Abstract
Lysosomes are catabolic organelles that contribute to the degradation of intracellular constituents through autophagy and of extracellular components through endocytosis, phagocytosis and macropinocytosis. They also have roles in secretory mechanisms, the generation of extracellular vesicles and certain cell death pathways. These functions make lysosomes central organelles in cell homeostasis, metabolic regulation and responses to environment changes including nutrient stresses, endoplasmic reticulum stress and defects in proteostasis. Lysosomes also have important roles in inflammation, antigen presentation and the maintenance of long-lived immune cells. Their functions are tightly regulated by transcriptional modulation via TFEB and TFE3, as well as by major signalling pathways that lead to activation of mTORC1 and mTORC2, lysosome motility and fusion with other compartments. Lysosome dysfunction and alterations in autophagy processes have been identified in a wide variety of diseases, including autoimmune, metabolic and kidney diseases. Deregulation of autophagy can contribute to inflammation, and lysosomal defects in immune cells and/or kidney cells have been reported in inflammatory and autoimmune pathologies with kidney involvement. Defects in lysosomal activity have also been identified in several pathologies with disturbances in proteostasis, including autoimmune and metabolic diseases such as Parkinson disease, diabetes mellitus and lysosomal storage diseases. Targeting lysosomes is therefore a potential therapeutic strategy to regulate inflammation and metabolism in a variety of pathologies.
Key points
-
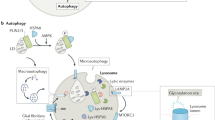
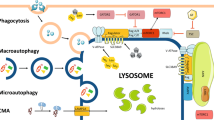
Lysosomes are versatile organelles that can associate with several other cell compartments, resulting in a variety of functions including roles in autophagy, endocytosis, phagocytosis, secretory mechanisms and cell death pathways.
-
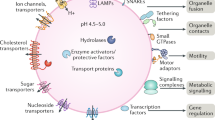
Lysosomes are dynamic, motile organelles that respond to intracellular and extracellular stimuli and are subject to numerous changes according to their environment; the composition, number and size of lysosomes are finely regulated.
-
Lysosomes have vital functions in cell homeostasis and metabolic regulation as well as in the immune system, including roles in phagocytosis, antigen processing and inflammation; disruption of these functions can lead to metabolic, autoimmune and kidney diseases.
-
Small molecules and peptides that can specifically target lysosomes might be valuable therapeutics, capable of correcting dysfunctions of crucial lysosome-dependent pathways and their immune and metabolic consequences.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Rousseau, A. & Bertolotti, A. Regulation of proteasome assembly and activity in health and disease. Nat. Rev. Mol. Cell Biol. 19, 697–712 (2018).
Hetz, C., Zhang, K. & Kaufman, R. J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 21, 421–438 (2020).
Dikic, I. & Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 19, 349–364 (2018).
Bonam, S. R., Wang, F. & Muller, S. Lysosomes as a therapeutic target. Nat. Rev. Drug. Discov. 18, 923–948 (2019).
Wang, L. et al. The emerging mechanisms and functions of microautophagy. Nat. Rev. Mol. Cell Biol. 24, 186–203 (2023).
Kaushik, S. & Cuervo, A. M. The coming of age of chaperone-mediated autophagy. Nat. Rev. Mol. Cell Biol. 19, 365–381 (2018).
Cullen, P. J. & Steinberg, F. To degrade or not to degrade: mechanisms and significance of endocytic recycling. Nat. Rev. Mol. Cell Biol. 19, 679–696 (2018).
Galluzzi, L. et al. Molecular definitions of autophagy and related processes. EMBO J. 36, 1811–1836 (2017).
Yang, C. & Wang, X. Lysosome biogenesis: regulation and functions. J. Cell Biol. 220, e202102001 (2021).
Heckmann, B. L. & Green, D. R. LC3-associated phagocytosis at a glance. J. Cell Sci. 132, jcs222984 (2019).
Ligeon, L.-A., Loi, M. & Münz, C. LC3-associated phagocytosis and antigen presentation. Curr. Protoc. Immunol. 123, e60 (2018).
Leidal, A. M. & Debnath, J. Emerging roles for the autophagy machinery in extracellular vesicle biogenesis and secretion. FASEB Bioadv. 3, 377–386 (2021).
Liu, G. Y. & Sabatini, D. M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 21, 183–203 (2020).
Shin, H. R. & Zoncu, R. The lysosome at the intersection of cellular growth and destruction. Dev. Cell 54, 226–238 (2020).
Ballabio, A. & Bonifacino, J. S. Lysosomes as dynamic regulators of cell and organismal homeostasis. Nat. Rev. Mol. Cell Biol. 21, 101–118 (2020).
Braulke, T. & Bonifacino, J. S. Sorting of lysosomal proteins. Biochim. Biophys. Acta 1793, 605–614 (2009).
Luzio, J. P., Hackmann, Y., Dieckmann, N. M. G. & Griffiths, G. M. The biogenesis of lysosomes and lysosome-related organelles. Cold Spring Harb. Perspect. Biol. 6, a016840 (2014).
Pols, M. S. et al. hVps41 and VAMP7 function in direct TGN to late endosome transport of lysosomal membrane proteins. Nat. Commun. 4, 1361 (2013).
Coutinho, M. F., Prata, M. J. & Alves, S. A shortcut to the lysosome: the mannose-6-phosphate-independent pathway. Mol. Genet. Metab. 107, 257–266 (2012).
Saftig, P. & Klumperman, J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat. Rev. Mol. Cell Biol. 10, 623–635 (2009).
Gan, N. et al. Structural mechanism of allosteric activation of TRPML1 by PI(3,5)P2 and rapamycin. Proc. Natl Acad. Sci. USA 119, e2120404119 (2022).
Talaia, G., Amick, J. & Ferguson, S. M. Receptor-like role for PQLC2 amino acid transporter in the lysosomal sensing of cationic amino acids. Proc. Natl Acad. Sci. USA 118, e2014941118 (2021).
Lee, J. H. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell 141, 1146–1158 (2010).
Verdon, Q. et al. SNAT7 is the primary lysosomal glutamine exporter required for extracellular protein-dependent growth of cancer cells. Proc. Natl Acad. Sci. USA 114, E3602–E3611 (2017).
Hong, W. & Lev, S. Tethering the assembly of SNARE complexes. Trends Cell Biol. 24, 35–43 (2014).
Luzio, J. P., Pryor, P. R. & Bright, N. A. Lysosomes: fusion and function. Nat. Rev. Mol. Cell Biol. 8, 622–632 (2007).
Bröcker, C., Engelbrecht-Vandré, S. & Ungermann, C. Multisubunit tethering complexes and their role in membrane fusion. Curr. Biol. 20, R943–R952 (2010).
Saxton, R. A. & Sabatini, D. M. mTOR signaling in growth, metabolism, and disease. Cell 169, 361–371 (2017).
Morgan, A. J., Platt, F. M., Lloyd-Evans, E. & Galione, A. Molecular mechanisms of endolysosomal Ca2+ signalling in health and disease. Biochem. J. 439, 349–378 (2011).
Reddy, A., Caler, E. V. & Andrews, N. W. Plasma membrane repair is mediated by Ca2+ regulated exocytosis of lysosomes. Cell 106, 157–169 (2001).
Maejima, I. Autophagy sequesters damaged lysosomes to control lysosomal biogenesis and kidney injury. EMBO J. 32, 2336–2347 (2013).
Skowyra, M. L., Schlesinger, P. H., Naismith, T. V. & Hanson, P. I. Triggered recruitment of ESCRT machinery promotes endolysosomal repair. Science 360, 6384 (2018).
Radulovic, M. ESCRT-mediated lysosome repair precedes lysophagy and promotes cell survival. EMBO J. 37, e99753 (2018).
Settembre, C., Fraldi, A., Medina, D. L. & Ballabio, A. Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat. Rev. Mol. Cell Biol. 14, 283–296 (2013).
Settembre, C. TFEB links autophagy to lysosomal biogenesis. Science 332, 1429–1433 (2011).
Sardiello, M. A gene network regulating lysosomal biogenesis and function. Science 325, 473–477 (2009).
Martina, J. A., Chen, Y., Gucek, M. & Puertollano, R. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy 8, 903–914 (2012).
Roczniak-Ferguson, A. The transcription factor TFEB links mTORC1 signalling to transcriptional control of lysosome homeostasis. Sci. Signal. 5, ra42 (2012).
Settembre, C. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 31, 1095–1108 (2012).
Medina, D. L. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat. Cell Biol. 17, 288–299 (2015).
Wang, W. Up-regulation of lysosomal TRPML1 channels is essential for lysosomal adaptation to nutrient starvation. Proc. Natl Acad. Sci. USA 112, E1373–E1381 (2015).
Li, Y. Protein kinase C controls lysosome biogenesis independently of mTORC1. Nat. Cell Biol. 18, 1065–1077 (2016).
Annunziata, I. MYC competes with MiT/TFE in regulating lysosomal biogenesis and autophagy through an epigenetic rheostat. Nat. Commun. 10, 3623 (2019).
Yang, L., Zhang, L., Wu, Q. & Boyd, D. D. Unbiased screening for transcriptional targets of ZKSCAN3 identifies integrin β4 and vascular endothelial growth factor as downstream targets. J. Biol. Chem. 283, 35295–35304 (2008).
Chauhan, S. ZKSCAN3 is a master transcriptional repressor of autophagy. Mol. Cell 50, 16–28 (2013).
Napolitano, G. et al. A substrate-specific mTORC1 pathway underlies Birt–Hogg–Dubé syndrome. Nature 585, 597–602 (2020).
Martínez-Fábregas, J. Lysosomal protease deficiency or substrate overload induces an oxidative-stress mediated STAT3-dependent pathway of lysosomal homeostasis. Nat. Commun. 9, 5343 (2018).
Liu, B. STAT3 associates with vacuolar H+-ATPase and regulates cytosolic and lysosomal pH. Cell Res. 28, 996–1012 (2018).
Hao, F. et al. Rheb localized on the Golgi membrane activates lysosome-localized mTORC1 at the Golgi-lysosome contact site. J. Cell Sci. 131, jcs208017 (2018).
Bonifacino, J. S. & Neefjes, J. Moving and positioning the endolysosomal system. Curr. Opin. Cell Biol. 47, 1–8 (2017).
Friedman, J. R., Dibenedetto, J. R., West, M., Rowland, A. A. & Voeltz, G. K. Endoplasmic reticulum-endosome contact increases as endosomes traffic and mature. Mol. Biol. Cell 24, 1030–1040 (2013).
Starling, G. P. Folliculin directs the formation of a Rab34-RILP complex to control the nutrient-dependent dynamic distribution of lysosomes. EMBO Rep. 17, 823–841 (2016).
Korolchuk, V. I. Lysosomal positioning coordinates cellular nutrient responses. Nat. Cell Biol. 13, 453–460 (2011).
Angarola, B. & Ferguson, S. M. Coordination of Rheb lysosomal membrane interactions with mTORC1 activation. F1000Res. 9(F1000 Faculty Rev), 450 (2020).
Angarola, B. & Ferguson, S. M. Weak membrane interactions allow Rheb to activate mTORC1 signaling without major lysosome enrichment. Mol. Biol. Cell 30, 2750–2760 (2019).
Jia, R. & Bonifacino, J. S. Lysosome positioning influences mTORC2 and AKT signaling. Mol. Cell 75, 26–38.e3 (2019).
Pu, J., Guardia, C. M., Keren-Kaplan, T. & Bonifacino, J. S. Mechanisms and functions of lysosome positioning. J. Cell Sci. 129, 4329–4339 (2016).
Wong, Y. C., Ysselstein, D. & Krainc, D. Mitochondria-lysosome contacts regulate mitochondrial fission via RAB7 GTP hydrolysis. Nature 554, 382–386 (2018).
Cioni, J. M. Late endosomes act as mRNA translation platforms and sustain mitochondria in axons. Cell 176, 56–72 (2019).
Liao, Y. C. RNA granules hitchhike on lysosomes for long-distance transport, using annexin A11 as a molecular tether. Cell 179, 147–164 (2019).
Wang, F. & Muller, S. Manipulating autophagic processes in autoimmune diseases: a special focus on modulating chaperone-mediated autophagy, an emerging therapeutic target. Front. Immunol. 6, 252 (2015).
Klionsky, D. J. et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy 17, 1–382 (2021).
Chen, C., Sidransky, E. & Chen, Y. Lyso-IP: uncovering pathogenic mechanisms of lysosomal dysfunction. Biomolecules 12, 616 (2022).
Barral, D. C. et al. Current methods to analyze lysosome morphology, positioning, motility and function. Traffic 23, 238–269 (2022).
Ferreira, A. P. A. & Boucrot, E. Mechanisms of carrier formation during clathrin-independent endocytosis. Trends Cell Biol. 28, 188–200 (2018).
Kaksonen, M. & Roux, A. Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 19, 313–326 (2018).
Rennick, J. J., Johnston, A. P. R. & Parton, R. G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol. 16, 266–276 (2021).
Reggiori, F. & Klumperman, J. in Lysosomes: Biology, Diseases, and Therapeutics (eds. Maxfield, F. R., Willard, J. M. & Lu, S.) 7–31 (Wiley, 2016).
Pickles, S., Vigié, P. & Youle, R. J. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr. Biol. 28, R170–R185 (2018).
Chino, H. & Mizushima, N. ER-phagy: quality control and turnover of endoplasmic reticulum. Trends Cell Biol. 30, 384–398 (2020).
Papadopoulos, C. & Meyer, H. Detection and clearance of damaged lysosomes by the endo-lysosomal damage response and lysophagy. Curr. Biol. 27, R1330–R1341 (2017).
Zechner, R., Madeo, F. & Kratky, D. Cytosolic lipolysis and lipophagy: two sides of the same coin. Nat. Rev. Mol. Cell Biol. 18, 671–684 (2017).
Martina, J. A. The nutrient-responsive transcription factor TFE3 promotes autophagy, lysosomal biogenesis, and clearance of cellular debris. Sci. Signal. 7, ra9 (2014).
Medina, D. L. Transcriptional activation of lysosomal exocytosis promotes cellular clearance. Dev. Cell 21, 421–430 (2011).
Buratta, S. et al. Lysosomal exocytosis, exosome release and secretory autophagy: the autophagic- and endo-lysosomal systems go extracellular. Int. J. Mol. Sci. 21, 2576 (2020).
DeSelm, C. J. et al. Autophagy proteins regulate the secretory component of osteoclastic bone resorption. Dev. Cell 21, 966–974 (2011).
Hessvik, N. P. & Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 75, 193–208 (2018).
Leidal, A. M. et al. The LC3-conjugation machinery specifies the loading of RNA-binding proteins into extracellular vesicles. Nat. Cell Biol. 22, 187–199 (2020).
Martinez, J. et al. Microtubule-associated protein 1 light chain 3 alpha (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proc. Natl Acad. Sci. USA 108, 17396–17401 (2011).
Stern, L. J., Potolicchio, I. & Santambrogio, L. MHC class II compartment subtypes: structure and function. Curr. Opin. Immunol. 18, 64–69 (2006).
Münz, C. The macroautophagy machinery in MHC restricted antigen presentation. Front. Immunol. 12, 628429 (2021).
& Münz, C. Autophagy proteins influence endocytosis for MHC restricted antigen presentation. Semin. Cancer Biol. 66, 110–115 (2020).
Peters, P. J. et al. Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J. Exp. Med 173, 1099–1109 (1991).
Yuseff, M. I. et al. Polarized secretion of lysosomes at the B cell synapse couples antigen extraction to processing and presentation. Immunity 35, 361–374 (2011).
Arbogast, F. et al. ATG5 is required for B cell polarization and presentation of particulate antigens. Autophagy 15, 280–294 (2019).
Arbogast, F. & Gros, F. Lymphocyte autophagy in homeostasis, activation, and inflammatory diseases. Front. Immunol. 9, 1801 (2018).
Clarke, A. J. & Simon, A. K. Autophagy in the renewal, differentiation and homeostasis of immune cells. Nat. Rev. Immunol. 19, 170–183 (2019).
Kimmey, J. M. & Stallings, C. L. Bacterial pathogens versus autophagy: implications for therapeutic interventions. Trends Mol. Med. 22, 1060–1076 (2016).
Gonzalez, C. D., Resnik, R. & Vaccaro, M. I. Secretory autophagy and its relevance in metabolic and degenerative disease. Front. Endocrinol. 11, 266 (2020).
Monteith, A. J. et al. Defects in lysosomal maturation facilitate the activation of innate sensors in systemic lupus erythematosus. Proc. Natl Acad. Sci. USA 113, E2142–E2151 (2016).
Lessard, C. J. et al. Identification of a systemic lupus erythematosus risk locus spanning ATG16L2, FCHSD2, and P2RY2 in Koreans. Arthritis Rheumatol. 68, 1197–1209 (2016).
Wang, F., Tasset, I., Cuervo, A. M. & Muller, S. In vivo remodeling of altered autophagy-lysosomal pathway by a phosphopeptide in lupus. Cells 9, E2328 (2020).
Cuervo, A. M. & Dice, J. F. Regulation of lamp2a levels in the lysosomal membrane. Traffic 1, 570–583 (2000).
Macri, C. et al. Modulation of deregulated chaperone-mediated autophagy by a phosphopeptide. Autophagy 11, 472–486 (2015).
An, N. et al. Chloroquine autophagic inhibition rebalances Th17/Treg-mediated immunity and ameliorates systemic lupus erythematosus. Cell Physiol. Biochem. 44, 412–422 (2017).
Li, B., Wang, F., Schall, N. & Muller, S. Rescue of autophagy and lysosome defects in salivary glands of MRL/lpr mice by a therapeutic phosphopeptide. J. Autoimmun. 90, 132–145 (2018).
Qi, Y.-Y. et al. Increased autophagy is cytoprotective against podocyte injury induced by antibody and interferon-α in lupus nephritis. Ann. Rheum. Dis. 77, 1799–1809 (2018).
Gros, F. et al. Macroautophagy is deregulated in murine and human lupus T lymphocytes. Autophagy 8, 1113–1123 (2012).
Alessandri, C. et al. T lymphocytes from patients with systemic lupus erythematosus are resistant to induction of autophagy. FASEB J. 26, 4722–4732 (2012).
Yuan, Q. et al. WDFY4 is involved in symptoms of systemic lupus erythematosus by modulating B cell fate via noncanonical autophagy. J. Immunol. 1950 201, 2570–2578 (2018).
Cappel, C., Gonzalez, A. C. & Damme, M. Quantification and characterization of the 5′ exonuclease activity of the lysosomal nuclease PLD3 by a novel cell-based assay. J. Biol. Chem. 296, 100152 (2021).
Zhou, X.-J., Klionsky, D. J. & Zhang, H. Podocytes and autophagy: a potential therapeutic target in lupus nephritis. Autophagy 15, 908–912 (2019).
Wilhelm, M. et al. Implication of a lysosomal antigen in the pathogenesis of lupus erythematosus. J. Autoimmun. 120, 102633 (2021).
Minota, S., Koyasu, S., Yahara, I. & Winfield, J. Autoantibodies to the heat-shock protein hsp90 in systemic lupus erythematosus. J. Clin. Invest. 81, 106–109 (1988).
Lassen, K. G. et al. Genetic coding variant in GPR65 alters lysosomal pH and links lysosomal dysfunction with colitis risk. Immunity 44, 1392–1405 (2016).
Wang, S.-L. et al. Intestinal autophagy links psychosocial stress with gut microbiota to promote inflammatory bowel disease. Cell Death Dis. 10, 391 (2019).
Hampe, J. et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat. Genet. 39, 207–211 (2007).
Rioux, J. D. et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat. Genet. 39, 596–604 (2007).
Momozawa, Y. et al. IBD risk loci are enriched in multigenic regulatory modules encompassing putative causative genes. Nat. Commun. 9, 2427 (2018).
Smillie, C. S. et al. Intra- and inter-cellular rewiring of the human colon during ulcerative colitis. Cell 178, 714–730.e22 (2019).
Xu, Y., Shen, J. & Ran, Z. Emerging views of mitophagy in immunity and autoimmune diseases. Autophagy 16, 3–17 (2020).
Shao, B.-Z. et al. The role of autophagy in inflammatory bowel disease. Front. Physiol. 12, 621132 (2021).
Foerster, E. G. et al. How autophagy controls the intestinal epithelial barrier. Autophagy 18, 86–103 (2022).
Tsuboi, K. et al. Autophagy protects against colitis by the maintenance of normal gut microflora and secretion of mucus. J. Biol. Chem. 290, 20511–20526 (2015).
Yang, L. et al. Impaired autophagy in intestinal epithelial cells alters gut microbiota and host immune responses. Appl. Environ. Microbiol. 84, e00880-18 (2018).
Mehto, S. et al. The Crohn’s disease risk factor IRGM limits NLRP3 inflammasome activation by impeding its assembly and by mediating its selective autophagy. Mol. Cell 73, 429–445.e7 (2019).
Retnakumar, S. V. et al. Targeting the endo-lysosomal autophagy pathway to treat inflammatory bowel diseases. J. Autoimmun. 128, 102814 (2022).
Aiyegbusi, O. et al. Renal disease in primary Sjögren’s syndrome. Rheumatol. Ther. 8, 63–80 (2021).
François, H. & Mariette, X. Renal involvement in primary Sjögren syndrome. Nat. Rev. Nephrol. 12, 82–93 (2016).
Tanaka, T. et al. LAMP3 inhibits autophagy and contributes to cell death by lysosomal membrane permeabilization. Autophagy 18, 1629–1647 (2022).
Alessandri, C. et al. CD4 T lymphocyte autophagy is upregulated in the salivary glands of primary Sjogren’s syndrome patients and correlates with focus score and disease activity. Arthritis Res. Ther. 19, 178 (2017).
Colafrancesco, S. et al. Autophagy occurs in lymphocytes infiltrating Sjögren’s syndrome minor salivary glands and correlates with histological severity of salivary gland lesions. Arthritis Res. Ther. 22, 238 (2020).
Voynova, E., Lefebvre, F., Qadri, A. & Muller, S. Correction of autophagy impairment inhibits pathology in the NOD.H-2h4 mouse model of primary Sjögren’s syndrome. J. Autoimmun. 108, 102418 (2020).
Weyand, C. M. & Goronzy, J. J. The immunology of rheumatoid arthritis. Nat. Immunol. 22, 10–18 (2021).
Baier, A., Meineckel, I., Gay, S. & Pap, T. Apoptosis in rheumatoid arthritis. Curr. Opin. Rheumatol. 15, 274–279 (2003).
van Loosdregt, J. et al. Increased autophagy in CD4+ T cells of rheumatoid arthritis patients results in T-cell hyperactivation and apoptosis resistance. Eur. J. Immunol. 46, 2862–2870 (2016).
Kumar, P. et al. Molecular mechanisms of autophagic memory in pathogenic T cells in human arthritis. J. Autoimmun. 94, 90–98 (2018).
Vomero, M. et al. Autophagy and rheumatoid arthritis: current knowledges and future perspectives. Front. Immunol. 9, 1577 (2018).
Jannat, A., John, P., Bhatti, A. & Hayat, M. Q. Tomorou attenuates progression of rheumatoid arthritis through alteration in ULK-1 independent autophagy pathway in collagen induced arthritis mice model. Cell Death Discov. 5, 142 (2019).
Zhu, L. et al. The autophagy level is increased in the synovial tissues of patients with active rheumatoid arthritis and is correlated with disease severity. Mediators Inflamm. 2017, 7623145 (2017).
Kato, M., Ospelt, C., Gay, R. E., Gay, S. & Klein, K. Dual role of autophagy in stress-induced cell death in rheumatoid arthritis synovial fibroblasts. Arthritis Rheumatol. 66, 40–48 (2014).
Kim, E. K. et al. IL-17-mediated mitochondrial dysfunction impairs apoptosis in rheumatoid arthritis synovial fibroblasts through activation of autophagy. Cell Death Dis. 8, e2565 (2017).
Aman, Y. et al. Autophagy in healthy aging and disease. Nat. Aging 1, 634–650 (2021).
Klionsky, D. J. et al. Autophagy in major human diseases. EMBO J. 40, e108863 (2021).
Chua, J. P., De Calbiac, H., Kabashi, E. & Barmada, S. J. Autophagy and ALS: mechanistic insights and therapeutic implications. Autophagy 18, 254–282 (2022).
Yuan, P. et al. PLD3 affects axonal spheroids and network defects in Alzheimer’s disease. Nature 612, 328–337 (2022).
Keller, C. W. & Lunemann, J. D. Autophagy and autophagy-related proteins in CNS autoimmunity. Front. Immunol. 8, 165 (2017).
Muller, S. et al. Autophagy in neuroinflammatory diseases. Autoimmun. Rev. 16, 856–874 (2017).
Misrielal, C., Mauthe, M., Reggiori, F. & Eggen, B. J. L. Autophagy in multiple sclerosis: two sides of the same coin. Front. Cell. Neurosci. 14, 603710 (2020).
van Beek, N., Klionsky, D. J. & Reggiori, F. Genetic aberrations in macroautophagy genes leading to diseases. Biochim. Biophys. Acta Mol. Cell Res. 1865, 803–816 (2018).
Igci, M. et al. Gene expression profiles of autophagy-related genes in multiple sclerosis. Gene 588, 38–46 (2016).
Bhattacharya, A., Parillon, X., Zeng, S., Han, S. & Eissa, N. T. Deficiency of autophagy in dendritic cells protects against experimental autoimmune encephalomyelitis. J. Biol. Chem. 289, 26525–26532 (2014).
Hassanpour, M. et al. Real-state of autophagy signaling pathway in neurodegenerative disease; focus on multiple sclerosis. J. Inflamm. (Lond.) 17, 6 (2020).
Brun, S. et al. An autophagy-targeting peptide to treat chronic inflammatory demyelinating polyneuropathies. J. Autoimmun. 92, 114–125 (2018).
Bonam, S. R., Tranchant, C. & Muller, S. Autophagy-lysosomal pathway as potential therapeutic target in Parkinson’s disease. Cells 10, 3547 (2021).
Orenstein, S. J. et al. Interplay of LRRK2 with chaperone-mediated autophagy. Nat. Neurosci. 16, 394–406 (2013).
Menzies, F. M. et al. Autophagy and neurodegeneration: pathogenic mechanisms and therapeutic opportunities. Neuron 93, 1015–1034 (2017).
Du, J., Ji, Y., Qiao, L., Liu, Y. & Lin, J. Cellular endo‐lysosomal dysfunction in the pathogenesis of non‐alcoholic fatty liver disease. Liver Int 40, 271–280 (2020).
Singh, R. et al. Autophagy regulates lipid metabolism. Nature 458, 1131–1135 (2009).
Czaja, M. J. et al. Functions of autophagy in normal and diseased liver. Autophagy 9, 1131–1158 (2013).
Pietrocola, F. & Bravo-San Pedro, J. M. Targeting autophagy to counteract obesity-associated oxidative stress. Antioxid. (Basel) 10, 102 (2021).
Yang, L., Li, P., Fu, S., Calay, E. S. & Hotamisligil, G. S. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 11, 467–478 (2010).
Guerrero-Ros, I. et al. The negative effect of lipid challenge on autophagy inhibits T cell responses. Autophagy 16, 223–238 (2020).
Madrigal-Matute, J. & Cuervo, A. M. Regulation of liver metabolism by autophagy. Gastroenterology 150, 328–339 (2016).
Hammoutene, A. et al. A defect in endothelial autophagy occurs in patients with non-alcoholic steatohepatitis and promotes inflammation and fibrosis. J. Hepatol. 72, 528–538 (2020).
Filali-Mouncef, Y. et al. The ménage à trois of autophagy, lipid droplets and liver disease. Autophagy 18, 50–72 (2022).
Kouroumalis, E., Voumvouraki, A., Augoustaki, A. & Samonakis, D. N. Autophagy in liver diseases. World J. Hepatol. 13, 6–65 (2021).
Kim, Y., Lee, D.-H., Park, S.-H., Jeon, T.-I. & Jung, C. H. The interplay of microRNAs and transcription factors in autophagy regulation in nonalcoholic fatty liver disease. Exp. Mol. Med. 53, 548–559 (2021).
Li, X., Wan, T. & Li, Y. Role of FoxO1 in regulating autophagy in type 2 diabetes mellitus (review). Exp. Ther. Med. 22, 707 (2021).
Wan, J. et al. LC3-associated phagocytosis in myeloid cells, a fireman that restrains inflammation and liver fibrosis, via immunoreceptor inhibitory signaling. Autophagy 16, 1526–1528 (2020).
Zeigerer, A. et al. Regulation of liver metabolism by the endosomal GTPase Rab5. Cell Rep. 11, 884–892 (2015).
Gosis, B. S. et al. Inhibition of nonalcoholic fatty liver disease in mice by selective inhibition of mTORC1. Science 376, eabf8271 (2022).
Mizunoe, Y. et al. Association between lysosomal dysfunction and obesity-related pathology: a key knowledge to prevent metabolic syndrome. Int. J. Mol. Sci. 20, 3688 (2019).
Zoncu, R. & Perera, R. M. Built to last: lysosome remodeling and repair in health and disease. Trends Cell Biol. 32, 597–610 (2022).
Platt, F. M. Emptying the stores: lysosomal diseases and therapeutic strategies. Nat. Rev. Drug. Discov. 17, 133–150 (2018).
Festa, B. P. et al. Impaired autophagy bridges lysosomal storage disease and epithelial dysfunction in the kidney. Nat. Commun. 9, 161 (2018).
Jouret, F. et al. Cystic fibrosis is associated with a defect in apical receptor-mediated endocytosis in mouse and human kidney. J. Am. Soc. Nephrol. 18, 707–718 (2007).
Ivanova, M. M., Changsila, E., Iaonou, C. & Goker-Alpan, O. Impaired autophagic and mitochondrial functions are partially restored by ERT in Gaucher and Fabry diseases. PLoS One 14, e0210617 (2019).
Meyer-Schwesinger, C. Lysosome function in glomerular health and disease. Cell Tissue Res 385, 371–392 (2021).
Lin, Q., Banu, K., Ni, Z., Leventhal, J. S. & Menon, M. C. Podocyte autophagy in homeostasis and disease. J. Clin. Med. 10, 1184 (2021).
Kimura, T., Isaka, Y. & Yoshimori, T. Autophagy and kidney inflammation. Autophagy 13, 997–1003 (2017).
Wang, L. & Law, H. K. W. The role of autophagy in lupus nephritis. Int. J. Mol. Sci. 16, 25154–25167 (2015).
Nakatsuka, A. et al. A vaspin–HSPA1L complex protects proximal tubular cells from organelle stress in diabetic kidney disease. Commun. Biol. 4, 373 (2021).
Kimura, T. et al. Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J. Am. Soc. Nephrol. 22, 902–913 (2011).
Sakashita, M., Tanaka, T. & Inagi, R. Metabolic changes and oxidative stress in diabetic kidney disease. Antioxidants 10, 1143 (2021).
Wu, M. et al. Relationship between lysosomal dyshomeostasis and progression of diabetic kidney disease. Cell Death Dis. 12, 958 (2021).
Brijmohan, A. S. et al. HDAC6 inhibition promotes transcription factor EB activation and is protective in experimental kidney disease. Front. Pharmacol. 9, 34 (2018).
Fukushima, K., Kitamura, S., Tsuji, K., Sang, Y. & Wada, J. Sodium glucose co-transporter 2 inhibitor ameliorates autophagic flux impairment on renal proximal tubular cells in obesity mice. Int. J. Mol. Sci. 21, E4054 (2020).
Gu, X. et al. Kidney disease genetic risk variants alter lysosomal beta-mannosidase (MANBA) expression and disease severity. Sci. Transl. Med. 13, eaaz1458 (2021).
Kim, H.-R., Jin, H.-S. & Eom, Y.-B. Association between MANBA gene variants and chronic kidney disease in a Korean population. J. Clin. Med. 10, 2255 (2021).
Gros, F. & Muller, S. Pharmacological regulators of autophagy and their link with modulators of lupus disease. Br. J. Pharmacol. 171, 4337–4359 (2014).
Palhegyi, A. M., Seranova, E., Dimova, S., Hoque, S. & Sarkar, S. Biomedical implications of autophagy in macromolecule storage disorders. Front. Cell Dev. Biol. 7, 179 (2019).
Panda, P. K. et al. Chemical screening approaches enabling drug discovery of autophagy modulators for biomedical applications in human diseases. Front. Cell Dev. Biol. 7, 38 (2019).
Retnakumar, S. V. & Muller, S. Pharmacological autophagy regulators as therapeutic agents for inflammatory bowel diseases. Trends Mol. Med. 25, 516–537 (2019).
Cao, M., Luo, X., Wu, K. & He, X. Targeting lysosomes in human disease: from basic research to clinical applications. Signal. Transduct. Target. Ther. 6, 379 (2021).
Gonzalez, C. D., Carro Negueruela, M. P., Nicora Santamarina, C., Resnik, R. & Vaccaro, M. I. Autophagy dysregulation in diabetic kidney disease: from pathophysiology to pharmacological interventions. Cells 10, 2497 (2021).
Whitmarsh-Everiss, T. & Laraia, L. Small molecule probes for targeting autophagy. Nat. Chem. Biol. 17, 653–664 (2021).
Rockel, J. S. & Kapoor, M. Autophagy: controlling cell fate in rheumatic diseases. Nat. Rev. Rheumatol. 13, 517–531 (2017).
Meng, X. et al. Gypenoside XVII enhances lysosome biogenesis and autophagy flux and accelerates autophagic clearance of amyloid-β through TFEB activation. J. Alzheimers Dis. 52, 1135–1150 (2016).
Renna, M. et al. Azithromycin blocks autophagy and may predispose cystic fibrosis patients to mycobacterial infection. J. Clin. Invest. 121, 3554–3563 (2011).
Cuddy, L. K. et al. Farnesyltransferase inhibitor LNK-754 attenuates axonal dystrophy and reduces amyloid pathology in mice. Mol. Neurodegener. 17, 54 (2022).
Hollywood, J. A. et al. Use of human induced pluripotent stem cells and kidney organoids to develop a cysteamine/mTOR inhibition combination therapy for cystinosis. J. Am. Soc. Nephrol. 31, 962–982 (2020).
Ma, T. et al. Low-dose metformin targets the lysosomal AMPK pathway through PEN2. Nature 603, 159–165 (2022).
Tsunemi, T. et al. Increased lysosomal exocytosis induced by lysosomal Ca2+ channel agonists protects human dopaminergic neurons from α-synuclein toxicity. J. Neurosci. 39, 5760–5772 (2019).
Chen, C.-C. et al. A small molecule restores function to TRPML1 mutant isoforms responsible for mucolipidosis type IV. Nat. Commun. 5, 4681 (2014).
Bonam, S. R., Muller, S., Bayry, J. & Klionsky, D. J. Autophagy as an emerging target for COVID-19: lessons from an old friend, chloroquine. Autophagy 16, 2260–2266 (2020).
Schrezenmeier, E. & Dörner, T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat. Rev. Rheumatol. 16, 155–166 (2020).
Li, M. et al. Gasdermin D maintains bone mass by rewiring the endo-lysosomal pathway of osteoclastic bone resorption. Dev. Cell 57, 2365–2380.e8 (2022).
Voss, M. D. et al. Discovery and pharmacological characterization of a novel small molecule inhibitor of phosphatidylinositol-5-phosphate 4-kinase, type II, beta. Biochem. Biophys. Res. Commun. 449, 327–331 (2014).
Korolenko, T. A., Johnston, T. P. & Vetvicka, V. Lysosomotropic features and autophagy modulators among medical drugs: evaluation of their role in pathologies. Molecules 25, E5052 (2020).
Zhao, Y. G., Codogno, P. & Zhang, H. Machinery, regulation and pathophysiological implications of autophagosome maturation. Nat. Rev. Mol. Cell Biol. 22, 733–750 (2021).
Yuan, Z., Wang, S., Tan, X. & Wang, D. New insights into the mechanisms of chaperon-mediated autophagy and implications for kidney diseases. Cells 11, 406 (2022).
Page, N. et al. HSC70 blockade by the therapeutic peptide P140 affects autophagic processes and endogenous MHCII presentation in murine lupus. Ann. Rheum. Dis. 70, 837–843 (2011).
Zimmer, R., Scherbarth, H. R., Rillo, O. L., Gomez-Reino, J. J. & Muller, S. Lupuzor/P140 peptide in patients with systemic lupus erythematosus: a randomised, double-blind, placebo-controlled phase IIb clinical trial. Ann. Rheum. Dis. 72, 1830–1835 (2013).
Schall, N., Talamini, L., Wilhelm, M., Jouvin-Marche, E. & Muller, S. P140 peptide leads to clearance of autoreactive lymphocytes and normalizes immune response in lupus-prone mice. Front. Immunol. 13, 904669 (2022).
Wilhelm, M. et al. Lupus regulator peptide P140 represses B-cell differentiation by reducing HLA class II molecule overexpression. Arthritis Rheumatol. 70, 1077–1088 (2018).
Galvão, I. et al. The therapeutic effect of phosphopeptide P140 attenuates inflammation induced by uric acid crystals in gout arthritis mouse model. Cells 11, 3709 (2022).
Akiyama, K. et al. Therapeutic effects of peptide P140 in a mouse periodontitis model. Cell. Mol. Life Sci. 79, 518 (2022).
Zhang, Z. et al. Role of lysosomes in physiological activities, diseases, and therapy. J. Hematol. Oncol. 14, 79 (2021).
Yim, W. W.-Y., Kurikawa, Y. & Mizushima, N. An exploratory text analysis of the autophagy research field. Autophagy 18, 1648–1661 (2022).
Grützkau, A. et al. LAMP-1 and LAMP-2, but not LAMP-3, are reliable markers for activation-induced secretion of human mast cells. Cytometry A 61, 62–68 (2004).
Yim, W. W.-Y. & Mizushima, N. Lysosome biology in autophagy. Cell Discov. 6, 6 (2020).
Juste, Y. R. et al. Reciprocal regulation of chaperone-mediated autophagy and the circadian clock. Nat. Cell Biol. 23, 1255–1270 (2021).
Seoane, P. I. et al. The NLRP3–inflammasome as a sensor of organelle dysfunction. J. Cell Biol. 219, e202006194 (2020).
Wang, H.-T. et al. Insights into ferroptosis, a novel target for the therapy of cancer. Front. Oncol. 12, 812534 (2022).
Gómez-Sintes, R., Ledesma, M. D. & Boya, P. Lysosomal cell death mechanisms in aging. Ageing Res. Rev. 32, 150–168 (2016).
Aits, S. & Jäättelä, M. Lysosomal cell death at a glance. J. Cell Sci. 126, 1905–1912 (2013).
Repnik, U., Stoka, V., Turk, V. & Turk, B. Lysosomes and lysosomal cathepsins in cell death. Biochim. Biophys. Acta 1824, 22–33 (2012).
Sargeant, T. J. et al. Stat3 controls cell death during mammary gland involution by regulating uptake of milk fat globules and lysosomal membrane permeabilization. Nat. Cell Biol. 16, 1057–1068 (2014).
Loison, F., Xu, Y. & Luo, H. R. Proteinase 3 and serpin B1: a novel pathway in the regulation of caspase-3 activation, neutrophil spontaneous apoptosis, and inflammation. Inflamm. Cell Signal. 1, e462 (2014).
Loison, F. et al. Proteinase 3-dependent caspase-3 cleavage modulates neutrophil death and inflammation. J. Clin. Invest. 124, 4445–4458 (2014).
Bewley, M. A. et al. A cardinal role for cathepsin D in co-ordinating the host-mediated apoptosis of macrophages and killing of pneumococci. PLOS Pathog. 7, e1001262 (2011).
Acknowledgements
The authors thank S. Blaise (Reims, France) and J. Wada (Okayama, Japan) for valuable suggestions. S.M. acknowledges the support of the French Centre National de la Recherche Scientifique (CNRS); the University of Strasbourg Institute for Advanced Study (USIAS); the ITI 2021-2028 programme, University of Strasbourg-CNRS-Inserm, IdEx Unistra (ANR-10-IDEX-0002) and SFRI (STRAT’US project, ANR-20-SFRI-0012); The French National Agency for Research (TopGum project ANR-21-CE17-0026); the European Regional Development Fund of the European Union in the context of the INTERREG V Upper Rhine programme; the FHU ARRIMAGE and the OMAGE project granted by Region Grand-Est of France and FEDER. S.M. also thanks the TRANSAUTOPHAGY COST Action (CA15138). F.G. thanks the French National Agency for Research (AURIGENE and AUTOMATE projects, ANR-20-CE93-001 and ANR-20-CE15-0018), the association des sclérodermiques de France (ASF) and the groupe français de recherche sur la sclérodermie (GFRS); F.G. and S.M. acknowledge the Club francophone de l’autophagie (CFATG).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Autophagy
-
A vital, finely regulated and evolutionarily conserved intracellular pathway that continuously degrades, recycles, and clears unnecessary or dysfunctional cellular components. Autophagy is crucial for cell adaptation to the environment and to maintain cell homeostasis, especially under stress conditions.
- Cathepsins
-
A large family of proteases that are mainly found in acidic endosomal and lysosomal compartments where they have a vital role in intracellular protein degradation, energy metabolism and immune responses.
- Efferocytosis
-
A rapid and efficient process of clearance of apoptotic cells occurring under the control of both professional and non-professional phagocytic cells.
- Endocytosis
-
A vesicle-mediated process by which cells engulf membrane and extracellular material.
- Endoplasmic reticulum
-
A network of tubular membranes that manufacture, process and transport chemical compounds for use inside and outside of the cell.
- Endosomal sorting complex required for transport
-
A multiprotein complex that mediates budding and abscission of intraluminal vesicles into multivesicular endosomes.
- Extracellular vesicles
-
Lipid-bound vesicles that are secreted by cells into the extracellular space. The three main subtypes of extracellular vesicles, microvesicles exosomes and apoptotic bodies, differ according to their biogenesis, release pathways, size, content and function.
- Hydroxychloroquine
-
A lysomotropic compound that increases pH within acidic vacuoles and alters protein degradation by lysosomal acidic hydrolases, assembly of macromolecules in the endosomes and post-translation modification of proteins in the Golgi apparatus.
- Lysomotropic agents
-
Drugs that selectively concentrate inside lysosomes after in vivo administration and exert their cellular effects via lysosomes.
- Lysosomal exocytosis
-
A process in which lysosomes fuse with the plasma membrane and empty their contents outside the cell. This process has an important role in plasma membrane repair, bone resorption, the immune response and elimination of pathogenic substrates.
- Mitophagy
-
A key process that selectively disrupts damaged mitochondria by autolysosomal degradation, preventing excessive generation of reactive oxygen species and activation of cell death.
- Phagocytosis
-
An endocytic process by which phagocytes (for example, macrophages) internalize large particles (>0.5 µm) such as bacteria, other microorganisms, foreign particles and aged red blood cells, to form a phagosome.
- Proteasome
-
A barrel-shaped multiprotein complex composed of proteases that is involved in the selective degradation of intracellular proteins, particularly those marked for proteolysis by ubiquitin. A human cell contains about 30,000 proteasomes.
- Trans-Golgi network
-
A cytosolic apparatus that sorts and transports proteins and some form of lipids to the other cytosolic compartments.
- Unfolded protein response
-
A conserved signalling network that is activated in response to misfolded or unfolded proteins and re-establishes protein homeostasis.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gros, F., Muller, S. The role of lysosomes in metabolic and autoimmune diseases. Nat Rev Nephrol 19, 366–383 (2023). https://doi.org/10.1038/s41581-023-00692-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-023-00692-2
This article is cited by
-
Development and validation of a novel lysosome-related LncRNA signature for predicting prognosis and the immune landscape features in colon cancer
Scientific Reports (2024)
-
Comprehensive Analysis and Experimental Validation of the Parkinson’s Disease Lysosomal Gene ACP2 and Pan-cancer
Biochemical Genetics (2024)
-
A simple and rapid assay of lysosomal-targeting CDy6 for long-term real-time viability assessments in 2D and 3D in vitro culture models
Scientific Reports (2023)
-
Identification a unique disulfidptosis classification regarding prognosis and immune landscapes in thyroid carcinoma and providing therapeutic strategies
Journal of Cancer Research and Clinical Oncology (2023)