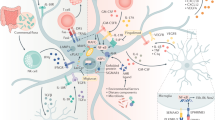
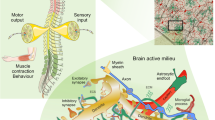
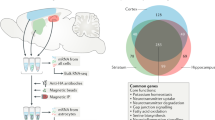
Abstract
Our understanding of astrocytes and their role in neurological diseases has increased considerably over the past two decades as the diverse roles of these cells have become recognized. Our evolving understanding of these cells suggests that they are more than support cells for neurons and that they play important roles in CNS homeostasis under normal conditions, in neuroprotection and in disease exacerbation. These multiple functions make them excellent candidates for targeted therapies to treat neurological disorders. New technological advances, including in vivo imaging, optogenetics and chemogenetics, have allowed us to examine astrocytic functions in ways that have uncovered new insights into the dynamic roles of these cells. Furthermore, the use of induced pluripotent stem cell-derived astrocytes from patients with a host of neurological disorders can help to tease out the contributions of astrocytes to human disease. In this Review, we explore some of the technological advances developed over the past decade that have aided our understanding of astrocyte function. We also highlight neurological disorders in which astrocyte function or dysfunction is believed to have a role in disease pathogenesis or propagation and discuss how the technological advances have been and could be used to study each of these diseases.
Key points
-
Astrocytes not only have key homeostatic functions in the CNS but also respond to neuronal injury in both neuroprotective and pathological manners.
-
Astrocytes have key roles in a broad spectrum of neurodevelopmental and neurodegenerative diseases.
-
New tools have been developed to evaluate the structural, functional and molecular mechanisms by which astrocytes respond to injury.
-
The in vivo methods by which astrocytes can be studied have revealed new layers of complexity in astrocyte function, which could not have been appreciated with the use of older experimental approaches.
-
The use of induced pluripotent stem cell-derived astrocytes could help with interpretation of preclinical observations as they are used to direct the design of human therapeutics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Sofroniew, M. V. & Vinters, H. V. Astrocytes: biology and pathology. Acta Neuropathol. 119, 7–35 (2010).
Pekny, M. & Pekna, M. Reactive gliosis in the pathogenesis of CNS diseases. Biochim. Biophys. Acta 1862, 483–491 (2016).
Khakh, B. S. & Sofroniew, M. V. Diversity of astrocyte functions and phenotypes in neural circuits. Nature Neurosci. 18, 942–952 (2015).
Molofsky, A. V. & Deneen, B. Astrocyte development: a guide for the perplexed. Glia 63, 1320–1329 (2015).
Pellerin, L. & Magistretti, P. J. Neuroenergetics: calling upon astrocytes to satisfy hungry neurons. Neuroscientist 10, 53–62 (2004).
Haydon, P. G. The evolving view of astrocytes. Cerebrum 1, 12–16 (2016).
Araque, A. et al. Gliotransmitters travel in time and space. Neuron 81, 728–739 (2014).
Alvarez, J. I., Katayama, T. & Prat, A. Glial influence on the blood brain barrier. Glia 61, 1939–1958 (2013).
Helmchen, F. & Kleinfeld, D. Chapter 10 in vivo measurements of blood flow and glial cell function with two-photon laser-scanning microscopy. Methods Enzymol. 444, 231–254 (2008).
Haber, M., Zhou, L. & Murai, K. K. Cooperative astrocyte and dendritic spine dynamics at hippocampal excitatory synapses. J. Neurosci. 26, 8881–8891 (2006).
Chung, W. S., Allen, N. J. & Eroglu, C. Astrocytes control synapse formation, function, and elimination. Cold Spring Harb. Perspect. Biol. 7, a020370 (2015).
Losi, G., Mariotti, L., Sessolo, M. & Carmignoto, G. New tools to study astrocyte Ca2+ signal dynamics in brain networks in vivo. Front. Cell Neurosci. 11, 134 (2017).
Li, D., Agulhon, C., Schmidt, E., Oheim, M. & Ropert, N. New tools for investigating astrocyte-to-neuron communication. Front. Cell Neurosci. 7, 193 (2013).
Bardehle, S. et al. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat. Neurosci. 16, 580–586 (2013).
Horton, N. G. et al. In vivo three-photon microscopy of subcortical structures within an intact mouse brain. Nat. Photonics 7, 205–209 (2013).
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Gourine, A. V. et al. Astrocytes control breathing through pH-dependent release of ATP. Science 329, 571–575 (2010).
Perea, G., Yang, A., Boyden, E. S. & Sur, M. Optogenetic astrocyte activation modulates response selectivity of visual cortex neurons in vivo. Nat. Commun. 5, 3262 (2014).
Nam, Y. et al. Reversible induction of pain hypersensitivity following optogenetic stimulation of spinal astrocytes. Cell Rep. 17, 3049–3061 (2016).
Poskanzer, K. E. & Yuste, R. Astrocytes regulate cortical state switching in vivo. Proc. Natl Acad. Sci. USA 113, E2675–E2684 (2016).
Beppu, K. et al. Optogenetic countering of glial acidosis suppresses glial glutamate release and ischemic brain damage. Neuron 81, 314–320 (2014).
Roth, B. L. DREADDs for Neuroscientists. Neuron 89, 683–694 (2016).
Armbruster, B. N., Li, X., Pausch, M. H., Herlitze, S. & Roth, B. L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl Acad. Sci. USA 104, 5163–5168 (2007).
Whissell, P. D., Tohyama, S. & Martin, L. J. The use of DREADDs to deconstruct behavior. Front. Genet. 7, 70 (2016).
Davila, D., Thibault, K., Fiacco, T. A. & Agulhon, C. Recent molecular approaches to understanding astrocyte function in vivo. Front. Cell Neurosci. 7, 272 (2013).
Agulhon, C. et al. Modulation of the autonomic nervous system and behaviour by acute glial cell Gq protein-coupled receptor activation in vivo. J. Physiol. 591, 5599–5609 (2013).
Scofield, M. D. et al. Gq-DREADD Selectively initiates glial glutamate release and inhibits cue-induced cocaine seeking. Biol. Psychiatry 78, 441–451 (2015).
Vardy, E. et al. A new DREADD facilitates the multiplexed chemogenetic interrogation of behavior. Neuron 86, 936–946 (2015).
Srinivasan, R. et al. Ca(2+) signaling in astrocytes from Ip3r2(−/−) mice in brain slices and during startle responses in vivo. Nat. Neurosci. 18, 708–717 (2015).
Shigetomi, E., Kracun, S. & Khakh, B. S. Monitoring astrocyte calcium microdomains with improved membrane targeted GCaMP reporters. Neuron Glia Biol. 6, 183–191 (2010).
Di Castro, M. A. et al. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat. Neurosci. 14, 1276–1284 (2011).
Bindocci, E. et al. Three-dimensional Ca2+ imaging advances understanding of astrocyte biology. Science 356, eaai8185 (2017).
Okubo, Y. et al. Imaging extrasynaptic glutamate dynamics in the brain. Proc. Natl Acad. Sci. USA 107, 6526–6531 (2010).
Marvin, J. S. et al. An optimized fluorescent probe for visualizing glutamate neurotransmission. Nat. Methods 10, 162–170 (2013).
Brancaccio, M., Patton, A. P., Chesham, J. E., Maywood, E. S. & Hastings, M. H. Astrocytes control circadian timekeeping in the suprachiasmatic nucleus via glutamatergic signaling. Neuron 93, 1420–1435 (2017).
Harada, K. et al. Red fluorescent protein-based cAMP indicator applicable to optogenetics and in vivo imaging. Sci. Rep. 7, 7351 (2017).
Rimmele, T. S. & Chatton, J. Y. A novel optical intracellular imaging approach for potassium dynamics in astrocytes. PLoS ONE 9, e109243 (2014).
Maragakis, N. J. & Rothstein, J. D. Mechanisms of disease: astrocytes in neurodegenerative disease. Nat. Clin. Pract. Neurol. 2, 679–689 (2006).
Haidet-Phillips, A. M. et al. Gene profiling of human induced pluripotent stem cell-derived astrocyte progenitors following spinal cord engraftment. Stem Cells Transl. Med. 3, 575–585 (2014).
Rinaldi, F., Motti, D., Ferraiuolo, L. & Kaspar, B. K. High content analysis in amyotrophic lateral sclerosis. Mol. Cell Neurosci. 80, 180–191 (2017).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Chandrasekaran, A., Avci, H. X., Leist, M., Kobolak, J. & Dinnyes, A. Astrocyte differentiation of human pluripotent stem cells: new tools for neurological disorder research. Front. Cell Neurosci. 10, 215 (2016).
Meyer, K. et al. Direct conversion of patient fibroblasts demonstrates non-cell autonomous toxicity of astrocytes to motor neurons in familial and sporadic ALS. Proc. Natl Acad. Sci. USA 111, 829–832 (2014).
Caiazzo, M. et al. Direct conversion of fibroblasts into functional astrocytes by defined transcription factors. Stem Cell Rep. 4, 25–36 (2015).
Tian, E. et al. Small-molecule-based lineage reprogramming creates functional astrocytes. Cell Rep. 16, 781–792 (2016).
Drouin-Ouellet, J. et al. REST suppression mediates neural conversion of adult human fibroblasts via microRNA-dependent and -independent pathways. EMBO Mol. Med. 9, 1117–1131 (2017).
Pasca, A. M. et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods 12, 671–678 (2015).
Sloan, S. A. et al. Human astrocyte maturation captured in 3D cerebral cortical spheroids derived from pluripotent stem cells. Neuron 95, 779–790 (2017).
Mertens, J., Marchetto, M. C., Bardy, C. & Gage, F. H. Evaluating cell reprogramming, differentiation and conversion technologies in neuroscience. Nat. Rev. Neurosci. 17, 424–437 (2016).
Myszczynska, M. & Ferraiuolo, L. New in vitro models to study amyotrophic lateral sclerosis. Brain Pathol. 26, 258–265 (2016).
Russo, L. S. Jr., Aron, A. & Anderson, P. J. Alexander’s disease: a report and reappraisal. Neurology 26, 607–614 (1976).
van der Knaap, M. S. et al. Alexander disease: ventricular garlands and abnormalities of the medulla and spinal cord. Neurology 66, 494–498 (2006).
Brenner, M. et al. Mutations in GFAP, encoding glial fibrillary acidic protein, are associated with Alexander disease. Nature Genet. 27, 117–120 (2001).
Li, R., Messing, A., Goldman, J. E. & Brenner, M. GFAP mutations in Alexander disease. Int. J. Dev. Neurosci. 20, 259–268 (2002).
Tang, G., Perng, M. D., Wilk, S., Quinlan, R. & Goldman, J. E. Oligomers of mutant glial fibrillary acidic protein (GFAP) Inhibit the proteasome system in alexander disease astrocytes, and the small heat shock protein alphaB-crystallin reverses the inhibition. J. Biol. Chem. 285, 10527–10537 (2010).
Messing, A., Brenner, M., Feany, M. B., Nedergaard, M. & Goldman, J. E. Alexander disease. J. Neurosci. 32, 5017–5023 (2012).
Messing, A. et al. Fatal encephalopathy with astrocyte inclusions in GFAP transgenic mice. Am. J. Pathol. 152, 391–398 (1998).
Walker, A. K. et al. Astrocytic TDP-43 pathology in Alexander disease. J. Neurosci. 34, 6448–6458 (2014).
Kondo, T. et al. Modeling Alexander disease with patient iPSCs reveals cellular and molecular pathology of astrocytes. Acta Neuropathol. Commun. 4, 69 (2016).
Amir, R. E. et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 23, 185–188 (1999).
Guy, J., Hendrich, B., Holmes, M., Martin, J. E. & Bird, A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat. Genet. 27, 322–326 (2001).
Patterson, K. C., Hawkins, V. E., Arps, K. M., Mulkey, D. K. & Olsen, M. L. MeCP2 deficiency results in robust Rett-like behavioural and motor deficits in male and female rats. Hum. Mol. Genet. 25, 3303–3320 (2016).
Okabe, Y. et al. Alterations of gene expression and glutamate clearance in astrocytes derived from an MeCP2-null mouse model of Rett syndrome. PLoS ONE 7, e35354 (2012).
Maezawa, I., Swanberg, S., Harvey, D., LaSalle, J. M. & Jin, L. W. Rett syndrome astrocytes are abnormal and spread MeCP2 deficiency through gap junctions. J. Neurosci. 29, 5051–5061 (2009).
Turovsky, E., Karagiannis, A., Abdala, A. P. & Gourine, A. V. Impaired CO2 sensitivity of astrocytes in a mouse model of Rett syndrome. J. Physiol. 593, 3159–3168 (2015).
Ballas, N., Lioy, D. T., Grunseich, C. & Mandel, G. Non-cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology. Nat. Neurosci. 12, 311–317 (2009).
Lioy, D. T. et al. A role for glia in the progression of Rett’s syndrome. Nature 475, 497–500 (2011).
Bebensee, D. F., Can, K. & Muller, M. Increased mitochondrial mass and cytosolic redox imbalance in hippocampal astrocytes of a mouse model of rett syndrome: subcellular changes revealed by ratiometric imaging of JC-1 and roGFP1 fluorescence. Oxid. Med. Cell. Longev. 2017, 3064016 (2017).
Delepine, C. et al. Altered microtubule dynamics and vesicular transport in mouse and human MeCP2-deficient astrocytes. Hum. Mol. Genet. 25, 146–157 (2016).
Olsen, M. L. et al. New insights on astrocyte ion channels: critical for homeostasis and neuron-glia signaling. J. Neurosci. 35, 13827–13835 (2015).
Williams, E. C. et al. Mutant astrocytes differentiated from Rett syndrome patients-specific iPSCs have adverse effects on wild-type neurons. Hum. Mol. Genet. 23, 2968–2980 (2014).
Thom, M. Review: hippocampal sclerosis in epilepsy: a neuropathology review. Neuropathol. Appl. Neurobiol. 40, 520–543 (2014).
Bedner, P. et al. Astrocyte uncoupling as a cause of human temporal lobe epilepsy. Brain 138, 1208–1222 (2015).
Tanaka, K. et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science 276, 1699–1702 (1997).
Bittner, C. X. et al. Fast and reversible stimulation of astrocytic glycolysis by K+ and a delayed and persistent effect of glutamate. J. Neurosci. 31, 4709–4713 (2011).
Hinterkeuser, S. et al. Astrocytes in the hippocampus of patients with temporal lobe epilepsy display changes in potassium conductances. Eur. J. Neurosci. 12, 2087–2096 (2000).
Schroder, W. et al. Functional and molecular properties of human astrocytes in acute hippocampal slices obtained from patients with temporal lobe epilepsy. Epilepsia 41 (Suppl. 6), S181–S184 (2000).
Bordey, A. & Sontheimer, H. Properties of human glial cells associated with epileptic seizure foci. Epilepsy Res. 32, 286–303 (1998).
Buono, R. J. et al. Association between variation in the human KCNJ10 potassium ion channel gene and seizure susceptibility. Epilepsy Res. 58, 175–183 (2004).
Dossi, E., Vasile, F. & Rouach, N. Human astrocytes in the diseased brain. Brain Res. Bull. 136, 139–156 (2017).
Hubbard, J. A., Szu, J. I. & Binder, D. K. The role of aquaporin-4 in synaptic plasticity, memory and disease. Brain Res. Bull. 136, 118–129 (2017).
Bedner, P. & Steinhauser, C. Altered Kir and gap junction channels in temporal lobe epilepsy. Neurochem. Int. 63, 682–687 (2013).
Kielbinski, M., Gzielo, K. & Soltys, Z. Review: roles for astrocytes in epilepsy: insights from malformations of cortical development. Neuropathol. Appl. Neurobiol. 42, 593–606 (2016).
Raimondo, J. V. et al. Tight coupling of astrocyte pH dynamics to epileptiform activity revealed by genetically encoded pH sensors. J. Neurosci. 36, 7002–7013 (2016).
Figueiredo, M. et al. Optogenetic experimentation on astrocytes. Exp. Physiol. 96, 40–50 (2011).
Ji, Z. G. & Wang, H. Optogenetic control of astrocytes: is it possible to treat astrocyte-related epilepsy? Brain Res. Bull. 110, 20–25 (2015).
Bristol, L. A. & Rothstein, J. D. Glutamate transporter gene expression in amyotrophic lateral sclerosis motor cortex. Ann. Neurol. 39, 676–679 (1996).
Lin, G., Bristol, L. A. & Rothstein, J. D. An abnormal mRNA leads to downregulation of glutamate transporter EAAT2 (GLT-1) expression in amyotrophic lateral sclerosis. Ann. Neurol. 40, 540–541 (1996).
Yamanaka, K. et al. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat. Neurosci. 11, 251–253 (2008).
Wang, L., Gutmann, D. H. & Roos, R. P. Astrocyte loss of mutant SOD1 delays ALS disease onset and progression in G85R transgenic mice. Hum. Mol. Genet. 20, 286–293 (2011).
Papadeas, S. T., Kraig, S. E., O’Banion, C., Lepore, A. C. & Maragakis, N. J. Astrocytes carrying the superoxide dismutase 1 (SOD1G93A) mutation induce wild-type motor neuron degeneration in vivo. Proc. Natl Acad. Sci. USA 108, 17803–17808 (2011).
Nagai, M. et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat. Neurosci. 10, 615–622 (2007).
Haidet-Phillips, A. M. et al. Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat. Biotechnol. 29, 824–828 (2011).
Re, D. B. et al. Necroptosis drives motor neuron death in models of both sporadic and familial ALS. Neuron 81, 1001–1008 (2014).
Almad, A. A. et al. Connexin 43 in astrocytes contributes to motor neuron toxicity in amyotrophic lateral sclerosis. Glia 64, 1154–1169 (2016).
Richard, J. P. & Maragakis, N. J. Induced pluripotent stem cells from ALS patients for disease modeling. Brain Res. 1607, 15–25 (2015).
Kawamata, H. et al. Abnormal intracellular calcium signaling and SNARE-dependent exocytosis contributes to SOD1G93A astrocyte-mediated toxicity in amyotrophic lateral sclerosis. J. Neurosci. 34, 2331–2348 (2014).
Agarwal, A. et al. Transient opening of the mitochondrial permeability transition pore induces microdomain calcium transients in astrocyte processes. Neuron 93, 587–605 (2017).
Bhat, R. et al. Astrocyte senescence as a component of Alzheimer’s disease. PLoS ONE 7, e45069 (2012).
Pike, C. J., Cummings, B. J., Monzavi, R. & Cotman, C. W. Beta-amyloid-induced changes in cultured astrocytes parallel reactive astrocytosis associated with senile plaques in Alzheimer’s disease. Neuroscience 63, 517–531 (1994).
Garwood, C. J. et al. Review: astrocytes in Alzheimer’s disease and other age-associated dementias: a supporting player with a central role. Neuropathol. Appl. Neurobiol. 43, 281–298 (2017).
Koistinaho, M. et al. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat. Med. 10, 719–726 (2004).
Alarcon, R., Fuenzalida, C., Santibanez, M. & von Bernhardi, R. Expression of scavenger receptors in glial cells. Comparing the adhesion of astrocytes and microglia from neonatal rats to surface-bound beta-amyloid. J. Biol. Chem. 280, 30406–30415 (2005).
Iram, T. et al. Astrocytes from old Alzheimer’s disease mice are impaired in Abeta uptake and in neuroprotection. Neurobiol. Dis. 96, 84–94 (2016).
Hartlage-Rubsamen, M. et al. Astrocytic expression of the Alzheimer’s disease beta-secretase (BACE1) is stimulus-dependent. Glia 41, 169–179 (2003).
Ben Haim, L. et al. The JAK/STAT3 pathway is a common inducer of astrocyte reactivity in Alzheimer’s and Huntington’s diseases. J. Neurosci. 35, 2817–2829 (2015).
Hefendehl, J. K. et al. Mapping synaptic glutamate transporter dysfunction in vivo to regions surrounding Abeta plaques by iGluSnFR two-photon imaging. Nat. Commun. 7, 13441 (2016).
Lim, D., Ronco, V., Grolla, A. A., Verkhratsky, A. & Genazzani, A. A. Glial calcium signalling in Alzheimer’s disease. Rev. Physiol. Biochem. Pharmacol. 167, 45–65 (2014).
Kuchibhotla, K. V., Lattarulo, C. R., Hyman, B. T. & Bacskai, B. J. Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science 323, 1211–1215 (2009).
Scott, H. A., Gebhardt, F. M., Mitrovic, A. D., Vandenberg, R. J. & Dodd, P. R. Glutamate transporter variants reduce glutamate uptake in Alzheimer’s disease. Neurobiol. Aging 32, 553.e1–553.e11 (2011).
Jo, S. et al. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nat. Med. 20, 886–896 (2014).
Acosta, C., Anderson, H. D. & Anderson, C. M. Astrocyte dysfunction in Alzheimer disease. J. Neurosci. Res. 95, 2430–2447 (2017).
Merlini, M., Meyer, E. P., Ulmann-Schuler, A. & Nitsch, R. M. Vascular beta-amyloid and early astrocyte alterations impair cerebrovascular function and cerebral metabolism in transgenic arcAbeta mice. Acta Neuropathol. 122, 293–311 (2011).
Orr, A. G. et al. Astrocytic adenosine receptor A2A and Gs-coupled signaling regulate memory. Nat. Neurosci. 18, 423–434 (2015).
Orellana, J. A. et al. ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. J. Neurochem. 118, 826–840 (2011).
Garwood, C. J., Pooler, A. M., Atherton, J., Hanger, D. P. & Noble, W. Astrocytes are important mediators of Abeta-induced neurotoxicity and tau phosphorylation in primary culture. Cell Death Dis. 2, e167 (2011).
Liddelow, S. A. et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017).
Jones, V. C., Atkinson-Dell, R., Verkhratsky, A. & Mohamet, L. Aberrant iPSC-derived human astrocytes in Alzheimer’s disease. Cell Death Dis. 8, e2696 (2017).
Liao, M. C. et al. Single-cell detection of secreted Abeta and sAPPalpha from human IPSC-derived neurons and astrocytes. J. Neurosci. 36, 1730–1746 (2016).
Kondo, T. et al. Modeling Alzheimer’s disease with iPSCs reveals stress phenotypes associated with intracellular Abeta and differential drug responsiveness. Cell Stem Cell 12, 487–496 (2013).
Zhang, Y. et al. Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron 89, 37–53 (2016).
Oberheim, N. A., Goldman, S. A. & Nedergaard, M. Heterogeneity of astrocytic form and function. Methods Mol. Biol. 814, 23–45 (2012).
Oberheim, N. A. et al. Uniquely hominid features of adult human astrocytes. J. Neurosci. 29, 3276–3287 (2009).
Han, X. et al. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell 12, 342–353 (2013).
Ben Haim, L. & Rowitch, D. H. Functional diversity of astrocytes in neural circuit regulation. Nat. Rev. Neurosci. 18, 31–41 (2017).
Wang, L. et al. Truncated N-terminal huntingtin fragment with expanded-polyglutamine (htt552-100Q) suppresses brain-derived neurotrophic factor transcription in astrocytes. Acta Biochim. Biophys. Sin. 44, 249–258 (2012).
Chou, S. Y. et al. Expanded-polyglutamine huntingtin protein suppresses the secretion and production of a chemokine (CCL5/RANTES) by astrocytes. J. Neurosci. 28, 3277–3290 (2008).
Ferraiuolo, L., Kirby, J., Grierson, A. J., Sendtner, M. & Shaw, P. J. Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis. Nat. Rev. Neurol. 7, 616–630 (2011).
Allaman, I. et al. Amyloid-beta aggregates cause alterations of astrocytic metabolic phenotype: impact on neuronal viability. J. Neurosci. 30, 3326–3338 (2010).
Oliveira, J. M. Mitochondrial bioenergetics and dynamics in Huntington’s disease: tripartite synapses and selective striatal degeneration. J. Bioenerg. Biomembr. 42, 227–234 (2010).
Cassina, P. et al. Mitochondrial dysfunction in SOD1G93A-bearing astrocytes promotes motor neuron degeneration: prevention by mitochondrial-targeted antioxidants. J. Neurosci. 28, 4115–4122 (2008).
Mei, X., Ezan, P., Giaume, C. & Koulakoff, A. Astroglial connexin immunoreactivity is specifically altered at beta-amyloid plaques in beta-amyloid precursor protein/presenilin1 mice. Neuroscience 171, 92–105 (2010).
Vis, J. C. et al. Connexin expression in Huntington’s diseased human brain. Cell Biol. Int. 22, 837–847 (1998).
Heuser, K. et al. Variants of the genes encoding AQP4 and Kir4.1 are associated with subgroups of patients with temporal lobe epilepsy. Epilepsy Res. 88, 55–64 (2010).
Tong, X. et al. Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington’s disease model mice. Nat. Neurosci. 17, 694–703 (2014).
Rossi, D. et al. Focal degeneration of astrocytes in amyotrophic lateral sclerosis. Cell Death Differ. 15, 1691–1700 (2008).
Rothstein, J. D., Martin, L. J. & Kuncl, R. W. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N. Engl. J. Med. 326, 1464–1468 (1992).
Arzberger, T., Krampfl, K., Leimgruber, S. & Weindl, A. Changes of NMDA receptor subunit (NR1, NR2B) and glutamate transporter (GLT1) mRNA expression in Huntington’s disease — an in situ hybridization study. J. Neuropathol. Exp. Neurol. 56, 440–454 (1997).
Jacob, C. P. et al. Alterations in expression of glutamatergic transporters and receptors in sporadic Alzheimer’s disease. J. Alzheimers Dis. 11, 97–116 (2007).
Gu, X. L. et al. Astrocytic expression of Parkinson’s disease-related A53T alpha-synuclein causes neurodegeneration in mice. Mol. Brain 3, 12 (2010).
Valenza, M. et al. Cholesterol defect is marked across multiple rodent models of Huntington’s disease and is manifest in astrocytes. J. Neurosci. 30, 10844–10850 (2010).
Bu, G. Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nat. Rev. Neurosci. 10, 333–344 (2009).
Delekate, A. et al. Metabotropic P2Y1 receptor signalling mediates astrocytic hyperactivity in vivo in an Alzheimer’s disease mouse model. Nat. Commun. 5, 5422 (2014).
Hauser, R. A. & Schwarzschild, M. A. Adenosine A2A receptor antagonists for Parkinson’s disease: rationale, therapeutic potential and clinical experience. Drugs Aging 22, 471–482 (2005).
Gandelman, M., Peluffo, H., Beckman, J. S., Cassina, P. & Barbeito, L. Extracellular ATP and the P2X7 receptor in astrocyte-mediated motor neuron death: implications for amyotrophic lateral sclerosis. J. Neuroinflamm. 7, 33 (2010).
Battaglia, G. et al. Early defect of transforming growth factor beta1 formation in Huntington’s disease. J. Cell. Mol. Med. 15, 555–571 (2011).
Shibata, N. et al. Persistent cleavage and nuclear translocation of apoptosis-inducing factor in motor neurons in the spinal cord of sporadic amyotrophic lateral sclerosis patients. Acta Neuropathol. 118, 755–762 (2009).
Johnson, J. A. et al. The Nrf2-ARE pathway: an indicator and modulator of oxidative stress in neurodegeneration. Ann. NY Acad. Sci. 1147, 61–69 (2008).
Rossi, D. et al. Defective tumor necrosis factor-alpha-dependent control of astrocyte glutamate release in a transgenic mouse model of Alzheimer disease. J. Biol. Chem. 280, 42088–42096 (2005).
Brambilla, L. et al. Disruption of the astrocytic TNFR1-GDNF axis accelerates motor neuron degeneration and disease progression in amyotrophic lateral sclerosis. Hum. Mol. Genet. 25, 3080–3095 (2016).
Frakes, A. E. et al. Microglia induce motor neuron death via the classical NF-kappaB pathway in amyotrophic lateral sclerosis. Neuron 81, 1009–1023 (2014).
Hsiao, H. Y., Chen, Y. C., Chen, H. M., Tu, P. H. & Chern, Y. A critical role of astrocyte-mediated nuclear factor-kappaB-dependent inflammation in Huntington’s disease. Hum. Mol. Genet. 22, 1826–1842 (2013).
Aebischer, J. et al. IFNgamma triggers a LIGHT-dependent selective death of motoneurons contributing to the non-cell-autonomous effects of mutant SOD1. Cell Death Differ. 18, 754–768 (2011).
Barcia, C. et al. IFN-gamma signaling, with the synergistic contribution of TNF-alpha, mediates cell specific microglial and astroglial activation in experimental models of Parkinson’s disease. Cell Death Dis. 2, e142 (2011).
Reviewer information
Nature Reviews Neurology thanks L. Barbeito, L. Ferraiuolo and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
Both authors contributed to all aspects of manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Tripartite synapse
-
A site at which three-way communication occurs between presynaptic, postsynaptic and astrocytic processes during synaptic transmission.
- Gliotransmission
-
The release of glutamate, ATP, d-serine and other neurotransmitters that are essential for synaptic transmission and plasticity from astrocytes.
- Microdialysis
-
A minimally invasive method of sampling in vivo concentrations of various analytes (neurotransmitters, peptides, glutamate, etc.) in the brain and spinal cord using a dialysis probe.
- Single-wavelength glutamate sensor
-
A fluorescent sensor based on a circularly permuted single fluorophore rather than Förster resonance energy transfer (FRET), which is based on ratiometric measurements at two different wavelengths.
- Human cortical spheroids
-
(hCSs). 3D cultures that produce laminated cerebral cortex-like structures that include astrocytes as part of a cortical neuronal circuit.
Rights and permissions
About this article
Cite this article
Almad, A., Maragakis, N.J. A stocked toolbox for understanding the role of astrocytes in disease. Nat Rev Neurol 14, 351–362 (2018). https://doi.org/10.1038/s41582-018-0010-2
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-018-0010-2
This article is cited by
-
Activated astrocytes attenuate neocortical seizures in rodent models through driving Na+-K+-ATPase
Nature Communications (2022)
-
Cancer microenvironment and genomics: evolution in process
Clinical & Experimental Metastasis (2022)
-
Chronic cortisol differentially impacts stem cell-derived astrocytes from major depressive disorder patients
Translational Psychiatry (2021)
-
Inhibitors of Src Family Kinases, Inducible Nitric Oxide Synthase, and NADPH Oxidase as Potential CNS Drug Targets for Neurological Diseases
CNS Drugs (2021)
-
Microglial Calcium: An Exquisite Sensor for Neuronal Activity
Neuroscience Bulletin (2021)