggesting that odour identification is a better indicator of hippocampal atrophy in patients with AD. A subsequent study reported that lower right hippocampal and left amygdala volume correlated with odour identification performance in amnestic MCI, and bilateral hippocampal and left amygdala volumes correlated with odour identification performance in AD, but no statistically significant correlations were found between MTL structures and University of Pennsylvania Smell Identification Test (UPSIT) scores in healthy control individuals79. In addition, reduced hippocampal volume and thinning of entorhinal cortex or a composite MTL signature has been associated with poor odour identification in cognitively normal individuals6,75. In one report75, abnormal hippocampal volume was significantly associated with odour identification score only in those individuals with abnormal amyloid PET scans, which suggests that amyloid accumulation might be required for odour identification performance to signal preclinical disease. In clinically normal individuals at risk of AD because of metabolic syndrome, odour identification is correlated with entorhinal thickness80. Longitudinal studies are needed to determine the predictive power of olfactory testing for hippocampal atrophy and other structural measures of MTL integrity; however, the current evidence suggests that olfactory tests might be a useful indicator of reduced hippocampal volume and thinning of the entorhinal cortex in preclinical AD, supporting the potential usefulness of this test in identifying individuals at risk of increasing cognitive impairment who might benefit from and enrich clinical trials of disease-modifying therapies.
Olfactory bulb
Olfactory receptor neurons from the olfactory epithelium project to the mitral cells in the glomeruli of the olfactory bulb. Early processing of olfactory information takes place in the bulb. Post-mortem studies indicate that the frequency and density of neurofibrillary tangles in the bulb is highly correlated with the frequency and density of tangles in the entorhinal cortex27,81. Whether changes in the bulb can be detected in vivo using structural MRI in preclinical and clinical stages of AD has been investigated, but findings have been inconsistent. One study that reported decreased olfactory bulb volume in AD82 and in MCI83 did not assess olfactory function. Another study reported no decrease in bulb volume in MCI or AD, and no correlation between bulb volume and olfactory function assessed by Sniffin’ Sticks (a commercially available odour identification test)84. This discrepancy might partially be attributable to the difficulty of measuring olfactory bulb volume. Studies on other patient groups with olfactory loss (for example after an upper respiratory infection, after head trauma or in patients with Parkinson disease) also show inconsistent results. Differences in scan type and disease severity might partially be responsible for the discrepancies; however, further research is warranted to clearly understand the potential for bulb volume to reflect early pathology and its relationship to olfactory performance.
Interventions
Cholinergic deficits underlying AD
Donepezil, a cholinesterase inhibitor, is effective in halting the progression of AD symptoms for ~6 months to 1 year and is the primary drug prescribed for AD in clinical practice. Improved odour identification has been suggested as a clinically useful measure to predict response to cholinesterase inhibitor treatment85. Two studies have shown improved odour identification with donepezil: a small sample study in patients with cognitive impairment86 and another in patients with mild to moderate AD87. In addition to detection of functional decline, the ability to reflect improvement is crucial in therapeutic trials, thus the positive effect of donepezil on olfactory function is of interest.
Hormonal replacement therapy
Hormonal replacement therapy (HRT) administered after AD diagnosis does not reverse the pathology of AD; however, several studies suggest that HRT might be effective in preventing, delaying or minimizing symptoms of AD if administered during the right window of opportunity88. Interestingly, olfactory function is impaired to a lesser degree in elderly women who have had HRT compared with other individuals. Patients with AD who received HRT outperformed those who did not receive HRT on odour memory, yet showed no advantage on visual memory89. Further study demonstrated a better odour threshold in APOE*ε4 carriers who did not have dementia and who had received HRT than in those without HRT90. Odour thresholds in HRT users with the APOE*ε4 allele were comparable to non-carriers, suggesting that HRT exerted a protective effect in APOE*ε4 carriers. No statistically significant differences in thresholds were found as a function of HRT use in non-carriers. A subsequent study also reported better odour memory scores in postmenopausal women on HRT than those with past HRT or no therapy, although sample sizes prevented investigation of an interaction between HRT and APOE*ε4 carrier status91.
Clinical application
Unawareness of olfactory impairment
Patients with AD are likely to be unaware of olfactory impairment. Unawareness of olfactory dysfunction is associated with cognitive decline in elderly adults without dementia92,93 and is associated with cognitive deficits in a number of domains, including verbal learning and memory, and attention and processing speed, as early as middle age94. This finding is important for neurologists who will use olfaction as an aid to diagnosis: asking a patient whether they can smell cannot be expected to produce valid and reliable information about whether they have olfactory impairment. To avoid a missed diagnosis, an objective assessment of olfactory function is necessary. Screening with a rapidly-administered test of odour identification is a first step that can reveal the need for more comprehensive testing of olfactory or cognitive function.
Instruments for odour identification screening
Several rapidly administered odour identification tests are available that can serve as screening instruments in a clinic setting (Box 1). In the USA, the NIH has developed an olfaction test within the NIH Toolbox that is rapidly and inexpensively administered95. The Odor Stick Identification Test for Japanese96, the San Diego Odor Identification Test97 and the Scandinavian Odor Identification Test developed in Sweden98 can be rapidly administered at minimal cost. The Sniffin’ Sticks Test and the UPSIT are commercially available products99,100,101. Notably, olfactory impairment is also a characteristic of other neurodegenerative diseases. Just as with cognitive screening instruments, positive findings on an odour identification test should prompt further testing for differential diagnoses.
Olfaction: conclusions and implications
AD pathogenesis begins decades before clinical symptoms manifest. FigURE 6 illustrates a proposed timeline for the emergence of olfactory functional impairment during disease progression. Olfactory tests reveal a substantially reduced sensitivity and ability for odour detection, identification and memory in people with AD and in populations at risk of AD, including carriers of the APOE*ε4 allele, individuals with MCI and cognitively normal individuals with increased amyloid burden. Studies suggest the utility of odour identification tests for early detection of amnestic disorders and suggest that such tests might be useful in identifying individuals at risk of cognitive decline and for enrichment of participants in clinical trials34. Notably, an elderly person with intact olfactory function is extremely unlikely to have AD or to develop dementia over a 5-year period51. The negative predictive value of performance on the San Diego Odor Identification test in the Beaver Dam study was 97.2%51 and another study found the negative predictive value of odour identification for AD to be 100%102. A limitation of current olfactory assessment, particularly odour identification, for AD lies in the odour identification impairment in non-AD causes of olfactory dysfunction, such as in Parkinson disease and Lewy body dementia. Accordingly, the sensitivity and specificity of odour identification tests currently used has negatively influenced their inclusion in clinical assessment and in clinical trials of disease-modifying therapy to date. Thus far, most olfactory testing in these instances has utilized odour identification tests, probably because they are inexpensive and rapidly administered by non-experts. Novel olfactory tests that target the specific brain areas and processes affected earliest in preclinical AD could hold additional promise for early disease detection.
Hearing
The first reports that hearing is impaired in AD were published in the 1970s28. Evidence from a number of epidemiological and population-based studies indicates an association between hearing impairment and dementia or AD, independent of age8,9,10. Hearing loss is highly prevalent in the elderly population, with implications for the sensitivity and specificity of hearing impairment as a potential biomarker.
A number of large cohort studies have now demonstrated an association between self-reported or measured hearing loss and cognitive impairment or dementia8,9,10,102. Cross-sectional assessment revealed a statistically significant association between hearing loss and scores on the Mini–Mental State Examination (MMSE), free recall and executive function in the Baltimore Longitudinal Study of Ageing102. The results suggested that the reduction in cognitive function associated with a 25 dB hearing loss was equivalent to an age-associated loss of 6.8 years of cognitive function. Self-reported hearing aid use was not associated with cognitive scores.
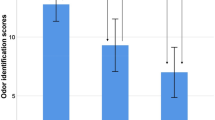
In a prospective study of the Baltimore participants, peripheral hearing loss at baseline was associated with incident dementia followed over time8. Greater hearing loss was associated with greater risk of dementia development. The authors raised the important question of whether hearing loss is a marker for early stage dementia or is a modifiable risk factor. The English Longitudinal Study of Ageing (ELSA), a large population study, assessed individuals ≥50 years of age and found that both self-reported hearing difficulty and objective hearing loss were associated with cumulative diagnosed dementia over a 10-year period. In this study, hearing aid use seemed to have a protective effect9. Another study analysed pure-tone air conduction thresholds in well-functioning, community-dwelling adults aged 70–79 years in the Health ABC Study, who were dementia-free at baseline and who self-reported no difficulty with mobility or activities of daily living. Results demonstrated that moderate to severe peripheral hearing loss was associated with a 55% increase in the risk of dementia after 9 years10. The rate of cognitive decline was unrelated to hearing impairment and no statistically significant effect was detected with regards to hearing aid use, although the authors note that in both instances the study might have been underpowered to detect these effects. Impaired hearing at baseline predicted cognitive impairment after 5 years in the Beaver Dam study, although impaired odour identification at baseline predicted cognitive impairment after 5 years more accurately than either impaired hearing or impaired vision52. A systematic review and meta-analysis of 40 studies that examined associations between hearing loss and cognitive function, cognitive impairment, dementia, vascular dementia or AD found statistically significant associations in cross-sectional studies examining the relationships between hearing loss and cognitive function, cognitive impairment and dementia; however, the associations between hearing loss and AD were not significant in either cross-sectional or cohort studies103.
Interventions
Given the association of hearing loss with cognitive impairment, two studies directly addressed the hypothesis that hearing aid use has the potential to reverse cognitive impairment104,105. Unfortunately, a 6-month randomized trial of hearing aid use did not produce an improvement in cognitive function in either study; however, further research investigating the parameters of treatment intervention is warranted.
Underlying mechanisms
A number of potential mechanisms have been proposed for the association between hearing loss and dementia. Evidence from the Baltimore Longitudinal Study of Ageing suggests an association between hearing impairment and the rate of decline in whole brain volume106. Regional loss of right temporal lobe volume in patients with dementia suggests a specific loss in areas important for speech processing. Other proposed mechanisms include the effects of social isolation102,107,108 and increased cognitive effort, secondary to hearing impairment108. Further research is warranted to develop an improved understanding of the mechanisms underlying the link between hearing loss and dementia.
Hearing: conclusions and implications
Evidence of hearing loss as a potential indicator of cognitive impairment and dementia is mounting. However, it is unclear whether hearing loss is associated with AD, is a causal factor in dementia or is associated with temporal lobe abnormalities or other factors. Whether hearing loss is a modifiable risk factor for AD is an important question. Additional information on potential mechanisms and interventions targeting hearing loss will be essential to a greater understanding of auditory impairment in dementia and AD.
Vision
The occurrence of AD pathology in the visual pathway is well established. Aβ plaques and oligomers have been identified both in post-mortem tissue from patients who had AD and in primary aqueous humour from individuals without AD undergoing cataract extraction109. The implications of this pathology require further investigation.
Retinal thinning
Multiple studies have reported retinal thinning in AD. Retinal nerve fibre layer thickness, ganglion cell layer thickness and macular volume have been examined with spectral domain optical coherence tomography in a number of studies in patients with AD, but the results for specific quadrants are mixed110,111. A 2016 study, powered to differentiate inter-group variances in thickness of 15 µm standard deviations from the mean, investigated retinal thinning in AD, non-AD dementia, amnestic MCI, Parkinson disease and age-matched healthy control individuals and found no difference in retinal markers, including retinal nerve fibre layer thickness, ganglion cell layer thickness and macular volume112. A subsequent study, with a larger sample size than the first, reported significant thinning of global and temporal superior quadrants of the peripapillary retinal nerve fibre layer in AD compared with controls113. Contrast sensitivity vision, which is poorer in patients with AD than in healthy controls, is significantly correlated with macular volume114. Results of studies of retinal thinning in MCI are emerging but a consistent picture is not yet clear. Retinal thinning has been observed in MCI and was found to be related to memory complaints in amnestic MCI115,116 However, patients with MCI had a greater macular volume than patients with AD or healthy control individuals, which was suggested to be due to inflammation and/or gliosis117. Another study found no statistically significant differences in thickness of inner retinal layers, or in macular or optic nerve volumes in patients with MCI or early–moderate AD118. The authors suggest a number of potential factors that might have influenced their findings, including a potentially insufficient sample size. The exclusion of patients with glaucoma from their study might have been of particular importance as the occurrence of glaucoma has been associated with AD. The degree to which retinal thinning distinguishes between healthy individuals, patients with MCI and patients with AD, and whether this thinning reflects cognitive function and disease severity are important unanswered questions.
Contrast sensitivity
Contrast sensitivity is impaired in AD119,120, in MCI and in individuals with cognitive complaints who do not have performance deficits120. The degree of impairment in contrast sensitivity is related to standard cognitive measures such as the California Verbal Learning Test (CVLT), CVLT total score and CVLT long delay recall scores, suggesting the potential for this impairment to signal cognitive decline. Increasing the contrast of visual stimuli to compensate for deficits in contrast sensitivity enables performance to be enhanced on tests of visual-dependent cognition such as letter identification, word reading and digit cancellation, suggesting that visual impairment is responsible for some of the apparent cognitive information processing difficulties. However, patients with AD continue to show poor performance regardless of enhanced contrast level, indicating the cognitive nature of their deficits121. Contrast sensitivity might also be associated with amyloid and tau deposition at preclinical stages, according to 18F-florbetapir and 18F-flortaucipir PET scans122.
Pupillary response
Patients with AD demonstrate abnormal pupillary responses. In fact, supersensitivity of the pupil dilation response to ocular administration of topicamide, an acetylcholine-blocker, has been suggested as a potential biomarker of AD123. The difference in the pupillary light reflex in patients with AD after treatment with tropicamide was reported to be significantly lower (1.01 mm) than in healthy controls (1.42 mm), and significantly correlated with DRS scores123. Changes in pupillary response might also be a biomarker of AD because they reflect the integrity of the locus coeruleus, which has been reported as the site of earliest AD neuropathology124.
Within the past few years, studies have focused on the pupillary response as a reflection of the ability to successfully compensate for increased cognitive load. During cognitive tasks the pupillary diameter reflects cognitive effort until the participant’s ability is exceeded. Thus, pupillary response measures have been studied in AD and MCI. Indeed, patients with MCI show changes in pupillary response and pupil size compared with healthy control individuals, with higher scores on the MMSE associated with a lower light reflex latency and greater increase in amplitude of pupil response125. In a study of adults aged 56–66 years, those with amnestic MCI had significantly greater alterations in pupil dilation than those with non-amnestic MCI and cognitively normal adults126. However, the sensitivity and specificity of pupillary response as a biomarker for AD is challenged by the fact that pupillary response is compromised in other conditions such as Parkinson disease.
Vision: conclusions and implications
The emergence of AD pathology in the peripheral and central visual systems is well established in patients with AD; however, the point at which pathology first appears in the visual system is a matter of discussion. Additional research into the sensitivity and specificity of visual dysfunction in longitudinal studies will be important to establish the clinical utility of visual system impairment in AD.
Conclusions
The evidence to date suggests that olfaction shows the greatest promise among all sensory biomarkers of AD. A substantial body of research indicates that the impairment of a number of measures of olfactory function can signal the development of the early stages of AD (Table 1). Odour identification, odour familiarity and odour recognition memory all show a robust ability to discriminate between cognitively normal individuals, patients with AD, patients with MCI and those at risk of AD14,35,38,39,40,41,42,43,44,45,46,47,48. Odour identification35,37 and odour familiarity (P. Wheeler and C.M., unpublished observations) predict transitions from MCI to AD, particularly in those with a genetic risk of AD, which suggests a potential utility for these measures in clinical trials35 . Olfactory impairment is associated with measures of reductions in hippocampal volume and entorhinal cortex thinning6,75, and may be related to the CSF levels of biomarkers of AD5. The emergence of olfactory impairment as tau levels increase is particularly suggestive of its potential as an early marker of disease. In addition to odour identification, the relative sensitivity and specificity of other promising olfactory tasks, particularly episodic odour recognition memory and remote odour memory, warrant further research.
Auditory impairment has also been associated with the development of cognitive impairment and dementia in epidemiological studies8,9,10, although the available evidence suggests that the sensitivity and specificity of auditory impairment as a marker of AD are lower than for olfaction52. To date, efforts to treat auditory impairment have not been shown to reverse or retard the development of dementia104,105. Visual deficits, specifically retinal thinning110,111,112,113, contrast sensitivity119,120,121 and pupillary response123,125,126 have received some attention as potential markers of AD. However, the extent to which these deficits emerge in the prodromal period is unclear. Additional studies of multiple sensory modalities in the same individuals are needed to further address the relative efficacies of measures of sensory impairment in detecting prodromal AD.
Tremendous interest exists in markers that can signal the early development of AD. Impairments in olfaction, hearing and vision have emerged as potential markers of prodromal AD, and these markers hold great promise as potential early indicators of disease. However, future research is necessary to chart the progression of sensory impairments in the prodromal period, to further examine their relationship to CSF biomarkers and emerging measures of structural and functional MRI, to assess their response to disease-modifying agents and to enhance their sensitivity and specificity for AD prediction.
References
Alzheimer’s Disease International. Dementia Statistics. ADI https://www.alz.co.uk/research/statistics (2017).
Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 11, 332–384 (2015).
Galasko, D. R. & Shaw, L. M. CSF biomarkers for Alzheimer disease — approaching consensus. Nat. Rev. Neurol. 13, 131–132 (2017).
Weiner, M. W. et al. The Alzheimer’s Disease Neuroimaging Initiative 3: continued improvement for clinical trial improvement. Alzheimers Dement. 13, 561–571 (2017).
Lafaille-Magnen, M.-E. et al. Odor identification as a biomarker of preclinical AD in older adults at risk. Neurology 89, 327–335 (2017).
Murphy, C., Jernigan, T. L. & Fennema-Notestine, C. Left hippocampal volume loss in Alzheimer’s disease is reflected in performance on odor identification: a structural MRI study. J. Int. Neuropsychol. Soc. 9, 459–471 (2003).
Growdon, M. E. et al. Odor identification and Alzheimer disease biomarkers in clinically normal elderly. Neurology 84, 2153–2160 (2015).
Lin, F. R. et al. Hearing loss and incident dementia. Arch. Neurol. 68, 214–220 (2011).
Davies, H. R., Cadar, D., Herbert, A., Orrell, M. & Steptoe, A. Hearing impairment and incident dementia: findings from the England Longitudinal Study of Ageing. J. Am. Geriatr. Soc. 65, 2074–2081 (2017).
Deal, J. A. et al. Hearing impairment and incident dementia and cognitive decline in older adults: the health ABC study. J. Gerontol. A Biol. Sci. Med. Sci. 72, 703–709 (2017).
Corder, E. H. et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261, 921–923 (1993).
Albert, M. S. et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 270–279 (2011).
Jak, A. J. et al. Contributions of neuropsychology and neuroimaging to understanding clinical subtypes of mild cognitive impairment. Neurobiol. Dementia 84, 81–103 (2009).
Roberts, R. O. et al. Association between olfactory dysfunction and amnestic mild cognitive impairment and Alzheimer disease dementia. JAMA Neurol. 16, 1–9 (2015).
Hyman, B. T., Arriagada, P. V. & Hoesen, G. W. Pathologic changes in the olfactory system in aging and Alzheimer’s disease. Ann. NY Acad. Sci. 640, 14–19 (1991).
Braak, H. & Braak, E. The human entorhinal cortex: normal morphology and lamina-specific pathology in various diseases. Neurosci. Res. 15, 6–31 (1992).
Braak, H. & Braak, E. Morphological criteria for the recognition of Alzheimer’s disease and the distribution pattern of cortical changes related to this disorder. Neurobiol. Aging 15, 355–356 (1994).
Braak, H. & Braak, E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol. Aging 18, 351–357 (1997).
Attems, J., Walker, L. & Jellinger, K. A. Olfactory bulb involvement in neurodegenerative diseases. Acta Neuropathol. 127, 459–475 (2014).
Hyman, B. T. & Tanzi, R. N. Amyloid, dementia and Alzheimer’s disease. Curr. Opin. Neurol. Neurosurgery 5, 88–93 (1992).
Hyman, B. T. The neuropathological diagnosis of Alzheimer’s disease: clinical-pathological studies. Neurobiol. Aging 18, 27–32 (1997).
Ohm, T. G. & Braak, H. Olfactory bulb changes in Alzheimer’s disease. Acta Neuropathol. 73, 365–369 (1987).
Struble, R. G. & Clark, H. B. Olfactory bulb lesions in Alzheimer’s disease. Neurobiol. Aging 13, 469–473 (1992).
Braak, H. & Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 237–259 (1991).
Braak, H. & Braak, E. Staging of Alzheimer-related cortical destruction. Int. Psychogeriatr. 9, 269–272 (1997).
Braak, H. & Del Tredici, K. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol. 121, 171–181 (2011).
Price, J. L., Davis, P. B., Morris, J. C. & White, D. L. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer’s disease. Neurobiol. Aging 12, 295–312 (1991).
Waldton, S. Clinical observations of impaired cranial nerve function in senile dementia. Acta Psychiat. Scand. 50, 539–547 (1974).
Serby, M. Olfaction and Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 10, 579–586 (1986).
Doty, R. L., Reyes, P. F. & Gregor, T. Presence of both odor identification and detection deficits in Alzheimer’s disease. Brain Res. Bull. 18, 597–600 (1987).
Chan, A., Tam, J., Murphy, C., Chiu, H. & Lam, L. Utility of olfactory identification test for diagnosing Chinese patients with Alzheimer’s disease. J. Clin. Exp. Neuropsychol. 24, 251–259 (2002).
Morgan, C. D., Nordin, S. & Murphy, C. Odor identification as an early marker for Alzheimer’s disease: impact of lexical functioning and detection sensitivity. J. Clin. Exp. Neuropsychol. 17, 793–803 (1995).
Oleson, S. & Murphy, C. Olfactory dysfunction in ApoE ε4/4 homozygotes with Alzheimer’s disease. J. Alzheimers Dis. 46, 791–803 (2015).
Woodward, M. R. et al. Validation of olfactory deficit as a biomarker of Alzheimer disease. Neurol. Clin. Practice 7, 5–14 (2017).
Albers, M. W. et al. At the interface of sensory and motor dysfunctions and Alzheimer’s disease. Alzheimers Dement. 11, 70–98 (2015).
Rahayel, S., Frasnelli, J. & Joubert, S. The effect of Alzheimer’s disease and Parkinson’s disease on olfaction: a meta-analysis. Behav. Brain Res. 231, 60–74 (2012).
Roalf, D. R. et al. A quantitative meta-analysis of olfactory dysfunction in mild cognitive impairment. J. Neurol. Neurosurg. Psychiatry 88, 226–232 (2017).
Murphy, C., Bacon, A. W., Bondi, M. W. & Salmon, D. P. Apolipoprotein E status is associated with odor identification deficits in nondemented older persons. Ann. NY Acad. Sci. 855, 744–750 (1998).
Graves, A. B. et al. Impaired olfaction as a marker for cognitive decline: interaction with apolipoprotein epsilon4 status. Neurology 53, 1480–1487 (1999).
Olofsson, J. K. et al. Odor identification impairment in carriers of ApoE-ε4 is independent of clinical dementia. Neurobiol. Aging 31, 567–577 (2010).
Mattis, S. in Geriatric Psychiatry: A Handbook for Psychiatrists and Primary Care Physicians (eds Bellak, L., Karasu, T. B. & Birenbaum, C.) 77–121 (Grune & Stratton, NY, 1976).
Calhoun-Haney, R. & Murphy, C. Apolipoprotein e4 is associated with more rapid decline in odor identification than in odor threshold or dementia rating scale scores. Brain Cogn. 58, 178–182 (2005).
Josefsson, M., Larsson, M., Nordin, S., Adolfsson, R. & Olofsson, J. APOE-e4 effects on longitudinal decline in olfactory and non-olfactory cognitive abilities in middle-aged and old adults. Scientif. Rep. 7, 1286 (2017).
Devanand, D. P. et al. Olfactory identification deficits and MCI in a multi-ethnic elderly community sample. Neurobiol. Aging 31, 1593–1600 (2010).
Djordjevic, J., Jones-Gotman, M., De Sousa, K. & Chertkow, H. Olfaction in patients with mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging 29, 693–706 (2008).
Wilson, R. S. et al. Olfactory identification and incidence of mild cognitive impairment in older age. Arch. General Psychiatry 64, 802–808 (2007).
Tabert, M. H. et al. A 10-item smell identification scale related to risk for Alzheimer’s disease. Ann. Neurol. 58, 155–160 (2005).
Yaffe, K., Freimer, D. & Chen, H. Olfaction and risk of dementia in a biracial cohort of older adults. Neurology 88, 456–462 (2017).
Lehrner, J., Pusswald, G., Gleiss, A., Auff, E. & Dal-Bianco, P. Odor identification and self-reported olfactory functioning in patients with subtypes of mild cognitive impairment. Clin. Neuropsychol. 23, 818–830 (2009).
Quarmley, M. et al. Odor identification screening improves diagnostic classification in incipient Alzheimer’s Disease. J. Alzheimers Dis. 55, 1497–1507 (2017).
Schubert, C. R. et al. Olfaction and the 5-year incidence of cognitive impairment in an epidemiological study of older adults. J. Am. Geriatr. Soc. 56, 1517–1521 (2008).
Fischer, M. E. et al. Age-related sensory impairments and risk of cognitive impairment. J. Am. Geriatr. Soc. 64, 1981–1987 (2016).
Devanand, D. P., Less, S. & Manly, J. Olfactory deficits predict cognitive decline and Alzheimer dementia in an urban community. Neurology 84, 182–189 (2015).
Murphy, C., Nordin, S. & Acosta, L. Odor learning, recall, and recognition memory in young and elderly adults. Neuropsychology 11, 126–137 (1997).
Delis, D. C., Kramer, J. H., Kaplan, E. & Ober, B. A. The California Verbal Learning Test (Psychological Corporation, 1987).
Nordin, S. & Murphy, C. Impaired sensory and cognitive olfactory function in questionable Alzheimer’s disease. Neuropsychology 10, 113–119 (1996).
Gilbert, P. E. & Murphy, C. The effect of the ApoE ε4 allele on recognition memory for olfactory and visual stimuli in patients with pathologically confirmed Alzheimer’s disease, probable Alzheimer’s disease and healthy elderly controls. J. Exp. Clin. Neuropsychol. 26, 779–794 (2004).
Gilbert, P. E. & Murphy, C. Differences between recognition memory and remote memory for olfactory and visual stimuli in nondemented elderly individuals genetically at risk for Alzheimer’s disease. Exp. Gerontol. 39, 433–441 (2004).
Sundermann, E., Gilbert, P. E. & Murphy, C. Apolipoprotein E ε4 genotype and gender: effects on memory. Am. J. Geriatr. Psychiatry 15, 869–878 (2007).
Albers, A. D. et al. Episodic memory of odors stratifies Alzheimer biomarkers in normal elderly. Ann. Neurol. 8, 846–857 (2016).
Gilbert, P. E., Barr, J. & Murphy, C. Differences in olfactory and visual memory in patients with pathologically confirmed Alzheimer’s disease and the Lewy body variant of Alzheimer’s disease. J. Int. Neuropsychol. Soc. 10, 835–842 (2004).
Haase, L., Wang, M., Green, E. & Murphy, C. Functional connectivity during recognition memory in individuals genetically at risk for Alzheimer’s disease. Hum. Brain Mapp. 34, 530–542 (2013).
Murphy, C. et al. Olfactory thresholds are associated with degree of dementia in Alzheimer’s disease. Neurobiol. Aging 11, 465–469 (1990).
Bacon, A. W., Bondi, M. W., Salmon, D. P. & Murphy, C. Very early changes in olfactory functioning due to Alzheimer’s disease and the role of apolipoprotein E in olfaction. Ann. NY Acad. Sci. 30, 723–731 (1998).
Lorig, T. S., Elmes, D. G., Zald, D. H. & Pardo, J. V. A computer-controlled olfactometer for fMRI and electrophysiological studies of olfaction. Behav. Res. Methods, Instruments, Computers 31, 370–375 (1999).
Morgan, C. D. & Murphy, C. Olfactory event-related potentials in Alzheimer’s disease. J. Int. Neuropsychol. Soc. 8, 753–763 (2002).
Morgan, C. & Murphy, C. Individuals at risk for Alzheimer’s disease show differential patterns of ERP brain activity during odour identification. Behav. Brain Funct. 8, 37 (2012).
Murphy, C., Solomon, E. S., Haase, L., Wang, M. & Morgan, C. D. Olfaction in aging and Alzheimer’s disease: event-related potentials to a cross-modal odor recognition memory task discriminate ApoE 4+ and ApoE 4- individuals. Ann. NY Acad. Sci. 1170, 647–657 (2009).
Kareken, D. A. et al. Olfactory system activation from sniffing: effects in piriform and orbitofrontal cortex. NeuroImage 22, 456–465 (2004).
Wang, J. E. et al. Olfactory deficit detected by fMRI in early Alzheimer’s disease. Brain Res. 1357, 184–194 (2010).
Vasavada, M. M. et al. Central olfactory dysfunction in Alzheimer’s disease and mild cognitive impairment: a functional MRI study. J. Alzheimers Dis. 59, 359–368 (2017).
Li, W., Howard, J. D. & Gottfried, J. A. Disruption of odour quality coding in piriform cortex mediates olfactory deficits in Alzheimer’s disease. Brain 133, 2714–2716 (2010).
Mormino, E. C. et al. Aβ deposition in aging is associated with increases in brain activation during successful memory encoding. Cerebral Cortex 22, 1813–1823 (2011).
Woodward, J. L. et al. Semantic memory activation in amnestic mild cognitive impairment. Brain 132, 20168–20178 (2009).
Vassilaki, M. et al. Neuroimaging biomarkers and impaired olfaction in cognitively normal individuals. Ann. Neurol. 81, 871–882 (2017).
Bahar-Fuchs, A. et al. Olfactory deficits and amyloid-β burden in Alzheimer’s disease, mild cognitive impairment, and healthy aging: a PiB PET study. J. Alzheimers Dis. 22, 1081–1087 (2010).
McEvoy, L. K. et al. Mild cognitive impairment: baseline and longitudinal structural MR imaging measures improve predictive prognosis. Radiology 259, 834–843 (2011).
McEvoy, L. K. et al. Alzheimer disease: quantitative structural neuroimaging for detection and prediction of clinical and structural changes in mild cognitive impairment. Radiology 251, 195–120 (2009).
Hagemeier, J. et al. Odor identification deficit in mild cognitive impairment and Alzheimer’s disease is associated with hippocampal and deep gray matter atrophy. Psychiatry Res.Neuroimaging 255, 87–93 (2016).
Murphy, C. et al. Olfactory function and structural integrity of entorhinal cortex and hippocampus in non-demented middle-aged and older adults at risk for Alzheimer’s disease [abstract 203]. Chem. Senses 41, e1–e110 (2016).
Christen-Zaech, S. et al. Early olfactory involvement in Alzheimer’s disease. Can. J. Neurol. Sci. 30, 20–25 (2003).
Thomann, P. A. et al. Reduced olfactory bulb and tract volume in early Alzheimer’s disease—a MRI study. Neurobiol. Aging 30, 838–841 (2009).
Thomann, P. A. et al. MRI-derived atrophy of the olfactory bulb and tract in mild cognitive impairment and Alzheimer’s disease. J. Alzheimers Dis. 17, 213–221 (2009).
Servello, A. et al. Olfactory dysfunction, olfactory bulb volume and Alzheimer’s disease: is there a correlation? a pilot study1. J. Alzheimers Dis. 48, 395–402 (2015).
Schofield, P. W., Ebrahimi, H., Jones, A. L., Bateman, G. A. & Murray, S. R. An olfactory ‘stress test’ may detect preclinical Alzheimer’s disease. BMC 12, 24 (2012).
Pelton, G. H., Soleimani, L., Roose, S. P., Tabert, M. H. & Devanand, M. D. Olfactory deficits predict cognitive improvement on donepezil in patients with depression and cognitive impairment: a randomized controlled pilot study. Alzheimer Dis. Assoc. Disord. 30, 67–69 (2016).
Velayudhan, L. & Lovestone, S. Smell identification test as a treatment response marker in patients with Alzhiemer disease receiving donepezil. J. Clin. Psychopharmacol. 29, 387–390 (2009).
Brinton, R. D. Estrogen regulation of glucose metabolism and mitochondrial function: therapeutic implications for prevention of Alzheimer’s disease. Adv. Drug Deliv. Rev. 60, 1504–1511 (2008).
Sundermann, E., Gilbert, P. E. & Murphy, C. Estrogen and performance in recognition memory for olfactory and visual stimuli in females diagnosed with Alzheimer’s disease. J. Int. Neuropsychol. Soc. 12, 400–404 (2006).
Sundermann, E. E., Gilbert, P. E. & Murphy, C. The effect of hormone therapy on olfactory sensitivity is dependent on apolipoprotein E genotype. Horm. Behav. 54, 528–533 (2008).
Doty, R. L. et al. Influences of hormone replacement therapy on olfactory and cognitive function in postmenopausal women. Neurobiol. Aging. 36, 2053–2059 (2015).
Nordin, S., Monsch, A. U. & Murphy, C. Unawareness of smell loss in normal aging and Alzheimer’s disease: discrepancy between self-reported and diagnosed smell sensitivity. J. Gerontol. B Psychol. Sci. Soc. Sci. 50, 187–192 (1995).
Devanand, D. P. et al. Olfactory deficits in patients with mild cognitive impairment predict Alzheimer’s disease at follow-up. Am. J. Psychiatry 157, 1399–1405 (2000).
Wehling, E., Nordin, S., Espeseth, T., Reinvang, I. & Lundervold, A. J. Unawareness of olfactory dysfunction and its association with cognitive functioning in middle aged and old adults. Arch. Clin. Neuropsychol. 26, 260–269 (2011).
Dalton, P. et al. Olfactory assessment using the NIH Toolbox. Neurology 80, 32–36 (2013).
Saito, S., Ayabe-Kanamura, S. & Takashima, Y. Development of a smell identification test using a novel stick-type odour presentation kit. Chem. Senses 31, 379–391 (2006).
Murphy, C. et al. Prevalence of olfactory impairment in older adults. JAMA 288, 2307–2312 (2002).
Nordin, S., Brämerson, A., Liden, E. & Bende, M. The Scandinavian Odor-Identification Test: development, reliability, validity and normative data. Acta Otolaryngol. 118, 226–234 (1998).
Hummel, T., Sekinger, B., Wolf, S. R., Pauli, E. & Kobal, G. ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem. Senses 22, 39–52 (1997).
Doty, R. L., Shaman, P. & Dann, M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol. Behav. 32, 489–502 (1984).
Christensen, T., Larsson, E.-M., Holm, I. D., Nielsen, O. B. F. & Andersen, S. Olfactory testing in consecutive patients referred with suspected dementia. BMC Geriatr. 17, 129 (2017).
Lin, F. R. et al. Hearing loss and cognition in the Baltimore Longitudinal Study of Aging. Neuropsychology 25, 763–770 (2011).
Loughrey, D. G., Kelly, M. E., Kelley, G. A., Brennan, S. & Lawlor, B. A. Association of age-related hearing loss with cognitive function, cognitive impairment, and dementia: a systematic review and meta-analysis. JAMA Otolaryngol. Head Neck Surg. 2513, 1–12 (2017).
Adrait, A. et al. Do hearing aids influence behavioral and psychological symptoms of dementia and quality of life in hearing impaired Alzheimer’s disease patients and their caregivers? J. Alzheimers Dis. 58, 109–121 (2017).
Nguyen, M. F. et al. Efficacy of hearing aids on the cognitive status of patients with Alzheimer’s disease and hearing loss: a multicenter controlled randomized trial. J. Alzheimers Dis. 58, 123–137 (2017).
Lin, F. R. et al. Association of hearing impairment with brain volume changes in older adults. Neuroimage 90, 84–92 (2014).
Weinstein, B. E. & Ventry, I. M. Hearing impairment and social isolation in the elderly. J. Speech Hear. Res. 25, 593–599 (1982).
Lin, F. R. & Albert, A. Hearing loss and dementia – who’s listening. Aging Mental Health, 18, 671–673 (2014).
Goldstein, L. E. et al. Cytosolic β-amyloid deposition and supranuclear cataracts in lenses from people with Alzheimer’s disease. Lancet 361, 1258–1265 (2003).
Iseri, P. K., Altinas, O., Tokay, T. & Yuksel, N. Relationship between cognitive impairment and retinal morphological and visual functional abnormalities in Alzheimer’s disease. J. Neuroophthalmol. 26, 18–24 (2006).
Beshira, F., Feke, G. T., Trempe, C. L., McMeel, J. W. & Schepens, C. L. Retinal abnormalities in early Alzheimer’s disease. Invest. Ophthalmol. Vis. Sci. 48, 2285–2289 (2007).
Pillai, J. A. et al. Retinal nerve fiber layer thinning in Alzheimer’s disease: a case-control study in comparison to normal aging, Parkinson’s disease, and non-Alzheimer’s dementia. Am. J. Alzheimers Dis. Other Demen. 31, 430–436 (2016).
Cunha, J. P. et al. OCT in Alzheimer’s disease: thinning of the RNFL and superior hemiretina. Graefes Arch. Clin. Exp. Ophthalmol. 255, 1827–1835 (2017).
Polo, V. et al. Visual function and its correlation with retinal changes in patients with Alzheimer’s disease. Eye 31, 1034–1041 (2017).
Paquet, C. et al. Abnormal retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Neurosci. Lett. 420, 97–99 (2007).
Kesler, A., Vakhapova, V., Korczyn, A. D., Naftaliev, E. & Neudorfer, M. Retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Clin. Neurol. Neurosurg. 113, 523–526 (2011).
Ascaso, F. J. et al. Reginal alterations in mild cognitive impairment and Alzheimer’s disease: an optical coherence tomography study. J. Neurol. 261, 1522–1530 (2014).
Lad, E. M. et al. Evaluation of inner retinal layers as biomarkers in mild cognitive impairment to moderate Alzheimer’s disease. PLOS ONE 13, e0192646 (2018).
Gilmore, G. C., Groth, K. E. & Thomas, C. W. Stimulus contrast and word reading speed in Alzheimer’s disease. Exp. Aging Res. 31, 15–33 (2005).
Risacher, S. L. et al. Visual contrast sensitivity in Alzheimer’s disease, mild cognitive impairement, and older adults with cognitive complaints. Neurobiol. Aging 34, 1133–1144 (2013).
Toner, C. K. et al. Vision-fair neuropsychological assessment in normal aging, Parkinson’s disease and Alzheimer’s disease. Psychol. Aging 27, 785–790 (2012).
Risacher, S. L. et al. Visual contrast sensitivity is associated with amyloid and tau deposition. Alzheimers Dement. 13, 154–155 (2017).
Granholm, E. et al. Tropicamide effects on pupil size and pupillary light reflexes in Alzheimer’s and Parkinson’s disease. Int. J. Psychophysiol. 47, 95–115 (2003).
Braak, H. & Del Tredici, K. Where, when, and in what form does sporadic Alzheimer’s disease begin? Curr. Opin. Neurol. 15, 708–714 (2012).
Bittner, D. M., Wieseler, I., Wilhelm, H., Riepe, M. W. & Müller, N. G. Repetitive pupil light reflex: potential marker in Alzheimer’s disease? J. Alzheimers Dis. 42, 1469–1477 (2014).
Granholm, E. L. et al. Pupillary responses as a biomarker of early risk for Alzheimer’s disease. J. Alzheimer’ Dis. 56, 1419–1428 (2017).
Jack, C. R. et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 9, 119–128 (2010).
Conti, M. Z. et al. Odor identification deficit predicts clinical conversion from mild cognitive impairment to dementia due to Alzheimer’s disease. Arch. Clin. Neuropsychol. 18, 391–399 (2013).
Eibenstein, A. et al. Olfactory screening test in mild cognitive impairment. Neurol. Sci. 26, 156–160 (2005).
Westervelt, H. J., Bruce, J. M., Coon, W. G. & Tremont, G. Odor identification in mild cognitive impairment subtypes. J. Clin. Exp. Neuropsychol. 30, 151–156 (2008).
Acknowledgements
The author is supported by NIH grant R01AG004085-26 from the National Institute on Aging. The author gratefully acknowledges C. Frank, A. Jacobson, M. Boquet, E. Farley and J. Liu for research assistance, and the patients and staff of the UCSD ADRC, particularly D. Salmon and D. Galasko and the late L. Thal and R. Katzman.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Murphy, C. Olfactory and other sensory impairments in Alzheimer disease. Nat Rev Neurol 15, 11–24 (2019). https://doi.org/10.1038/s41582-018-0097-5
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-018-0097-5
This article is cited by
-
Profiling of long non-coding RNAs in hippocampal–entorhinal system subfields: impact of RN7SL1 on neuroimmune response modulation in Alzheimer’s disease
Journal of Neuroinflammation (2024)
-
Influence of age on nicotinic cholinergic regulation of blood flow in rat’s olfactory bulb and neocortex
The Journal of Physiological Sciences (2024)
-
Odor identification score as an alternative method for early identification of amyloidogenesis in Alzheimer’s disease
Scientific Reports (2024)
-
Post-COVID-19 Hyposmia Does Not Exhibit Main Neurodegeneration Markers in the Olfactory Pathway
Molecular Neurobiology (2024)
-
Predicting the efficacy of donepezil intervention in Alzheimer’s disease patients using regional homogeneity in the inferior orbitofrontal cortex
Aging Clinical and Experimental Research (2024)