Abstract
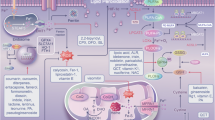
Ferroptosis is a mechanism of regulated necrotic cell death characterized by iron-dependent, lipid peroxidation-driven membrane destruction that can be inhibited by glutathione peroxidase 4. Morphologically, it is characterized by cellular, organelle and cytoplasmic swelling and the loss of plasma membrane integrity, with the release of intracellular components. Ferroptosis is triggered in cells with dysregulated iron and thiol redox metabolism, whereby the initial robust but selective accumulation of hydroperoxy polyunsaturated fatty acid-containing phospholipids is further propagated through enzymatic and non-enzymatic secondary mechanisms, leading to formation of oxidatively truncated electrophilic species and their adducts with proteins. Thus, ferroptosis is dependent on the convergence of iron, thiol and lipid metabolic pathways. The kidney is particularly susceptible to redox imbalance. A growing body of evidence has linked ferroptosis to acute kidney injury in the context of diverse stimuli, such as ischaemia–reperfusion, sepsis or toxins, and to chronic kidney disease, suggesting that ferroptosis may represent a novel therapeutic target for kidney disease. However, further work is needed to address gaps in our understanding of the triggers, execution and spreading mechanisms of ferroptosis.
Key points
-
Multiple cell death pathways contribute to the pathogenesis of acute and chronic kidney injury; ferroptosis is one such mechanism of regulated necrotic cell death, characterized by marked oxidation of phospholipids containing polyunsaturated fatty acids.
-
Ferroptotic death programs are triggered in cells with dysregulated iron and thiol redox metabolism, leading to robust but selective lipid peroxidation, which is propagated through enzymatic and non-enzymatic mechanisms.
-
The excessive accumulation of hydroperoxy-derivatives of arachidonoyl- and adrenoyl-phosphatidylethanolamines is catalysed by a complex of 15-lipoxygenase with phosphatidylethanolamine-binding protein 1 and represents a potential therapeutic target.
-
GPX4 detoxifies phospholipid hydroperoxides to non-toxic phospholipid alcohols using glutathione as a source of reducing equivalents, thus preventing ferroptotic cell death.
-
iNOS, NO•, Ca2+-independent phospholipase A2β and FSP1 can eliminate ferroptotic lipid peroxidation products and prevent ferroptosis in cells that lack GPX4 catalytic activity.
-
Increased levels of urinary ferroptotic phospholipid hydroperoxides are associated with non-recovery of kidney function in patients with acute kidney injury.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Green, D. R. The coming decade of cell death research: five riddles. Cell 177, 1094–1107 (2019).
Kerr, J. F. History of the events leading to the formulation of the apoptosis concept. Toxicology 181–182, 471–474 (2002).
Laster, S. M., Wood, J. G. & Gooding, L. R. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J. Immunol. 141, 2629–2634 (1988).
Galluzzi, L. et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 25, 486–541 (2018).
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Stockwell, B. R. et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171, 273–285 (2017).
Wiernicki, B. et al. Excessive phospholipid peroxidation distinguishes ferroptosis from other cell death modes including pyroptosis. Cell Death Dis. 11, 922 (2020).
Poulianiti, K. P. et al. Systemic redox imbalance in chronic kidney disease: a systematic review. Oxid. Med. Cell Longev. 2016, 8598253 (2016).
Otasevic, V., Vucetic, M., Grigorov, I., Martinovic, V. & Stancic, A. Ferroptosis in different pathological contexts seen through the eyes of mitochondria. Oxid. Med. Cell Longev. 2021, 5537330 (2021).
Linkermann, A. et al. Synchronized renal tubular cell death involves ferroptosis. Proc. Natl Acad. Sci. USA 111, 16836–16841 (2014).
Riegman, M. et al. Ferroptosis occurs through an osmotic mechanism and propagates independently of cell rupture. Nat. Cell Biol. 22, 1042–1048 (2020).
van Swelm, R. P. L., Wetzels, J. F. M. & Swinkels, D. W. The multifaceted role of iron in renal health and disease. Nat. Rev. Nephrol. 16, 77–98 (2020).
Stoyanovsky, D. A. et al. Iron catalysis of lipid peroxidation in ferroptosis: regulated enzymatic or random free radical reaction? Free. Radic. Biol. Med. 133, 153–161 (2019).
Lai, C. S. & Piette, L. H. Spin-trapping studies of hydroxyl radical production involved in lipid peroxidation. Arch. Biochem. Biophys. 190, 27–38 (1978).
Leaf, D. E. & Swinkels, D. W. Catalytic iron and acute kidney injury. Am. J. Physiol. Renal Physiol. 311, F871–F876 (2016).
Lane, D. J., Robinson, S. R., Czerwinska, H., Bishop, G. M. & Lawen, A. Two routes of iron accumulation in astrocytes: ascorbate-dependent ferrous iron uptake via the divalent metal transporter (DMT1) plus an independent route for ferric iron. Biochem. J. 432, 123–132 (2010).
Fuqua, B. K. et al. The multicopper ferroxidase hephaestin enhances intestinal iron absorption in mice. PLoS One 9, e98792 (2014).
Swaminathan, S. Iron homeostasis pathways as therapeutic targets in acute kidney injury. Nephron 140, 156–159 (2018).
Ramey, G. et al. Hepcidin targets ferroportin for degradation in hepatocytes. Haematologica 95, 501–504 (2010).
Xu, Y., Alfaro-Magallanes, V. M. & Babitt, J. L. Physiological and pathophysiological mechanisms of hepcidin regulation: clinical implications for iron disorders. Br. J. Haematol. 193, 882–893 (2021).
Pinto, J. P. et al. Erythropoietin mediates hepcidin expression in hepatocytes through EPOR signaling and regulation of C/EBPα. Blood 111, 5727–5733 (2008).
Kautz, L. et al. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat. Genet. 46, 678–684 (2014).
Van Coillie, S. et al. Targeting ferroptosis protects against experimental (multi)organ dysfunction and death. Nat. Commun. 13, 1046 (2022).
Hvidberg, V. et al. Identification of the receptor scavenging hemopexin-heme complexes. Blood 106, 2572–2579 (2005).
Nielsen, M. J., Andersen, C. B. F. & Moestrup, S. K. CD163 binding to haptoglobin-hemoglobin complexes involves a dual-point electrostatic receptor-ligand pairing. J. Biol. Chem. 288, 18834–18841 (2013).
Drakesmith, H., Nemeth, E. & Ganz, T. Ironing out ferroportin. Cell Metab. 22, 777–787 (2015).
Philpott, C. C. Coming into view: eukaryotic iron chaperones and intracellular iron delivery. J. Biol. Chem. 287, 13518–13523 (2012).
Lawson, D. M. et al. Identification of the ferroxidase centre in ferritin. FEBS Lett. 254, 207–210 (1989).
Chen, H. et al. Hephaestin is a ferroxidase that maintains partial activity in sex-linked anemia mice. Blood 103, 3933–3939 (2004).
Ito, F., Kato, K., Yanatori, I., Murohara, T. & Toyokuni, S. Ferroptosis-dependent extracellular vesicles from macrophage contribute to asbestos-induced mesothelial carcinogenesis through loading ferritin. Redox Biol. 47, 102174 (2021).
Namgaladze, D., Fuhrmann, D. C. & Brune, B. Interplay of Nrf2 and BACH1 in inducing ferroportin expression and enhancing resistance of human macrophages towards ferroptosis. Cell Death Discov. 8, 327 (2022).
Fuhrmann, D. C., Mondorf, A., Beifuss, J., Jung, M. & Brune, B. Hypoxia inhibits ferritinophagy, increases mitochondrial ferritin, and protects from ferroptosis. Redox Biol. 36, 101670 (2020).
Yang, W. S. & Stockwell, B. R. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem. Biol. 15, 234–245 (2008).
Gao, M., Monian, P., Quadri, N., Ramasamy, R. & Jiang, X. Glutaminolysis and transferrin regulate ferroptosis. Mol. Cell 59, 298–308 (2015).
Ohgami, R. S., Campagna, D. R., McDonald, A. & Fleming, M. D. The Steap proteins are metalloreductases. Blood 108, 1388–1394 (2006).
Gunshin, H. et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388, 482–488 (1997).
Wang, C.-Y. et al. ZIP8 is an iron and zinc transporter whose cell-surface expression is up-regulated by cellular iron loading. J. Biol. Chem. 287, 34032–34043 (2012).
Kozyraki, R. et al. Megalin-dependent cubilin-mediated endocytosis is a major pathway for the apical uptake of transferrin in polarized epithelia. Proc. Natl Acad. Sci. USA 98, 12491–12496 (2001).
Jenkitkasemwong, S., Wang, C.-Y., Mackenzie, B. & Knutson, M. D. Physiologic implications of metal-ion transport by ZIP14 and ZIP8. Biometals 25, 643–655 (2012).
Haldar, S. et al. Prion protein promotes kidney iron uptake via its ferrireductase activity. J. Biol. Chem. 290, 5512–5522 (2015).
Jin, J. et al. Machine learning classifies ferroptosis and apoptosis cell death modalities with TfR1 immunostaining. ACS Chem. Biol. 17, 654–660 (2022).
Hider, R. C. & Kong, X. Iron speciation in the cytosol: an overview. Dalton Trans. 42, 3220–3229 (2013).
Hider, R. C. & Kong, X. L. Glutathione: a key component of the cytoplasmic labile iron pool. Biometals 24, 1179–1187 (2011).
Patel, S. J. et al. A PCBP1-BolA2 chaperone complex delivers iron for cytosolic [2Fe-2S] cluster assembly. Nat. Chem. Biol. 15, 872–881 (2019).
Philpott, C. C., Ryu, M.-S., Frey, A. & Patel, S. Cytosolic iron chaperones: proteins delivering iron cofactors in the cytosol of mammalian cells. J. Biol. Chem. 292, 12764–12771 (2017).
Shi, H., Bencze, K. Z., Stemmler, T. L. & Philpott, C. C. A cytosolic iron chaperone that delivers iron to ferritin. Science 320, 1207–1210 (2008).
Leidgens, S. et al. Each member of the Poly-r(C)-binding protein 1 (PCBP) family exhibits iron chaperone activity toward ferritin. J. Biol. Chem. 288, 17791–17802 (2013).
Frey, A. G. et al. Iron chaperones PCBP1 and PCBP2 mediate the metallation of the dinuclear iron enzyme deoxyhypusine hydroxylase. Proc. Natl Acad. Sci. USA 111, 8031–8036 (2014).
Nandal, A. et al. Activation of the HIF prolyl hydroxylase by the iron chaperones PCBP1 and PCBP2. Cell Metab. 14, 647–657 (2011).
McCullough, K. & Bolisetty, S. Ferritins in kidney disease. Semin. Nephrol. 40, 160–172 (2020).
Wang, X. et al. Mitochondrial ferritin deficiency promotes osteoblastic ferroptosis via mitophagy in type 2 diabetic osteoporosis. Biol. Trace Elem. Res. 200, 298–307 (2022).
Wang, P. et al. Mitochondrial ferritin attenuates cerebral ischaemia/reperfusion injury by inhibiting ferroptosis. Cell Death Dis. 12, 447 (2021).
Santambrogio, P. et al. Mitochondrial ferritin expression in adult mouse tissues. J. Histochem. Cytochem. 55, 1129–1137 (2007).
Mancias, J. D., Wang, X., Gygi, S. P., Harper, J. W. & Kimmelman, A. C. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 509, 105–109 (2014).
Yanatori, I., Richardson, D. R., Imada, K. & Kishi, F. Iron export through the transporter ferroportin 1 is modulated by the iron chaperone PCBP2*. J. Biol. Chem. 291, 17303–17318 (2016).
Gao, M. et al. Ferroptosis is an autophagic cell death process. Cell Res. 26, 1021–1032 (2016).
Hou, W. et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 12, 1425–1428 (2016).
Imoto, S. et al. Labile iron, ROS, and cell death are prominently induced by haemin, but not by non-transferrin-bound iron. Transfus. Apher. Sci. 61, 103319 (2022).
Norden, A. G. W. et al. Glomerular protein sieving and implications for renal failure in Fanconi syndrome. Kidney Int. 60, 1885–1892 (2001).
Prinsen, B. et al. Transferrin synthesis is increased in nephrotic patients insufficiently to replace urinary losses. J. Am. Soc. Nephrol. 12, 1017–1025 (2001).
Raaij, S. E. G. V. et al. Iron handling by the human kidney: glomerular filtration and tubular reabsorption both contribute to urinary iron excretion. Am. J. Physiol. Renal Physiol. 316, F606–F614 (2019).
Jaszai, J. et al. Prominin-2 is a novel marker of distal tubules and collecting ducts of the human and murine kidney. Histochem. Cell Biol. 133, 527–539 (2010).
Brown, C. W. et al. Prominin2 drives ferroptosis resistance by stimulating iron export. Dev. Cell 51, 575–586 e574 (2019).
Macdougall, I. C. Intravenous iron therapy in patients with chronic kidney disease: recent evidence and future directions. Clin. Kidney J. 10, i16–i24 (2017).
Portoles, J., Martin, L., Broseta, J. J. & Cases, A. Anemia in chronic kidney disease: from pathophysiology and current treatments, to future agents. Front. Med. 8, 642296 (2021).
McMurray, J. et al. Kidney disease: Improving global outcomes (KDIGO) anemia work group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int. Suppl. 2, 279–335 (2012).
Locatelli, F. et al. Anaemia management in patients with chronic kidney disease: a position statement by the anaemia working group of European Renal Best Practice (ERBP). Nephrol. Dial. Transplant. 24, 348–354 (2008).
Gupta, N. & Wish, J. B. Hypoxia-inducible factor prolyl hydroxylase inhibitors: a potential new treatment for anemia in patients with CKD. Am. J. Kidney Dis. 69, 815–826 (2017).
Li, X. et al. Pretreatment with roxadustat (FG-4592) attenuates folic acid-induced kidney injury through antiferroptosis via Akt/GSK-3β/Nrf2 pathway. Oxid. Med. Cell Longev. 2020, 6286984 (2020).
Ho, J. et al. Mass spectrometry-based proteomic analysis of urine in acute kidney injury following cardiopulmonary bypass: a nested case-control study. Am. J. Kidney Dis. 53, 584–595 (2009).
Prowle, J. R. et al. Greater increase in urinary hepcidin predicts protection from acute kidney injury after cardiopulmonary bypass. Nephrol. Dial. Transpl. 27, 595–602 (2012).
Scindia, Y. et al. Hepcidin mitigates renal ischemia-reperfusion injury by modulating systemic iron homeostasis. J. Am. Soc. Nephrol. 26, 2800–2814 (2015).
van Swelm, R. P. et al. Renal handling of circulating and renal-synthesized hepcidin and its protective effects against hemoglobin-mediated kidney injury. J. Am. Soc. Nephrol. 27, 2720–2732 (2016).
Brown, R. J. & Gray, J. D. The mechanism of acute ferrous sulphate poisoning. Can. Med. Assoc. J. 73, 192–197 (1955).
Sochaski, M. A. et al. Lipid peroxidation and protein modification in a mouse model of chronic iron overload. Metabolism 51, 645–651 (2002).
Valerio, L. G. & Petersen, D. R. Characterization of hepatic iron overload following dietary administration of dicyclopentadienyl iron (Ferrocene) to mice: cellular, biochemical, and molecular aspects. Exp. Mol. Pathol. 68, 1–12 (2000).
Kozlov, A. V. et al. ‘Free’ iron, as detected by electron paramagnetic resonance spectroscopy, increases unequally in different tissues during dietary iron overload in the rat. Biometals 9, 98–103 (1996).
Burns, D. L. & Pomposelli, J. J. Toxicity of parenteral iron dextran therapy. Kidney Int. Suppl. 69, S119–S124 (1999).
Uhlén, M. et al. Tissue-based map of the human proteome. Science 347, 1260419 (2015).
Protchenko, O. et al. Iron chaperone poly rc binding protein 1 protects mouse liver from lipid peroxidation and steatosis. Hepatology 73, 1176–1193 (2021).
Arosio, P., Ingrassia, R. & Cavadini, P. Ferritins: a family of molecules for iron storage, antioxidation and more. Biochim. Biophys. Acta 1790, 589–599 (2009).
Ferreira, C. et al. Early embryonic lethality of H ferritin gene deletion in mice. J. Biol. Chem. 275, 3021–3024 (2000).
Zarjou, A. et al. Proximal tubule H-ferritin mediates iron trafficking in acute kidney injury. J. Clin. Invest. 123, 4423–4434 (2013).
Hatcher, H. C., Tesfay, L., Torti, S. V. & Torti, F. M. Cytoprotective effect of ferritin H in renal ischemia reperfusion injury. PLoS One 10, e0138505 (2015).
Zille, M. et al. Neuronal death after hemorrhagic stroke in vitro and in vivo shares features of ferroptosis and necroptosis. Stroke 48, 1033–1043 (2017).
Bergmann, C. et al. Polycystic kidney disease. Nat. Rev. Dis. Prim. 4, 50 (2018).
Zhang, X. et al. Ferroptosis promotes cyst growth in autosomal dominant polycystic kidney disease mouse models. J. Am. Soc. Nephrol. 32, 2759–2776 (2021).
Ide, S. et al. Ferroptotic stress promotes the accumulation of pro-inflammatory proximal tubular cells in maladaptive renal repair. Elife https://doi.org/10.7554/eLife.68603 (2021).
Wagner, B. A., Buettner, G. R. & Burns, C. P. Free radical-mediated lipid peroxidation in cells: oxidizability is a function of cell lipid bis-allylic hydrogen content. Biochemistry 33, 4449–4453 (1994).
Shchepinov, M. S. Polyunsaturated fatty acid deuteration against neurodegeneration. Trends Pharmacol. Sci. 41, 236–248 (2020).
Kuhn, H., Banthiya, S. & van Leyen, K. Mammalian lipoxygenases and their biological relevance. Biochim. Biophys. Acta 1851, 308–330 (2015).
Kagan, V. E. et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 13, 81–90 (2017).
Seiler, A. et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 8, 237–248 (2008).
Shintoku, R. et al. Lipoxygenase-mediated generation of lipid peroxides enhances ferroptosis induced by erastin and RSL3. Cancer Sci. 108, 2187–2194 (2017).
Wenzel, S. E. et al. PEBP1 wardens ferroptosis by enabling lipoxygenase generation of lipid death signals. Cell 171, e626 (2017).
Lamade, A. M. et al. Inactivation of RIP3 kinase sensitizes to 15LOX/PEBP1-mediated ferroptotic death. Redox Biol. 50, 102232 (2022).
Shah, R., Shchepinov, M. S. & Pratt, D. A. Resolving the role of lipoxygenases in the initiation and execution of ferroptosis. ACS Cent. Sci. 4, 387–396 (2018).
Friedmann Angeli, J. P. et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 16, 1180–1191 (2014).
Anthonymuthu, T. S. et al. Empowerment of 15-lipoxygenase catalytic competence in selective oxidation of membrane ETE-PE to ferroptotic death signals, HpETE-PE. J. Am. Chem. Soc. 140, 17835–17839 (2018).
Chu, B. et al. ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway. Nat. Cell Biol. 21, 579–591 (2019).
Dar, H. H. et al. Pseudomonas aeruginosa utilizes host polyunsaturated phosphatidylethanolamines to trigger theft-ferroptosis in bronchial epithelium. J. Clin. Invest. 128, 4639–4653 (2018).
Kim, S. et al. Characterization of ferroptosis in kidney tubular cell death under diabetic conditions. Cell Death Dis. 12, 160 (2021).
Martin-Saiz, L. et al. Ferrostatin-1 modulates dysregulated kidney lipids in acute kidney injury. J. Pathol. 257, 285–299 (2022).
Tonnus, W. & Meyer, C. Dysfunction of the key ferroptosis-surveilling systems hypersensitizes mice to tubular necrosis during acute kidney injury. Nat. Commun. 12, 4402 (2021).
Anthonymuthu, T. S. et al. Resolving the paradox of ferroptotic cell death: ferrostatin-1 binds to 15LOX/PEBP1 complex, suppresses generation of peroxidized ETE-PE, and protects against ferroptosis. Redox Biol. 38, 101744 (2021).
Davydov, R., Gilep, A. A., Strushkevich, N. V., Usanov, S. A. & Hoffman, B. M. Compound I is the reactive intermediate in the first monooxygenation step during conversion of cholesterol to pregnenolone by cytochrome P450scc: EPR/ENDOR/cryoreduction/annealing studies. J. Am. Chem. Soc. 134, 17149–17156 (2012).
Veglia, F. et al. Fatty acid transport protein 2 reprograms neutrophils in cancer. Nature 569, 73–78 (2019).
Codreanu, S. G. & Liebler, D. C. Novel approaches to identify protein adducts produced by lipid peroxidation. Free. Radic. Res. 49, 881–887 (2015).
Conrad, M. & Pratt, D. A. The chemical basis of ferroptosis. Nat. Chem. Biol. 15, 1137–1147 (2019).
Bayir, H. et al. Achieving life through death: redox biology of lipid peroxidation in ferroptosis. Cell Chem. Biol. 27, 387–408 (2020).
Farmer, L. A. et al. Intrinsic and extrinsic limitations to the design and optimization of inhibitors of lipid peroxidation and associated cell death. J. Am. Chem. Soc. 144, 14706–14721 (2022).
Doll, S. et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 13, 91–98 (2017).
Lagrost, L. & Masson, D. The expanding role of lyso-phosphatidylcholine acyltransferase-3 (LPCAT3), a phospholipid remodeling enzyme, in health and disease. Curr. Opin. Lipidol. 33, 193–198 (2022).
Gimeno, R. E. Fatty acid transport proteins. Curr. Opin. Lipidol. 18, 271–276 (2007).
Qiu, P. et al. FATP2-targeted therapies — a role beyond fatty liver disease. Pharmacol. Res. 161, 105228 (2020).
Chen, Y. et al. Involvement of FATP2-mediated tubular lipid metabolic reprogramming in renal fibrogenesis. Cell Death Dis. 11, 994 (2020).
Khan, S. et al. Kidney proximal tubule lipoapoptosis is regulated by fatty acid transporter-2 (FATP2. J. Am. Soc. Nephrol. 29, 81–91 (2018).
Khan, S. et al. Fatty acid transport protein-2 regulates glycemic control and diabetic kidney disease progression. JCI Insight https://doi.org/10.1172/jci.insight.136845 (2020).
Magtanong, L. et al. Context-dependent regulation of ferroptosis sensitivity. Cell Chem. Biol. 29, 1409–1418.e1406 (2022).
Adeshakin, A. O. et al. Lipidomics data showing the effect of lipofermata on myeloid-derived suppressor cells in the spleens of tumor-bearing mice. Data Brief. 35, 106882 (2021).
Ursini, F. et al. A white paper on phospholipid hydroperoxide glutathione peroxidase (GPx4) forty years later. Free Radic. Biol. Med. 188, 117–133 (2022).
Stockwell, B. R. Ferroptosis turns 10: EMERGING mechanisms, physiological functions, and therapeutic applications. Cell 185, 2401–2421 (2022).
Angeli, J. P. F., Shah, R., Pratt, D. A. & Conrad, M. Ferroptosis inhibition: mechanisms and opportunities. Trends Pharmacol. Sci. 38, 489–498 (2017).
Bowry, V. W., Ingold, K. U. & Stocker, R. Vitamin E in human low-density lipoprotein. When and how this antioxidant becomes a pro-oxidant. Biochem. J. 288, 341–344 (1992).
Miotto, G. et al. Insight into the mechanism of ferroptosis inhibition by ferrostatin-1. Redox Biol. 28, 101328 (2020).
Kagan, V., Serbinova, E. & Packer, L. Antioxidant effects of ubiquinones in microsomes and mitochondria are mediated by tocopherol recycling. Biochem. Biophys. Res. Commun. 169, 851–857 (1990).
Kagan, V. E. et al. Plasma membrane NADH-coenzyme Q0 reductase generates semiquinone radicals and recycles vitamin E homologue in a superoxide-dependent reaction. FEBS Lett. 428, 43–46 (1998).
Kagan, V. E., Serbinova, E. A., Forte, T., Scita, G. & Packer, L. Recycling of vitamin E in human low density lipoproteins. J. Lipid Res. 33, 385–397 (1992).
Cossins, E., Lee, R. & Packer, L. ESR studies of vitamin C regeneration, order of reactivity of natural source phytochemical preparations. Biochem. Mol. Biol. Int. 45, 583–597 (1998).
Maguire, J. J., Kagan, V., Ackrell, B. A., Serbinova, E. & Packer, L. Succinate-ubiquinone reductase linked recycling of alpha-tocopherol in reconstituted systems and mitochondria: requirement for reduced ubiquinone. Arch. Biochem. Biophys. 292, 47–53 (1992).
Ernster, L. & Dallner, G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim. Biophys. Acta 1271, 195–204 (1995).
Mukai, K., Morimoto, H., Kikuchi, S. & Nagaoka, S. Kinetic study of free-radical-scavenging action of biological hydroquinones (reduced forms of ubiquinone, vitamin K and tocopherol quinone) in solution. Biochim. Biophys. Acta 1157, 313–317 (1993).
Bersuker, K. et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575, 688–692 (2019).
Doll, S. et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 575, 693–698 (2019).
Jang, S. et al. Elucidating the contribution of mitochondrial glutathione to ferroptosis in cardiomyocytes. Redox Biol. 45, 102021 (2021).
Solmonson, A. & DeBerardinis, R. J. Lipoic acid metabolism and mitochondrial redox regulation. J. Biol. Chem. 293, 7522–7530 (2018).
Anthonymuthu, T. S., Kenny, E. M., Lamade, A. M., Kagan, V. E. & Bayir, H. Oxidized phospholipid signaling in traumatic brain injury. Free. Radic. Biol. Med. https://doi.org/10.1016/j.freeradbiomed.2018.06.031 (2018).
Hogg, N. & Kalyanaraman, B. Nitric oxide and lipid peroxidation. Biochim. Biophys. Acta 1411, 378–384 (1999).
O’Donnell, V. B. & Freeman, B. A. Interactions between nitric oxide and lipid oxidation pathways: implications for vascular disease. Circ. Res. 88, 12–21 (2001).
Kagan, V. E. et al. Redox epiphospholipidome in programmed cell death signaling: catalytic mechanisms and regulation. Front. Endocrinol. 11, 628079 (2020).
Kapralov, A. A. et al. Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death. Nat. Chem. Biol. 16, 278–290 (2020).
Dar, H. H. et al. A new thiol-independent mechanism of epithelial host defense against Pseudomonas aeruginosa: iNOS/NO(•) sabotage of theft-ferroptosis. Redox Biol. 45, 102045 (2021).
Mikulska-Ruminska, K. et al. NO• represses the oxygenation of arachidonoyl PE by 15LOX/PEBP1: mechanism and role in ferroptosis. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22105253 (2021).
Charbe, N. B. et al. A new era in oxygen therapeutics? From perfluorocarbon systems to haemoglobin-based oxygen carriers. Blood Rev. 54, 100927 (2022).
Ekmekci, Y. et al. Thrombophilia and avascular necrosis of femoral head in kidney allograft recipients. Nephrol. Dial. Transpl. 21, 3555–3558 (2006).
Jenkins, C. M., Yan, W., Mancuso, D. J. & Gross, R. W. Highly selective hydrolysis of fatty acyl-CoAs by calcium-independent phospholipase A2β. Enzyme autoacylation and acyl-CoA-mediated reversal of calmodulin inhibition of phospholipase A2 activity. J. Biol. Chem. 281, 15615–15624 (2006).
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. https://www.ncbi.nlm.nih.gov/books/NBK547852/ (National Institute of Diabetes and Digestive and Kidney Diseases, 2012).
Jenkins, C. M., Yang, J. & Gross, R. W. Mechanism-based inhibition of iPLA2β demonstrates a highly reactive cysteine residue (C651) that interacts with the active site: mass spectrometric elucidation of the mechanisms underlying inhibition. Biochemistry 52, 4250–4263 (2013).
Aguilera, A. et al. C-ferroptosis is an iron-dependent form of regulated cell death in cyanobacteria. J. Cell Biol. https://doi.org/10.1083/jcb.201911005 (2022).
Beharier, O. et al. PLA2G6 guards placental trophoblasts against ferroptotic injury. Proc. Natl Acad. Sci. USA 117, 27319–27328 (2020).
Chen, D. et al. iPLA2β-mediated lipid detoxification controls p53-driven ferroptosis independent of GPX4. Nat. Commun. 12, 3644 (2021).
Sun, W. Y. et al. Phospholipase iPLA2β averts ferroptosis by eliminating a redox lipid death signal. Nat. Chem. Biol. 17, 465–476 (2021).
Brennan, E., Kantharidis, P., Cooper, M. E. & Godson, C. Pro-resolving lipid mediators: regulators of inflammation, metabolism and kidney function. Nat. Rev. Nephrol. 17, 725–739 (2021).
Gai, Z. et al. Lipid accumulation and chronic kidney disease. Nutrients https://doi.org/10.3390/nu11040722 (2019).
Schelling, J. R. The contribution of lipotoxicity to diabetic kidney disease. Cells https://doi.org/10.3390/cells11203236 (2022).
Li, L. O., Klett, E. L. & Coleman, R. A. Acyl-CoA synthesis, lipid metabolism and lipotoxicity. Biochim. Biophys. Acta 1801, 246–251 (2010).
Proneth, B. & Conrad, M. Ferroptosis and necroinflammation, a yet poorly explored link. Cell Death Differ. 26, 14–24 (2019).
Sarhan, M., von Massenhausen, A., Hugo, C., Oberbauer, R. & Linkermann, A. Immunological consequences of kidney cell death. Cell Death Dis. 9, 114 (2018).
Martin-Sanchez, D. et al. Ferroptosis, but not necroptosis, is important in nephrotoxic folic acid-induced AKI. J. Am. Soc. Nephrol. 28, 218–229 (2017).
Mishima, E. et al. Drugs repurposed as antiferroptosis agents suppress organ damage, including AKI, by functioning as lipid peroxyl radical scavengers. J. Am. Soc. Nephrol. 31, 280–296 (2020).
Zhang, J., Wang, B., Yuan, S., He, Q. & Jin, J. The role of ferroptosis in acute kidney injury. Front. Mol. Biosci. 9, 951275 (2022).
Jiang, X., Stockwell, B. R. & Conrad, M. Ferroptosis: mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 22, 266–282 (2021).
Ardaillou, R., Baud, L. & Sraer, J. Leukotrienes and other lipoxygenase products of arachidonic acid synthesized in the kidney. Am. J. Med. 81, 12–22 (1986).
Afshinnia, F. et al. Elevated lipoxygenase and cytochrome P450 products predict progression of chronic kidney disease. Nephrol. Dial. Transpl. 35, 303–312 (2020).
Wang, Y. et al. ACSL4 deficiency confers protection against ferroptosis-mediated acute kidney injury. Redox Biol. 51, 102262 (2022).
Bolignano, D. et al. Antioxidant agents for delaying diabetic kidney disease progression: a systematic review and meta-analysis. PLoS One 12, e0178699 (2017).
Lippa, S., Colacicco, L., Calla, C., Sagliaschi, G. & Angelitti, A. G. Coenzyme Q10 levels, plasma lipids and peroxidation extent in renal failure and in hemodialytic patients. Mol. Asp. Med. 15, s213–s219 (1994).
Peerapanyasut, W., Thamprasert, K. & Wongmekiat, O. Ubiquinol supplementation protects against renal ischemia and reperfusion injury in rats. Free Radic. Res. 48, 180–189 (2014).
Nielsen, M. B. et al. Elevated plasma free thiols are associated with early and one-year graft function in renal transplant recipients. PLoS One 16, e0255930 (2021).
Ozkok, A. & Edelstein, C. L. Pathophysiology of cisplatin-induced acute kidney injury. BioMed. Res. Int. 2014, 967826 (2014).
van der Slikke, E. C. et al. Plasma free thiol levels during early sepsis predict future renal function decline. Antioxidants https://doi.org/10.3390/antiox11050800 (2022).
Zhang, M. Z. et al. IL-4/IL-13-mediated polarization of renal macrophages/dendritic cells to an M2a phenotype is essential for recovery from acute kidney injury. Kidney Int. 91, 375–386 (2017).
Li, X. et al. Role of M2 macrophages in sepsis-induced acute kidney injury. Shock 50, 233–239 (2018).
Kinsey, G. R. et al. Identification and distribution of endoplasmic reticulum iPLA2. Biochem. Biophys. Res. Commun. 327, 287–293 (2005).
Cohen, D. et al. Role of calcium-independent phospholipase A2 in complement-mediated glomerular epithelial cell injury. Am. J. Physiol. Renal Physiol. 294, F469–F479 (2008).
Van Laer, K., Hamilton, C. J. & Messens, J. Low-molecular-weight thiols in thiol-disulfide exchange. Antioxid. Redox Signal. 18, 1642–1653 (2013).
Salinas, G. & Comini, M. A. Alternative thiol-based redox systems. Antioxid. Redox Signal. 28, 407–409 (2018).
Abu-Remaileh, M. et al. Lysosomal metabolomics reveals V-ATPase- and mTOR-dependent regulation of amino acid efflux from lysosomes. Science 358, 807–813 (2017).
Stipanuk, M. H., Dominy, J. E. Jr., Lee, J. I. & Coloso, R. M. Mammalian cysteine metabolism: new insights into regulation of cysteine metabolism. J. Nutr. 136, 1652S–1659S (2006).
Lieberman, M. W. et al. Growth retardation and cysteine deficiency in γ-glutamyl transpeptidase-deficient mice. Proc. Natl Acad. Sci. USA 93, 7923–7926 (1996).
Nakagawa, H. et al. Assignment of the human renal dipeptidase gene (DPEP1) to band q24 of chromosome 16. Cytogenet. Cell Genet. 59, 258–260 (1992).
Kaur, A. et al. ChaC2, an enzyme for slow turnover of cytosolic glutathione. J. Biol. Chem. 292, 638–651 (2017).
Dixon, S. J. et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife 3, e02523 (2014).
Poltorack, C. D. & Dixon, S. J. Understanding the role of cysteine in ferroptosis: progress & paradoxes. FEBS J. https://doi.org/10.1111/febs.15842 (2021).
Combs, J. A. & DeNicola, G. M. The non-essential amino acid cysteine becomes essential for tumor proliferation and survival. Cancers https://doi.org/10.3390/cancers11050678 (2019).
Asantewaa, G. & Harris, I. S. Glutathione and its precursors in cancer. Curr. Opin. Biotechnol. 68, 292–299 (2021).
Dolma, S., Lessnick, S. L., Hahn, W. C. & Stockwell, B. R. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 3, 285–296 (2003).
Huang, S. et al. N-Acetylcysteine attenuates cisplatin-induced acute kidney injury by inhibiting the C5a receptor. Biomed. Res. Int. 2019, 4805853 (2019).
Louandre, C. et al. Iron-dependent cell death of hepatocellular carcinoma cells exposed to sorafenib. Int. J. Cancer 133, 1732–1742 (2013).
Zheng, J. et al. Sorafenib fails to trigger ferroptosis across a wide range of cancer cell lines. Cell Death Dis. 12, 698 (2021).
Pader, I. et al. Thioredoxin-related protein of 14 kDa is an efficient L-cystine reductase and S-denitrosylase. Proc. Natl Acad. Sci. USA 111, 6964–6969 (2014).
Harris, I. S. et al. Deubiquitinases maintain protein homeostasis and survival of cancer cells upon glutathione depletion. Cell Metab. 29, 1166–1181 e1166 (2019).
Badgley, M. A. et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science 368, 85–89 (2020).
Elmarakby, A. A. et al. A dual role of 12/15-lipoxygenase in LPS-induced acute renal inflammation and injury. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1864, 1669–1680 (2019).
Shimada, K. et al. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat. Chem. Biol. 12, 497–503 (2016).
Lee, S. J. et al. Impact of case volume on outcome and performance of targeted temperature management in out-of-hospital cardiac arrest survivors. Am. J. Emerg. Med. 33, 31–36 (2015).
Barayeu, U. et al. Hydropersulfides inhibit lipid peroxidation and ferroptosis by scavenging radicals. Nat. Chem. Biol. https://doi.org/10.1038/s41589-022-01145-w (2022).
Alvarez, S. W. et al. NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis. Nature 551, 639–643 (2017).
Kang, Y. P. et al. Non-canonical glutamate-cysteine ligase activity protects against ferroptosis. Cell Metab. 33, 174–189 e177 (2021).
Sato, H. et al. Redox imbalance in cystine/glutamate transporter-deficient mice. J. Biol. Chem. 280, 37423–37429 (2005).
Chintala, S. et al. Slc7a11 gene controls production of pheomelanin pigment and proliferation of cultured cells. Proc. Natl Acad. Sci. USA 102, 10964–10969 (2005).
De Bundel, D. et al. Loss of system xc− does not induce oxidative stress but decreases extracellular glutamate in hippocampus and influences spatial working memory and limbic seizure susceptibility. J. Neurosci. 31, 5792–5803 (2011).
Daher, B. et al. Genetic ablation of the cystine transporter xCT in PDAC cells inhibits mTORC1, growth, survival, and tumor formation via nutrient and oxidative stresses. Cancer Res. 79, 3877–3890 (2019).
Sosnoski, H. M., Sears, S. M. S., He, Y., Frare, C. & Hewett, S. J. Sexually dimorphic and brain region-specific transporter adaptations in system xc− null mice. Neurochem. Int. 141, 104888 (2020).
Meira, W. et al. A Cystine-cysteine intercellular shuttle prevents ferroptosis in xCT(KO) pancreatic ductal adenocarcinoma cells. Cancers https://doi.org/10.3390/cancers13061434 (2021).
Fang, D. et al. Corrigendum to “Calorie restriction protects against contrast-induced nephropathy via SIRT1/GPX4 activation”. Oxid. Med. Cell. Longev. 2022, 9846101 (2022).
Yant, L. J. et al. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic. Biol. Med. 34, 496–502 (2003).
Seibt, T. M., Proneth, B. & Conrad, M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic. Biol. Med. 133, 144–152 (2019).
von Massenhausen, A. et al. Dexamethasone sensitizes to ferroptosis by glucocorticoid receptor-induced dipeptidase-1 expression and glutathione depletion. Sci. Adv. 8, eabl8920 (2022).
Guan, Y. et al. A single genetic locus controls both expression of DPEP1/CHMP1A and kidney disease development via ferroptosis. Nat. Commun. 12, 5078 (2021).
Li, S. et al. Inhibiting Rab27a in renal tubular epithelial cells attenuates the inflammation of diabetic kidney disease through the miR-26a-5p/CHAC1/NF-kB pathway. Life Sci. 261, 118347 (2020).
Arensman, M. D. et al. Cystine-glutamate antiporter xCT deficiency suppresses tumor growth while preserving antitumor immunity. Proc. Natl Acad. Sci. USA 116, 9533–9542 (2019).
Zhang, L. et al. Hypersensitivity to ferroptosis in chromophobe RCC is mediated by a glutathione metabolic dependency and cystine import via solute carrier family 7 member 11. Proc. Natl Acad. Sci. USA 119, e2122840119 (2022).
Gopal, R. K. et al. Early loss of mitochondrial complex I and rewiring of glutathione metabolism in renal oncocytoma. Proc. Natl Acad. Sci. USA 115, E6283–E6290 (2018).
Priolo, C. et al. Impairment of gamma-glutamyl transferase 1 activity in the metabolic pathogenesis of chromophobe renal cell carcinoma. Proc. Natl Acad. Sci. USA 115, E6274–E6282 (2018).
Bansal, A. et al. γ-Glutamyltransferase 1 promotes clear cell renal cell carcinoma initiation and progression. Mol. Cancer Res. 17, 1881–1892 (2019).
Van den Branden, C. et al. Vitamin E protects renal antioxidant enzymes and attenuates glomerulosclerosis in adriamycin-treated rats. Nephron 91, 129–133 (2002).
Ikeda, Y. et al. Role of ferroptosis in cisplatin-induced acute nephrotoxicity in mice. J. Trace Elem. Med. Biol. 67, 126798 (2021).
Qin, L. Y. et al. Therapeutic potential of astragaloside IV against adriamycin-induced renal damage in rats via ferroptosis. Front. Pharmacol. 13, 812594 (2022).
van Raaij, S. et al. Tubular iron deposition and iron handling proteins in human healthy kidney and chronic kidney disease. Sci. Rep. 8, 9353 (2018).
Ikeda, Y. et al. Iron chelation by deferoxamine prevents renal interstitial fibrosis in mice with unilateral ureteral obstruction. PLoS One 9, e89355 (2014).
Naito, Y. et al. Effect of iron restriction on renal damage and mineralocorticoid receptor signaling in a rat model of chronic kidney disease. J. Hypertens. 30, 2192–2201 (2012).
Wang, J. et al. Ferroptosis, a new target for treatment of renal injury and fibrosis in a 5/6 nephrectomy-induced CKD rat model. Cell Death Discov. 8, 127 (2022).
Fang, X., Ardehali, H., Min, J. & Wang, F. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat. Rev. Cardiol. https://doi.org/10.1038/s41569-022-00735-4 (2022).
Ichikawa, Y. et al. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J. Clin. Invest. 124, 617–630 (2014).
Lyu, Y. L. et al. Topoisomerase IIβ mediated DNA double-strand breaks: implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Cancer Res. 67, 8839–8846 (2007).
Hassannia, B. et al. Nano-targeted induction of dual ferroptotic mechanisms eradicates high-risk neuroblastoma. J. Clin. Invest. 128, 3341–3355 (2018).
Wang, L., Chen, X. & Yan, C. Ferroptosis: an emerging therapeutic opportunity for cancer. Genes Dis. 9, 334–346 (2022).
Trujillo-Alonso, V. et al. FDA-approved ferumoxytol displays anti-leukaemia efficacy against cells with low ferroportin levels. Nat. Nanotechnol. 14, 616–622 (2019).
Zanganeh, S. et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 11, 986–994 (2016).
Okazaki, Y. The role of ferric nitrilotriacetate in renal carcinogenesis and cell death: from animal models to clinical implications. Cancers 14, 1495 (2022).
Rushworth, G. F. & Megson, I. L. Existing and potential therapeutic uses for N-acetylcysteine: the need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol. Ther. 141, 150–159 (2014).
Anderson, M. E., Powrie, F., Puri, R. N. & Meister, A. Glutathione monoethyl ester: preparation, uptake by tissues, and conversion to glutathione. Arch. Biochem. Biophys. 239, 538–548 (1985).
Hagos, F. T. et al. Probenecid, an organic anion transporter 1 and 3 inhibitor, increases plasma and brain exposure of N-acetylcysteine. Xenobiotica 47, 346–353 (2017).
Samuni, Y., Goldstein, S., Dean, O. M. & Berk, M. The chemistry and biological activities of N-acetylcysteine. Biochim. Biophys. Acta 1830, 4117–4129 (2013).
Schöneich, C. Thiyl radical reactions in the chemical degradation of pharmaceutical proteins. Molecules https://doi.org/10.3390/molecules24234357 (2019).
Hurst, G. A., Shaw, P. B. & LeMaistre, C. A. Laboratory and clinical evaluation of the mucolytic properties of acetylcysteine. Am. Rev. Respir. Dis. 96, 962–970 (1967).
Prescott, L. F. et al. Intravenous N-acetylcystine: the treatment of choice for paracetamol poisoning. Br. Med. J. 2, 1097–1100 (1979).
Karuppagounder, S. S. et al. N-acetylcysteine targets 5 lipoxygenase-derived, toxic lipids and can synergize with prostaglandin E2 to inhibit ferroptosis and improve outcomes following hemorrhagic stroke in mice. Ann. Neurol. 84, 854–872 (2018).
Wang, H. Z. et al. N-acetylcysteine is effective for prevention but not for treatment of folic acid-induced acute kidney injury in mice. Crit. Care Med. 39, 2487–2494 (2011).
Ergin, B. et al. Effects of N-acetylcysteine (NAC) supplementation in resuscitation fluids on renal microcirculatory oxygenation, inflammation, and function in a rat model of endotoxemia. Intensive Care Med. Exp. 4, 29 (2016).
Adedoyin, O. et al. Heme oxygenase-1 mitigates ferroptosis in renal proximal tubule cells. Am. J. Physiol. Renal Physiol. 314, F702–F714 (2018).
Li, Q. et al. NAC alleviative ferroptosis in diabetic nephropathy via maintaining mitochondrial redox homeostasis through activating SIRT3-SOD2/Gpx4 pathway. Free Radic. Biol. Med. 187, 158–170 (2022).
Ratliff, B. B., Abdulmahdi, W., Pawar, R. & Wolin, M. S. Oxidant mechanisms in renal injury and disease. Antioxid. Redox Signal. 25, 119–146 (2016).
Rachoin, J.-S., Wolfe, Y., Patel, S. & Cerceo, E. Contrast associated nephropathy after intravenous administration: what is the magnitude of the problem? Ren. Fail. 43, 1311–1321 (2021).
Steele, M. L. et al. Effect of Nrf2 activators on release of glutathione, cysteinylglycine and homocysteine by human U373 astroglial cells. Redox Biol. 1, 441–445 (2013).
Liebman, S. E. & Le, T. H. Eat your broccoli: oxidative stress, NRF2, and sulforaphane in chronic kidney disease. Nutrients 13, 266 (2021).
Wu, Y. et al. Selenoprotein gene mRNA expression evaluation during renal ischemia–reperfusion injury in rats and ebselen intervention effects. Biol. Trace Elem. Res. https://doi.org/10.1007/s12011-022-03275-7 (2022).
Müller, A., Cadenas, E., Graf, P. & Sies, H. A novel biologically active seleno-organic compound — 1: Glutathione peroxidase-like activity in vitro and antioxidant capacity of PZ 51 (Ebselen). Biochem. Pharmacol. 33, 3235–3239 (1984).
Cramer, S. L. et al. Systemic depletion of L-cyst(e)ine with cyst(e)inase increases reactive oxygen species and suppresses tumor growth. Nat. Med. 23, 120–127 (2017).
Mukhopadhyay, S. et al. Autophagy is required for proper cysteine homeostasis in pancreatic cancer through regulation of SLC7A11. Proc Natl Acad Sci USA 118, https://doi.org/10.1073/pnas.2021475118 (2021).
Kim, R. et al. Ferroptosis of tumour neutrophils causes immune suppression in cancer. Nature 612, 338–346 (2022).
Nabeyama, A. et al. xCT deficiency accelerates chemically induced tumorigenesis. Proc. Natl Acad. Sci. USA 107, 6436–6441 (2010).
Zilka, O. et al. On the mechanism of cytoprotection by ferrostatin-1 and liproxstatin-1 and the role of lipid peroxidation in ferroptotic cell death. ACS Cent. Sci. 3, 232–243 (2017).
Skouta, R. et al. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J. Am. Chem. Soc. 136, 4551–4556 (2014).
Takahashi, N. et al. Necrostatin-1 analogues: critical issues on the specificity, activity and in vivo use in experimental disease models. Cell Death Dis. 3, e437 (2012).
Fiore, A. & Murray, P. J. Tryptophan and indole metabolism in immune regulation. Curr. Opin. Immunol. 70, 7–14 (2021).
Agledal, L., Niere, M. & Ziegler, M. The phosphate makes a difference: cellular functions of NADP. Redox Rep. 15, 2–10 (2010).
Dong, C., Liu, S., Cui, Y. & Guo, Q. 12-Lipoxygenase as a key pharmacological target in the pathogenesis of diabetic nephropathy. Eur. J. Pharmacol. 879, 173122 (2020).
Camara, N. O., Martins, J. O., Landgraf, R. G. & Jancar, S. Emerging roles for eicosanoids in renal diseases. Curr. Opin. Nephrol. Hypertens. 18, 21–27 (2009).
Faulkner, J., Pye, C., Al-Shabrawey, M. & Elmarakby, A. A. Inhibition of 12/15-lipoxygenase reduces renal inflammation and injury in streptozotocin-induced diabetic mice. J. Diabetes Metab. https://doi.org/10.4172/2155-6156.1000555 (2015).
Hao, C. M. & Breyer, M. D. Physiologic and pathophysiologic roles of lipid mediators in the kidney. Kidney Int. 71, 1105–1115 (2007).
Po, L. S., Chen, Z. Y., Tsang, D. S. & Leung, L. K. Baicalein and genistein display differential actions on estrogen receptor (ER) transactivation and apoptosis in MCF-7 cells. Cancer Lett. 187, 33–40 (2002).
Liao, J. F., Hung, W. Y. & Chen, C. F. Anxiolytic-like effects of baicalein and baicalin in the Vogel conflict test in mice. Eur. J. Pharmacol. 464, 141–146 (2003).
Patel, N. S. et al. Reduction of renal ischemia-reperfusion injury in 5-lipoxygenase knockout mice and by the 5-lipoxygenase inhibitor zileuton. Mol. Pharmacol. 66, 220–227 (2004).
Badr, K. F. Five-lipoxygenase products in glomerular immune injury. J. Am. Soc. Nephrol. 3, 907–915 (1992).
Serhan, C. N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101 (2014).
Famulski, K. S. et al. Molecular phenotypes of acute kidney injury in kidney transplants. J. Am. Soc. Nephrol. 23, 948–958 (2012).
Müller, T. et al. Necroptosis and ferroptosis are alternative cell death pathways that operate in acute kidney failure. Cell. Mol. Life Sci. 74, 3631–3645 (2017).
Bao, Z. et al. Prokineticin-2 prevents neuronal cell deaths in a model of traumatic brain injury. Nat. Commun. 12, 4220 (2021).
Sun, Z., Wu, J., Bi, Q. & Wang, W. Exosomal lncRNA TUG1 derived from human urine-derived stem cells attenuates renal ischemia/reperfusion injury by interacting with SRSF1 to regulate ASCL4-mediated ferroptosis. Stem Cell Res. Ther. 13, 297 (2022).
Tao, W. H. et al. Dexmedetomidine attenuates ferroptosis-mediated renal ischemia/reperfusion injury and inflammation by inhibiting ACSL4 via alpha2-AR. Front. Pharmacol. 13, 782466 (2022).
Setti, G. et al. Peroxisome proliferator-activated receptor-γ agonist rosiglitazone prevents albuminuria but not glomerulosclerosis in experimental diabetes. Am. J. Nephrol. 32, 393–402 (2010).
Calkin, A. C. et al. PPAR-α and -γ agonists attenuate diabetic kidney disease in the apolipoprotein E knockout mouse. Nephrol. Dial. Transpl. 21, 2399–2405 (2006).
Gumieniczek, A. Effect of the new thiazolidinedione-pioglitazone on the development of oxidative stress in liver and kidney of diabetic rabbits. Life Sci. 74, 553–562 (2003).
Bakris, G. et al. Rosiglitazone reduces urinary albumin excretion in type II diabetes. J. Hum. Hypertens. 17, 7–12 (2003).
Nakamura, T. et al. Comparative effects of pioglitazone, glibenclamide, and voglibose on urinary endothelin-1 and albumin excretion in diabetes patients. J. Diabetes Complicat. 14, 250–254 (2000).
Agarwal, R. et al. A pilot randomized controlled trial of renal protection with pioglitazone in diabetic nephropathy. Kidney Int. 68, 285–292 (2005).
Hsu, Y.-W. et al. Dexmedetomidine pharmacodynamics: part I: crossover comparison of the respiratory effects of dexmedetomidine and remifentanil in healthy volunteers. Anesthesiology 101, 1066–1076 (2004).
Toto, R. D. Treatment of hypertension in chronic kidney disease. Semin. Nephrol. 25, 435–439 (2005).
Kawagishi, H. & Finkel, T. Unraveling the truth about antioxidants: ROS and disease: finding the right balance. Nat. Med. 20, 711–713 (2014).
O’Donnell, V. B. & Aldrovandi, M. Enzymatically oxidized phospholipids assume center stage as essential regulators of innate immunity and cell death., https://doi.org/10.1126/scisignal.aau2293 (2019).
Pang, H. et al. Multiple-ascending-dose pharmacokinetics and safety evaluation of baicalein chewable tablets in healthy Chinese volunteers. Clin. Drug Investig. 36, 713–724 (2016).
Acknowledgements
The work of the authors is supported by the NIH (R01GM122923 to S.J.D.; U01AI156924, P01HL114453, R01CA165065, R01CA266342 and R01AI145406 to V.E.K.; R01NS076511, R37NS061817 and U01AI156923 to H.B.; and UG3DK114861 to J.A.K.) and by the American Cancer Society (RSG-21-017-01-CCG to S.J.D.).
Author information
Authors and Affiliations
Contributions
H.B. and V.E.K. designed and conceptualized the review. All authors contributed to writing of the manuscript and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
S.J.D. is a founder of Prothegen Inc., a member of the scientific advisory board for Ferro Therapeutics and Hillstream BioPharma, and an inventor on patents related to ferroptosis (patent numbers 10597381, 10233171 and 9580398). J.A.K. is currently Chief Medical Officer of Spectral Medical and Dialco Medical Inc. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks Tomokazu Souma, Brent Stockwell and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bayır, H., Dixon, S.J., Tyurina, Y.Y. et al. Ferroptotic mechanisms and therapeutic targeting of iron metabolism and lipid peroxidation in the kidney. Nat Rev Nephrol 19, 315–336 (2023). https://doi.org/10.1038/s41581-023-00689-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-023-00689-x
This article is cited by
-
Unraveling the interplay of ferroptosis and immune dysregulation in diabetic kidney disease: a comprehensive molecular analysis
Diabetology & Metabolic Syndrome (2024)
-
SKP alleviates the ferroptosis in diabetic kidney disease through suppression of HIF-1α/HO-1 pathway based on network pharmacology analysis and experimental validation
Chinese Medicine (2024)
-
Loss of AR-regulated AFF3 contributes to prostate cancer progression and reduces ferroptosis sensitivity by downregulating ACSL4 based on single-cell sequencing analysis
Apoptosis (2024)
-
Ferroptosis: potential targets and emerging roles in pancreatic diseases
Archives of Toxicology (2024)
-
The mechanism of ferroptosis and its related diseases
Molecular Biomedicine (2023)