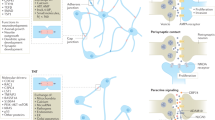
Abstract
Glioblastomas are heterogeneous and invariably lethal tumours. They are characterized by genetic and epigenetic variations among tumour cells, which makes the development of therapies that eradicate all tumour cells challenging and currently impossible. An important component of glioblastoma growth is communication with and manipulation of other cells in the brain environs, which supports tumour progression and resistance to therapy. Glioblastoma cells recruit innate immune cells and change their phenotype to support tumour growth. Tumour cells also suppress adaptive immune responses, and our increasing understanding of how T cells access the brain and how the tumour thwarts the immune response offers new strategies for mobilizing an antitumour response. Tumours also subvert normal brain cells — including endothelial cells, neurons and astrocytes — to create a microenviron that favours tumour success. Overall, after glioblastoma-induced phenotypic modifications, normal cells cooperate with tumour cells to promote tumour proliferation, invasion of the brain, immune suppression and angiogenesis. This glioblastoma takeover of the brain involves multiple modes of communication, including soluble factors such as chemokines and cytokines, direct cell–cell contact, extracellular vesicles (including exosomes and microvesicles) and connecting nanotubes and microtubes. Understanding these multidimensional communications between the tumour and the cells in its environs could open new avenues for therapy.
Key Points
-
Glioblastomas use numerous forms of communication to hijack many different cell types in the brain environs to support tumour progression.
-
Communication routes include secreted proteins and molecules, gap junctions between cells, extracellular vesicles, tunnelling nanotubes and microtubes.
-
Tumour cells co-opt microglia and infiltrating macrophages for their own benefit through the release of cytokines and extracellular vesicles.
-
Glioblastomas and pericytes generate a state of reduced T cell effector function that is commonly referred to as T cell exhaustion or dysfunction.
-
The interaction of tumour cells with normal brain cells, such as neurons, is not unidirectional, and neuronal activity is subverted to promote glioblastoma progression.
-
Comprehension and disruption of tumour directives in the glioblastoma microenvironment could improve therapeutic intervention for these lethal tumours.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Stupp, R. et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 10, 459–466 (2009).
Jhaveri, N., Chen, T. C. & Hofman, F. M. Tumor vasculature and glioma stem cells: contributions to glioma progression. Cancer Lett. 380, 545–551 (2016).
See, A. P., Parker, J. J. & Waziri, A. The role of regulatory T cells and microglia in glioblastoma-associated immunosuppression. J. Neurooncol. 23, 405–412 (2015).
Roesch, S., Rapp, C., Dettling, S. & Herold-Mende, C. When immune cells turn bad-tumor-associated microglia/macrophages in glioma. Int. J. Mol. Sci. 19, E436 (2018).
Okolie, O. et al. Reactive astrocytes potentiate tumor aggressiveness in a murine glioma resection and recurrence model. Neuro Oncol. 18, 1622–1633 (2016).
Pencheva, N. et al. Identification of a druggable pathway controlling glioblastoma invasiveness. Cell Rep. 20, 48–60 (2017).
Aspelund, A. et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 212, 991–999 (2015).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015).
Boussiotis, V. A. & Charest, A. Immunotherapies for malignant glioma. Oncogene 15, 1121–1141 (2017).
Thuringer, D. et al. Transfer of functional microRNAs between glioblastoma and microvascular endothelial cells through gap junctions. Oncotarget 7, 73925–73934 (2016).
Hong, X., Sin, W. C., Harris, A. L. & Naus, C. C. Gap junctions modulate glioma invasion by direct transfer of microRNA. Oncotarget 6, 15566–15577 (2015).
Balça-Silva, J. et al. The expression of connexins and SOX2 reflects the plasticity of glioma stem-like cells. Transl Oncol. 10, 555–569 (2017).
Tkach, M. & Théry, C. Communication by extracellular vesicles: where we are and where we need to go. Cell 164, 1226–1232 (2016).
Maas, S. L., Breakefield, X. O. & Weaver, A. M. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 27, 172–188 (2017).
Minciacchi, V. R. et al. Large oncosomes contain distinct protein cargo and represent a separate functional class of tumor-derived extracellular vesicles. Oncotarget 6, 11327–11341 (2015).
Rilla, K. et al. Hyaluronan production enhances shedding of plasma membrane-derived microvesicles. Exp. Cell Res. 319, 2006–2018 (2013).
Lai, C. P. et al. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano 8, 483–494 (2014).
Fonseca, P., Vardaki, I., Occhionero, A. & Panaretakis, T. Metabolic and signaling functions of cancer cell-derived extracellular vesicles. Int. Rev. Cell. Mol. Biol. 326, 175–199 (2016).
D’Asti, E., Chennakrishnaiah, S., Lee, T. H. & Rak, J. Extracellular vesicles in brain tumor progression. Cell. Mol. Neurobiol. 36, 383–407 (2016).
Redzic, J., Balaj, L., van der Vos, K. & Breakefield, X. O. Extracellular RNA mediates and marks cancer progression. Semin. Cancer Biol. 28, 14–23 (2014).
Wang, X., Veruki, M. L., Bukoreshtliev, N. V., Hartveit, E. & Gerdes, H. H. Animal cells connected by nanotubes can be electrically coupled through interposed gap-junction channels. Proc. Natl Acad. Sci. USA 107, 17194–17199 (2010).
Vignais, M. L., Caicedo, A., Brondello, J. M. & Jorgensen, C. Cell connections by tunneling nanotubes: effects of mitochondrial trafficking on target cell metabolism, homeostasis, and response to therapy. Stem Cells Int. 2017, 6917941 (2017).
Osswald, M. et al. Brain tumour cells interconnect to a functional and resistant network. Nature 528, 93–98 (2015).
Weil, S. et al. Tumor microtubes convey resistance to surgical lesions and chemotherapy in gliomas. Neuro Oncol. 19, 1316–1326 (2017).
van der Vos, K. E. et al. Directly visualized glioblastoma-derived extracellular vesicles transfer RNA to microglia/macrophages in the brain. Neuro Oncol. 18, 58–69 (2016).
Bowman, R. L. et al. Macrophage ontogeny underlies differences in tumor-specific education in brain malignancies. Cell Rep. 17, 2445–2459 (2016).
Chen, Z. et al. Cellular and molecular identity of tumor-associated macrophages in glioblastoma. Cancer Res. 77, 2266–2278 (2017).
Ajami, B., Bennett, J. L., Krieger, C., Tetzlaff, W. & Rossi, F. M. V. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 10, 1538–1543 (2007).
Ginhoux, F. et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845 (2010).
Hickman, S. E. et al. The microglial sensome revealed by direct RNA sequencing. Nat. Neurosci. 16, 1896–1905 (2013).
Müller, A., Brandenburg, S., Turkowski, K., Müller, S. & Vajkoczy, P. Resident microglia, and not peripheral macrophages, are the main source of brain tumor mononuclear cells. Int. J. Cancer 137, 278–288 (2015).
Hambardzumyan, D., Gutmann, D. H. & Kettenmann, H. The role of microglia and macrophages in glioma maintenance and progression. Nat. Neurosci. 19, 20–27 (2016).
Li, W. & Graeber, M. B. The molecular profile of microglia under the influence of glioma. Neuro Oncol. 14, 958–978 (2012).
Zhou, W. et al. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat. Cell Biol. 17, 170–182 (2015).
Alieva, M. et al. Preventing inflammation inhibits biopsy-mediated changes in tumor cell behavior. Sci. Rep. 7, 7529 (2017).
Chang, A. L. et al. CCL2 produced by the glioma microenvironment is essential for the recruitment of regulatory T cells and myeloid-derived suppressor cells. Cancer Res. 76, 5671–5682 (2016).
Wurdinger, T., Deumelandt, K., van der Vliet, H. J., Wesseling, P. & de Gruijl, T. D. Mechanisms of intimate and long-distance cross-talk between glioma and myeloid cells: how to break a vicious cycle. Biochim. Biophys. Acta 1846, 560–575 (2014).
de Vrij, J. et al. Glioblastoma-derived extracellular vesicles modify the phenotype of monocytic cells. Int. J. Cancer 137, 1630–1642 (2015).
Ransohoff, R. M. A polarizing question: do M1 and M2 microglia exist? Nat. Neurosci. 19, 987–991 (2016).
Mantovani, A., Sozzani, S., Locati, M., Allavena, P. & Sica, A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 23, 549–555 (2002).
Xue, J. et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 40, 274–288 (2014).
Gabrusiewicz, K. et al. Characteristics of the alternative phenotype of microglia/macrophages and its modulation in experimental gliomas. PLoS ONE 6, e23902 (2011).
Szulzewsky, F. et al. Glioma-associated microglia/macrophages display an expression profile different from M1 and M2 polarization and highly express Gpnmb and Spp1. PLoS ONE 10, e0116644 (2015).
Kim, C. C., Nakamura, M. C. & Hsieh, C. L. Brain trauma elicits non-canonical macrophage activation states. J. Neuroinflamm. 13, 117 (2016).
Kessenbrock, K., Plaks, V. & Werb, Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141, 52–67 (2010).
Du, R. et al. Matrix metalloproteinase-2 regulates vascular patterning and growth affecting tumor cell survival and invasion in GB. Neuro Oncol. 10, 254–264 (2008).
Hu, F. et al. Glioma-derived versican promotes tumor expansion via glioma-associated microglial/macrophages Toll-like receptor 2 signaling. Neuro Oncol. 17, 200–210 (2015).
Brandenburg, S. et al. Resident microglia rather than peripheral macrophages promote vascularization in brain tumors and are source of alternative pro-angiogenic factors. Acta Neuropathol. 131, 365–378 (2016).
Nijaguna, M. B. et al. Glioblastoma-derived macrophage colony-stimulating factor (MCSF) induces microglial release of insulin-like growth factor-binding protein 1 (IGFBP1) to promote angiogenesis. J. Biol. Chem. 290, 23401–23415 (2015).
Chen, X. et al. RAGE expression in tumor-associated macrophages promotes angiogenesis in glioma. Cancer Res. 74, 7285–7297 (2014).
Fossati, G. et al. Neutrophil infiltration into human gliomas. Acta Neuropathol. 98, 349–354 (1999).
Põlajeva, J. et al. Mast cell accumulation in glioblastoma with a potential role for stem cell factor and chemokine CXCL12. PLoS ONE 6, e25222 (2011).
Kolaczkowska, E. & Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175 (2013).
Sionov, R. V., Fridlender, Z. G. & Granot, Z. The multifaceted roles neutrophils play in the tumor microenvironment. Cancer Microenviron. 8, 125–158 (2015).
Põlajeva, J. et al. Glioma-derived macrophage migration inhibitory factor (MIF) promotes mast cell recruitment in a STAT5-dependent manner. Mol. Oncol. 8, 50–58 (2014).
Roy, A. et al. Glioma-derived plasminogen activator inhibitor-1 (PAI-1) regulates the recruitment of LRP1 positive mast cells. Oncotarget 6, 23647–23661 (2015).
Attarha, S., Roy, A., Westermark, B. & Tchougounova, E. Mast cells modulate proliferation, migration and stemness of glioma cells through downregulation of GSK3β expression and inhibition of STAT3 activation. Cell. Signal. 37, 81–92 (2017).
Compston, A. & Coles, A. Multiple sclerosis. Lancet 372, 1502–1517 (2008).
Dalmau, J. & Rosenfeld, M. R. Paraneoplastic syndromes of the CNS. Lancet Neurol. 7, 327–340 (2008).
Berger, J. R. & Koralnik, I. J. Progressive multifocal leukoencephalopathy and natalizumab—unforeseen consequences. N. Engl. J. Med. 353, 414–416 (2005).
Calzascia, T. et al. Homing phenotypes of tumor-specific CD8 T cells are predetermined at the tumor site by crosspresenting APCs. Immunity 22, 175–184 (2005).
Galea, I., Bechmann, I. & Perry, V. H. What is immune privilege (not)? Trends Immunol. 28, 12–18 (2007).
Alexandrov, L. B. et al. Signatures of mutational processes in human cancer. Nature 500, 415–421 (2013).
Lohr, J. et al. Effector T cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-β. Clin. Cancer Res. 17, 4296–4308 (2011).
Kim, Y. H. et al. Tumour-infiltrating T cell subpopulations in glioblastomas. Br. J. Neurosurg. 26, 21–27 (2012).
Kmiecik, J. et al. Elevated CD3+ and CD8+ tumor-infiltrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level. J. Neuroimmunol. 264, 71–83 (2013).
Han, S. et al. Tumour-infiltrating CD4(+) and CD8(+) lymphocytes as predictors of clinical outcome in glioma. Br. J. Cancer 110, 2560–2568 (2014).
Donson, A. M. et al. Increased immune gene expression and immune cell infiltration in high-grade astrocytoma distinguish long-term from short-term survivors. J. Immunol. 189, 1920–2197 (2012).
Cserr, H. F. & Knopf, P. M. Cervical lymphatics, the blood-brain barrier and the immunoreactivity of the brain: a new view. Immunol. Today 13, 507–512 (1992).
Reboldi, A. et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat. Immunol. 10, 514–523 (2009).
Schwyzer, M. & Fontana, A. Partial purification and biochemical characterization of a T cell suppressor factor produced by human glioblastoma cells. J. Immunol. 134, 1003–1009 (1985).
Masson, F. et al. Brain microenvironment promotes the final functional maturation of tumor-specific effector CD8+ T cells. J. Immunol. 179, 845–853 (2007).
Thomas, D. A. & Massagué, J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 8, 369–380 (2005).
Weller, M. et al. CD95-dependent T cell killing by glioma cells expressing CD95 ligand: more on tumor immune escape, the CD95 counterattack, and the immune privilege of the brain. Cell Physiol. Biochem. 7, 282–288 (1997).
Berghoff, A. S. et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol. 17, 1064–1075 (2015).
Wainwright, D. A. et al. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin. Cancer Res. 18, 6110–6121 (2012).
Bao, S. et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444, 756–760 (2006).
Valdor, R. et al. Glioblastoma progression is assisted by induction of immunosuppressive function of pericytes through interaction with tumor cells. Oncotarget 8, 68614–68626 (2017).
Speiser, D. E., Ho, P. C. & Verdeil, G. Regulatory circuits of T cell function in cancer. Nat. Rev. Immunol. 16, 599–6110 (2016).
Wherry, E. J. & Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 15, 486–499 (2015).
Martinez, G. J. et al. The transcription factor NFAT promotes exhaustion of activated CD8+ T cells. Immunity 42, 265–278 (2015).
Bauer, C. A. et al. Dynamic Treg interactions with intratumoral APCs promote local CTL dysfunction. J. Clin. Invest. 124, 2425–2450 (2014).
Park, B. V. et al. TGFβ1-mediated SMAD3 enhances PD-1 expression on antigen-specific T cells in cancer. Cancer Discov. 6, 1366–1381 (2016).
Voron, T. et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J. Exp. Med. 212, 139–148 (2015).
Spranger, S. et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci. Transl Med. 5, 200ra116 (2013).
Parsa, A. T. et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat. Med. 13, 84–88 (2007).
Reardon, D. A. et al. Glioblastoma eradication following immune checkpoint blockade in an orthotopic, immunocompetent model. Cancer Immunol. Res. 4, 124–135 (2016).
Snyder, A., Wolchok, J. D. & Chan, T. A. Genetic basis for clinical response to CTLA-4 blockade. N. Engl. J. Med. 372, 783 (2015).
Daud, A. I. et al. Tumor immune profiling predicts response to anti-PD-1 therapy in human melanoma. J. Clin. Invest. 126, 3447–3452 (2016).
Omuro, A. et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: results from exploratory phase 1 cohorts of CheckMate 143. Neuro. Oncol. 20, 674–686 (2017).
Reardon, D. A. et al. OS10.3 randomized phase 3 study evaluating the efficacy and safety of nivolumab versus bevacizumab in patients with recurrent glioblastoma: CheckMate 143. Neuro. Oncol. 19 (Suppl. 3), iii21 (2017).
Long, G. V. et al. A randomized phase II study of nivolumab or nivolumab combined with ipilimumab in patients (pts) with melanoma brain metastases (mets): the Anti-PD1 Brain Collaboration (ABC) (abstract 9508). J. Clin. Oncol. 35 (Suppl. 15), 9508 (2017).
Sharma, A. & Shiras, A. Cancer stem cell-vascular endothelial cell interactions in glioblastoma. Biochem. Biophys. Res. Commun. 473, 688–692 (2016).
Treps, L., Perret, R., Edmond, S., Ricard, D. & Gavard, J. Glioblastoma stem-like cells secrete the pro-angiogenic VEGF-A factor in extracellular vesicles. J. Extracell. Vesicles 6, 1359479 (2017).
Rupp, T. et al. Tenascin-C orchestrates glioblastoma angiogenesis by modulation of pro- and anti-angiogenic signaling. Cell Rep. 17, 2607–2619 (2016).
Soda, Y. et al. Transdifferentiation of glioblastoma cells into vascular endothelial cells. Proc. Natl Acad. Sci. USA 108, 4274–4280 (2011).
Guelfi, S., Duffau, H., Bauchet, L., Rothhut, B. & Hugnot, J. P. Vascular transdifferentiation in the CNS: a focus on neural and glioblastoma stem-like cells. Stem Cells Int. 2016, 2759403 (2016).
Mei, X., Chen, Y. S., Chen, F. R., Xi, S. Y. & Chen, Z. P. Glioblastoma stem cell differentiation into endothelial cells evidenced through live-cell imaging. Neuro Oncol. 19, 1109–1118 (2017).
Hu, B. et al. Epigenetic activation of WNT5A drives glioblastoma stem cell differentiation and invasive growth. Cell 167, 1281–1295.e1218 (2016).
Peterson, T. E. et al. Dual inhibition of Ang-2 and VEGF receptors normalizes tumor vasculature and prolongs survival in glioblastoma by altering macrophages. Proc. Natl Acad. Sci. USA 113, 4470–4475 (2016).
Watkins, S. et al. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat. Commun. 5, 4196 (2014).
Wen, L. et al. VEGF-mediated tight junctions pathological fenestration enhances doxorubicin-loaded glycolipid-like nanoparticles traversing BBB for glioblastoma-targeting therapy. Drug Deliv. 24, 1843–1855 (2017).
Treps, L. et al. Extracellular vesicle-transported semaphorin3A promotes vascular permeability in glioblastoma. Oncogene 35, 2615–2623 (2016).
Xu, B. et al. Neurons secrete miR-132-containing exosomes to regulate brain vascular integrity. Cell Rep. 27, 882–897 (2017).
Miller, J. J. & Wen, P. Y. Emerging targeted therapies for glioma. Expert Opin. Emerg. Drugs 21, 441–452 (2016).
Buckingham, S. C. et al. Glutamate release by primary brain tumors induces epileptic activity. Nat. Med. 17, 1269–1274 (2011).
Sattler, R. et al. Increased expression of glutamate transporter GLT-1 in peritumoral tissue associated with prolonged survival and decreases in tumor growth in a rat model of experimental malignant glioma. J. Neurosurg. 119, 878–886 (2013).
Vazana, U. et al. Glutamate-mediated blood-brain barrier opening: implications for neuroprotection and drug delivery. J. Neurosci. 36, 7727–7739 (2016).
Wei, Z. et al. Full-coverage landscape of extracellular RNAs, coding and non-coding, released by human glioma stem cells. Nat. Commun. https://doi.org/10.1038/s41467-017-01196-x (2017).
Kim, H. et al. Integrative genome analysis reveals an oncomir/oncogene cluster regulating glioblastoma survivorship. Proc. Natl Acad. Sci. USA 107, 2183–2188 (2010).
Teplyuk, N. M. et al. MicroRNA-10b inhibition reduces E2F1-mediated transcription and miR-15/16 activity in glioblastoma. Oncotarget 6, 3770–3783 (2015).
Herrup, K. & Yang, Y. Cell cycle regulation in the postmitotic neuron: oxymoron or new biology? Nat. Rev. Neurosci. 8, 368–378 (2007).
Absalon, S., Kochanek, D. M., Raghavan, V. & Krichevsky, A. M. MiR-26b, upregulated in Alzheimer’s disease, activates cell cycle entry, tau-phosphorylation, and apoptosis in postmitotic neurons. J. Neurosci. 33, 14645–14659 (2013).
Takano, T. et al. Glutamate release promotes growth of malignant gliomas. Nat. Med. 7, 1010–1015 (2001).
Ishiuchi, S. et al. Ca2+-permeable AMPA receptors regulate growth of human glioblastoma via Akt activation. J. Neurosci. 27, 7987–8001 (2007).
El-Habr, E. A. et al. A driver role for GABA metabolism in controlling stem and proliferative cell state through GHB production in glioma. Acta Neuropathol. 133, 645–660 (2017).
Venkatesh, H. S. et al. Neuronal activity promotes glioma growth through neuroligin-3 secretion. Cell 161, 803–816 (2015).
Venkatesh, H. S. et al. Targeting neuronal activity-regulated neuroligin-3 dependency in high-grade glioma. Nature 549, 533–537 (2017).
Li, F., Liu, X., Sampson, J. H., Bigner, D. D. & Li, C. Y. Rapid reprogramming of primary human astrocytes into potent tumor-initiating cells with defined genetic factors. Cancer Res. 76, 5143–5150 (2016).
Jahani-Asl, A. et al. Control of glioblastoma tumorigenesis by feed-forward cytokine signaling. Nat. Neurosci. 19, 798–806 (2016).
Biasoli, D. et al. Glioblastoma cells inhibit astrocytic p53-expression favoring cancer malignancy. Oncogenesis 3, e123 (2014).
Lemée, J. M., Clavreul, A. & Menei, P. Intratumoral heterogeneity in glioblastoma: don’t forget the peritumoral brain zone. Neuro Oncol. 17, 1322–1332 (2015).
Mangiola, A. et al. Gene expression profile of glioblastoma peritumoral tissue: an ex vivo study. PLoS ONE 8, e57145 (2013).
Leiss, L. et al. Tumour-associated glial host cells display a stem-like phenotype with a distinct gene expression profile and promote growth of GB xenografts. BMC Cancer 17, 108 (2017).
Iwadate, Y., Fukuda, K., Matsutani, T. & Saeki, N. Intrinsic protective mechanisms of the neuron-glia network against glioma invasion. J. Clin. Neurosci. 26, 19–25 (2016).
El Fatimy, R., Subramanian, S., Uhlmann, E. J. & Krichevsky, A. M. Genome editing reveals glioblastoma addiction to microRNA-10b. Mol. Ther. 25, 368–378 (2017).
Yuan, J. X., Bafakih, F. F., Mandell, J. W., Horton, B. J. & Munson, J. M. Quantitative analysis of the cellular microenvironment of glioblastoma to develop predictive statistical models of overall survival. J. Neuropathol. Exp. Neurol. 75, 1110–1123 (2016).
Rath, B. H., Fair, J. M., Jamal, M., Camphausen, K. & Tofilon, P. J. Astrocytes enhance the invasion potential of glioblastoma stem-like cells. PLoS ONE 8, e54752 (2013).
Roos, A., Ding, Z., Loftus, J. C. & Tran, N. L. Molecular and microenvironmental determinants of glioma stem-like cell survival and invasion. Front. Oncol. 7, 120 (2017).
Asslaber, M. et al. Native oligodendrocytes in astrocytomas might inhibit tumor proliferation by WIF1 expression. J. Neuropathol. Exp. Neurol. 76, 16–26 (2017).
Peferoen, L., Kipp, M., van der Valk, P., van Noort, J. M. & Amor, S. Oligodendrocyte-microglia cross-talk in the central nervous system. Immunology 141, 302–313 (2014).
Liddelow, S. A. et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017).
Trajkovic, K. et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319, 1244–1247 (2008).
Dinkins, M. B., Dasgupta, S., Wang, G., Zhu, G. & Bieberich, E. Exosome reduction in vivo is associated with lower amyloid plaque load in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol. Aging 35, 1792–1800 (2014).
Asai, H. et al. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat. Neurosci. 18, 1584–1593 (2015).
Phuyal, S., Hessvik, N. P., Skotland, T., Sandvig, K. & Llorente, A. Regulation of exosome release by glycosphingolipids and flotillins. FEBS J. 281, 2214–2227 (2014).
Atai, N. A. et al. Heparin blocks transfer of extracellular vesicles between donor and recipient cells. J. Neurooncol 115, 343–351 (2013).
Jansen, F. et al. Endothelial microparticle uptake in target cells is annexin I/phosphatidylserine receptor dependent and prevents apoptosis. Arterioscler Thromb. Vasc. Biol. 32, 1925–1935 (2012).
Chen, Q. et al. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature 533, 493–498 (2016).
Ulrich, T. A., de Juan Pardo, E. M. & Kumar, S. The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res. 69, 4167–4174 (2009).
Nuti, E. et al. Bifunctional inhibitors as a new tool to reduce cancer cell invasion by impairing MMP-9 homodimerization. ACS Med. Chem. Lett. 8, 293–298 (2017).
Barker, H. E., Paget, J. T., Khan, A. A. & Harrington, K. J. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat. Rev. Cancer 15, 409–425 (2015).
Pyonteck, S. M. et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 19, 1264–1272 (2013).
Quail, D. F. et al. The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science 352, aad3018 (2016).
Butowski, N. et al. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an ivy foundation early phase clinical trials consortium phase II study. Neuro Oncol. 18, 557–564 (2016).
Brennan, C. W. et al. The somatic genomic landscape of glioblastoma. Cell 155, 462–477 (2013).
Prins, R. M. et al. Gene expression profile correlates with T cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin. Cancer Res. 17, 1603–1615 (2011).
Reardon, D. A. et al. Randomized phase 3 study evaluating the efficacy and safety of nivolumab versus bevacizumab in patients with recurrent glioblastoma: checkmate 143. Neuro. Oncol. 19 (suppl. 3), iii21 (2017).
Sampson, J. H. et al. A randomized, phase 3, open-label study of nivolumab versus temozolomide (TMZ) in combination with radiotherapy (RT) in adult patients (pts) with newly diagnosed, O-6-methylguanine DNA methyltransferase (MGMT)-unmethylated glioblastoma (GBM): CheckMate-498. J. Clin. Oncol. 34 (Suppl. 15), TPS2079 (2016).
Weller, M. et al. A randomized phase 2, single-blind study of temozolomide (TMZ) and radiotherapy (RT) combined with nivolumab or placebo (PBO) in newly diagnosed adult patients (pts) with tumor O6-methylguanine DNA methyltransferase (MGMT)-methylated glioblastoma (GBM)—CheckMate-548. Ann. Oncol. 27 (Suppl. 6), 356TiP (2016).
Bouffet, E. et al. Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J. Clin. Oncol. 34, 2206–2211 (2016).
Saha, D., Martuza, R. L. & Rabkin, S. D. Macrophage polarization contributes to glioblastoma eradication by combination immunovirotherapy and immune checkpoint blockade. Cancer Cell 32, 253–267.e255 (2017).
Jiang, H. et al. Oncolytic adenovirus and tumor-targeting immune modulatory therapy improve autologous cancer vaccination. Cancer Res. 77, 3894–3907 (2017).
Khasraw, M., Ameratunga, M. S., Grant, R., Wheeler, H. & Pavlakis, N. Antiangiogenic therapy for high-grade glioma. Cochrane Database Syst. Rev. 9, CD008218 (2014).
Park, J. S. et al. Normalization of tumor vessels by Tie2 activation and Ang2 inhibition enhances drug delivery and produces a favorable tumor microenvironment. Cancer Cell 30, 953–967 (2016).
Infante, J., Burris, H. A. & Lewis, N. A multicenter phase Ib study of the safety, pharmacokinetics, biological activity and clinical efficacy of INCB7839, a potent and selective inhibitor of ADAM10 and ADAM17. Breast Cancer Res. Treat. 106, S269 (2007).
Friedman, S. et al. Clinical benefit of INCB7839, a potent and selective inhibitor of ADAM10 and ADAM17, in combination with trastuzumab in metastatic HER2 positive breast cancer patients. Cancer Res. 69, 5056 (2014).
Kim, S. S., Pirollo, K. F. & Chang, E. H. Isolation and culturing of glioma cancer stem cells. Curr. Protoc. Cell Biol. 67, 10.21–10 (2015).
Hubert, C. G. et al. A three-dimensional organoid culture system derived from human glioblastomas recapitulates the hypoxic gradients and cancer stem cell heterogeneity of tumors found in vivo. Cancer Res. 76, 2465–2477 (2016).
Hira, V. V. V. et al. Periarteriolar glioblastoma stem cell niches express bone marrow hematopoietic stem cell niche proteins. J. Histochem. Cytochem. 66, 155–173 (2018).
Calabrese, C. et al. A perivascular niche for brain tumor stem cells. Cancer Cell 11, 69–82 (2007).
Xu, Z., Kader, M., Sen, R. & Placantonakis, D. G. Orthotopic patient-derived glioblastoma xenografts in mice. Methods Mol. Biol. 1741, 183–190 (2018).
William, D. et al. Optimized creation of glioblastoma patient derived xenografts for use in preclinical studies. J. Transl Med. 15, 27 (2017).
Oh, T. et al. Immunocompetent murine models for the study of glioblastoma immunotherapy. J. Transl Med. 12, 107 (2014).
Hambardzumyan, D., Parada, L. F., Holland, E. C. & Charest, A. Genetic modeling of gliomas in mice: new tools to tackle old problems. Glia 59, 1155–1168 (2011).
Ben-David, U. et al. Patient-derived xenografts undergo mouse-specific tumor evolution. Nat. Genet. 49, 1567–1575 (2017).
Baysan, M. et al. Micro-environment causes reversible changes in DNA methylation and mRNA expression profiles in patient-derived glioma stem cells. PLoS ONE 9, e94045 (2014).
Bigner, S. H., Mark, J. & Bigner, D. D. Chromosomal progression of malignant human gliomas from biopsy to establishment as permanent lines in vitro. Cancer Genet. Cytogenet. 24, 163–176 (1987).
Beutler, A. S., Banck, M. S., Wedekind, D. & Hedrich, H. J. Tumor gene therapy made easy: allogeneic major histocompatibility complex in the C6 rat gliomamodel. Hum. Gene Ther. 10, 95–101 (1999).
Patel, A. P. et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 344, 1396–1401 (2014).
Wang, Q. et al. Tumor evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell 32, 42–56.e46 (2017).
Louis, D. N. et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 131, 803–820 (2016).
Flavahan, W. A. et al. Insulator dysfunction and oncogene activation in IDH mutant gliomas. Nature 529, 110–114 (2016).
Parsons, D. W. et al. An integrated genomic analysis of human glioblastoma multiforme. Science 321, 1807–1812 (2008).
Frattini, V. et al. The integrated landscape of driver genomic alterations in glioblastoma. Nat. Genet. 45, 1141–1149 (2013).
Acknowledgements
The authors thank S. McDavitt for her skilled editorial assistance. This work was supported by U19 CA179563 by the US NIH Common Fund, through the Office of Strategic Coordination/Office of the NIH Director (X.O.B., A.M.K. and T.R.M.), and the US NIH National Cancer Institute (P01 CA069246 (X.O.B.), R01 AI123349 (T.R.M.) and R21 NS098051 (A.M.K.)).
Reviewer information
Nature Reviews Neurology thanks W. Wick and the other, anonymous reviewers for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
All authors contributed to researching data, discussion of content, writing and review of the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Broekman, M.L., Maas, S.L.N., Abels, E.R. et al. Multidimensional communication in the microenvirons of glioblastoma. Nat Rev Neurol 14, 482–495 (2018). https://doi.org/10.1038/s41582-018-0025-8
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-018-0025-8
This article is cited by
-
Mesenchymal glioma stem cells trigger vasectasia—distinct neovascularization process stimulated by extracellular vesicles carrying EGFR
Nature Communications (2024)
-
Shooting the messenger: a systematic review investigating extracellular vesicle isolation and characterisation methods and their influence on understanding extracellular vesicles-radiotherapy interactions in glioblastoma
BMC Cancer (2023)
-
On the origin and development of glioblastoma: multifaceted role of perivascular mesenchymal stromal cells
Acta Neuropathologica Communications (2023)
-
A transformer-based multi-task deep learning model for simultaneous infiltrated brain area identification and segmentation of gliomas
Cancer Imaging (2023)
-
Effects of RNA methylation on Tumor angiogenesis and cancer progression
Molecular Cancer (2023)