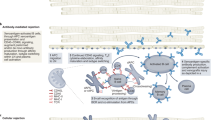
Abstract
A major limitation of organ allotransplantation is the insufficient supply of donor organs. Consequently, thousands of patients die every year while waiting for a transplant. Progress in xenotransplantation that has permitted pig organ graft survivals of years in non-human primates has led to renewed excitement about the potential of this approach to alleviate the organ shortage. In 2022, the first pig-to-human heart transplant was performed on a compassionate use basis, and xenotransplantation experiments using pig kidneys in deceased human recipients provided encouraging data. Many advances in xenotransplantation have resulted from improvements in the ability to genetically modify pigs using CRISPR–Cas9 and other methodologies. Gene editing has the capacity to generate pig organs that more closely resemble those of humans and are hence more physiologically compatible and less prone to rejection. Despite such modifications, immune responses to xenografts remain powerful and multi-faceted, involving innate immune components that do not attack allografts. Thus, the induction of innate and adaptive immune tolerance to prevent rejection while preserving the capacity of the immune system to protect the recipient and the graft from infection is desirable to enable clinical xenotransplantation.
Key points
-
As the demand for human organs for transplantation far exceeds the supply, many patients die while waiting for a transplant; xenotransplantation provides a potential near-term solution to the organ shortage.
-
Advances in genetic engineering, immunosuppression and tolerance approaches have contributed to improved pig organ survival in non-human primates (NHPs).
-
Functioning pig islet and heart grafts have survived in NHPs for months and functioning pig kidney grafts have survived in NHPs for years.
-
In 2022, a pig heart was transplanted into a patient with heart failure and functioned for 7 weeks and xenotransplantation experiments using pig kidneys in deceased human recipients provided encouraging data.
-
Mixed haematopoietic chimerism and thymic transplantation approaches have been successfully used to tolerize human T cells, B cells and natural killer cells in human immune system mice, providing proof of their potential to induce immune tolerance.
-
Advances in applying tolerance approaches in NHPs, including genetic engineering of source pigs, support their potential to provide a long-term solution to the powerful immune barriers to xenotransplantation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
OPTN. http://optn.transplant.hrsa.gov (2022).
Levitt, M. Could the organ shortage ever be met? Life Sci. Soc. Policy 11, 6 (2015).
Kang, H. W., Yoo, J. J. & Atala, A. Bioprinted scaffolds for cartilage tissue engineering. Methods Mol. Biol. 1340, 161–169 (2015).
Gilpin, S. E. & Ott, H. C. Using nature’s platform to engineer bio-artificial lungs. Ann. Am. Thorac. Soc. 12, S45–S49 (2015).
Reemtsma, K., McCracken, B. H. & Schlegel, J. U. Renal heterotransplantation in man. Ann. Surg. 160, 384 (1964).
Hardy, J. D. et al. Heart transplantation. J. Miss. State Med. Assoc. 9, 105–110 (1968).
Starzl, T. E., Marchioro, T. L. & Peters, G. N. Renal heterotransplantation from baboon to man: experience with six cases. Transplantation 2, 752 (1964).
Fu, Y. et al. Selective rejection of porcine islet xenografts by macrophages. Xenotransplantation 15, 307–312 (2008).
Abe, M. et al. Elimination of porcine hematopoietic cells by macrophages in mice. J. Immunol. 168, 621–628 (2002).
Basker, M. et al. Clearance of mobilized porcine peripheral blood progenitor cells is delayed by depletion of the phagocytic reticuloendothelial system in baboons. Transplantation 72, 1278–1285 (2001).
Candinas, D. et al. T cell independence of macrophage and natural killer cell infiltration, cytokine production, and endothelial activation during delayed xenograft rejection. Transplantation 62, 1920–1927 (1996).
Yi, S. et al. CD4+ T cells initiate pancreatic islet xenograft rejection via an interferon-gamma-dependent recruitment of macrophages and natural killer cells. Transplantation 73, 437–446 (2002).
Benda, B., Karlsson-Parra, A., Ridderstad, A. & Korsgren, O. Xenograft rejection of porcine islet-like cell clusters in immunoglobulin- or Fc-receptor γ-deficient mice. Transplantation 62, 1207–1211 (1996).
Gill, R. G., Wolf, L., Daniel, D. & Coulombe, M. CD4+ T cells are both necessary and sufficient for islet xenograft rejection. Transplant. Proc. 26, 1203 (1994).
Yi, S. et al. T cell-activated macrophages are capable of both recognition and rejection of pancreatic islet xenografts. J. Immunol. 170, 2750–2758 (2003).
Millan, M. T. et al. Human monocytes activate porcine endothelial cells, resulting in increased E-selectin, interleukin-8, monocyte chemotactic protein-1, and plasminogen activator inhibitor-type-1 expression. Transplantation 63, 421–429 (1997).
Shimizu, A., Yamada, K., Robson, S. C., Sachs, D. H. & Colvin, R. B. Pathologic characteristics of transplanted kidney xenografts. J. Am. Soc. Nephrol. 23, 225–235 (2012).
Hisashi, Y. et al. Rejection of cardiac xenografts transplanted from α1,3-galactosyltransferase gene-knockout (GalT-KO) pigs to baboons. Am. J. Transplant. 8, 2516–2526 (2008).
Seebach, J. D. & Waneck, G. L. Natural killer cells in xenotransplantation. Xenotransplantation 4, 201–211 (1997).
Middleton, D., Curran, M. & Maxwell, L. Natural killer cells and their receptors. Transpl. Immunol. 10, 147–164 (2002).
Yang, Y. G. & Sykes, M. Xenotransplantation: current status and a perspective on the future. Nat. Rev. Immunol. 7, 519–531 (2007).
Donnelly, C. E., Yatko, C., Johnson, E. W. & Edge, A. S. Human natural killer cells account for non-MHC class I-restricted cytolysis of porcine cells. Cell. Immunol. 175, 171–178 (1997).
Seebach, J. D. et al. HLA-Cw3 expression on porcine endothelial cells protects against xenogeneic cytotoxicity mediated by a subset of human NK cells. J. Immunol. 159, 3655–3661 (1997).
Forte, P., Baumann, B. C., Schneider, M. K. & Seebach, J. D. HLA-Cw4 expression on porcine endothelial cells reduces cytotoxicity and adhesion mediated by CD158a+ human NK cells. Xenotransplantation 16, 19–26 (2009).
Lilienfeld, B. G., Crew, M. D., Forte, P., Baumann, B. C. & Seebach, J. D. Transgenic expression of HLA-E single chain trimer protects porcine endothelial cells against human natural killer cell-mediated cytotoxicity. Xenotransplantation 14, 126–134 (2007).
Weiss, E. H. et al. HLA-E/human beta2-microglobulin transgenic pigs: protection against xenogeneic human anti-pig natural killer cell cytotoxicity. Transplantation 87, 35–43 (2009).
Seebach, J. D., Yamada, K., McMorrow, I., Sachs, D. H. & DerSimonian, H. D.Xenogeneic human anti-pig cytotoxicity mediated by activated natural killer cells. Xenotransplantation 3, 188–197 (1996).
Watier, H. et al. Human NK cell-mediated direct and IgG-dependent cytotoxicity against xenogeneic porcine endothelial cells. Transpl. Immunol. 4, 293–299 (1996).
Nikolic, B., Cooke, D. T., Zhao, G. & Sykes, M. Both γδ T cells and NK cells inhibit the engraftment of xenogeneic rat bone marrow cells and the induction of xenograft tolerance in mice. J. Immunol. 166, 1398–1404 (2001).
Sacks, S. H., Chowdhury, P. & Zhou, W. Role of the complement system in rejection. Curr. Opin. Immunol. 15, 487–492 (2003).
Java, A., Atkinson, J. & Salmon, J. Defective complement inhibitory function predisposes to renal disease. Annu. Rev. Med. 64, 307–324 (2013).
Cozzi, E. & White, D. J. G. The generation of transgenic pigs as potential organ donors for humans. Nat. Med. 1, 964–967 (1995).
Robson, S. C., Cooper, D. K. & d’Apice, A. J. Disordered regulation of coagulation and platelet activation in xenotransplantation. Xenotransplantation 7, 166–176 (2000).
Shimizu, A. et al. Thrombotic microangiopathy associated with humoral rejection of cardiac xenografts from α1,3-galactosyltransferase gene-knockout pigs in baboons. Am. J. Pathol. 172, 1471–1481 (2008).
Shimizu, A. et al. Thrombotic microangiopathic glomerulopathy in human decay accelerating factor-transgenic swine-to-baboon kidney xenografts. J. Am. Soc. Nephrol. 16, 2732–2745 (2005).
Knosalla, C. et al. Renal and cardiac endothelial heterogeneity impact acute vascular rejection in pig-to-baboon xenotransplantation. Am. J. Transplant. 9, 1006–1016 (2009).
Yang, Y.-G., Chen, A. M., Sergio, J. J., Zhou, Y. & Sykes, M. Role of antibody-independent complement activation in rejection of porcine bone marrow cells in mice. Transplantation 69, 163–190 (1999).
Galili, U., Shohet, S. B., Kobrin, E., Stults, C. L. & Macher, B. A. Man, apes, and old world monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J. Biol. Chem. 263, 17755–17762 (1988).
Parker, W., Bruno, D., Holzknecht, Z. E. & Platt, J. L. Characterization and affinity isolation of xenoreactive human natural antibodies. J. Immunol. 153, 3791–3803 (1994).
McMorrow, I. M., Comrack, C. A., Sachs, D. H. & DerSimonian, H. Heterogeneity of human anti-pig natural antibodies cross-reactive with the Gal(α1,3)Galactose epitope. Transplantation 64, 501–510 (1997).
Lai, L. et al. Production of {α}-1,3-Galactosyltransferase knockout pigs by nuclear transfer cloning. Science 295, 1089–1092 (2002).
Phelps, C. J. et al. Production of α 1,3-galactosyltransferase-deficient pigs. Science 299, 411–414 (2003).
Kolber-Simonds, D. et al. Production of {α}-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc. Natl Acad. Sci. USA. 101, 7335–7340 (2004).
Chen, G. et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat. Med. 11, 1295–1298 (2005).
Le Bas-Bernardet, S. et al. Bortezomib, C1-inhibitor and plasma exchange do not prolong the survival of multi-transgenic GalT-KO pig kidney xenografts in baboons. Am. J. Transplant. 15, 358–370 (2015).
Le Bas-Bernardet, S. et al. Xenotransplantation of galactosyl-transferase knockout, CD55, CD59, CD39, and fucosyl-transferase transgenic pig kidneys into baboons. Transplant. Proc. 43, 3426–3430 (2011).
Byrne, G. W. et al. Proteomic identification of non-Gal antibody targets after pig-to-primate cardiac xenotransplantation. Xenotransplantation 15, 268–276 (2008).
Byrne, G. W., Stalboerger, P. G., Du, Z., Davis, T. R. & McGregor, C. G. Identification of new carbohydrate and membrane protein antigens in cardiac xenotransplantation. Transplantation 91, 287–292 (2011).
Miwa, Y. et al. Are N-glycolylneuraminic acid (Hanganutziu-Deicher) antigens important in pig-to-human xenotransplantation? Xenotransplantation 11, 247–253 (2004).
Zhu, A. & Hurst, R. Anti-N-glycolylneuraminic acid antibodies identified in healthy human serum. Xenotransplantation 9, 376–381 (2002).
Irie, A., Koyama, S., Kozutsumi, Y., Kawasaki, T. & Suzuki, A. The molecular basis for the absence of N-glycolylneuraminic acid in humans. J. Biol. Chem. 273, 15866–15871 (1998).
Yamamoto, T. et al. Old World Monkeys are less than ideal transplantation models for testing pig organs lacking three carbohydrate antigens (Triple-Knockout). Sci. Rep. 10, 9771 (2020).
Platt, J. L. et al. The role of natural antibodies in the activation of xenogeneic endothelial cells. Transplantation 52, 1037–1043 (1991).
Grehan, J. F., Levay-Young, B. K., Benson, B. A., Abrahamsen, M. S. & Dalmasso, A. P. αGal ligation of pig endothelial cells induces protection from complement and apoptosis independently of NF-κB and inflammatory changes. Am. J. Transplant. 5, 712–719 (2005).
Magee, J. C. et al. Prevention of hyperacute xenograft rejection by intravenous immunoglobulin. Transplant. Proc. 27, 271–271 (1995).
Dalmasso, A. P., He, T. & Benson, B. A. Human IgM xenoreactive natural antibodies can induce resistance of porcine endothelial cells to complement-mediated injury. Xenotransplantation 3, 54–62 (1996).
Ladowski, J. M. et al. Swine leukocyte antigen (SLA) class II is a xenoantigen. Transplantation 102, 249–254 (2017).
Martens, G. R. et al. Humoral reactivity of renal transplant-waitlisted patients to cells from GGTA1/CMAH/B4GalNT2, and SLA class I knockout pigs. Transplantation 101, e86–e92 (2017).
Martens, G. R. et al. HLA class I-sensitized renal transplant patients have antibody binding to SLA class I epitopes. Transplantation 103, 1620–1629 (2019).
Ladowski, J. M. et al. Examining epitope mutagenesis as a strategy to reduce and eliminate human antibody binding to class II swine leukocyte antigens. Immunogenetics 71, 479–487 (2019).
Barcy, S. et al. γδ+ T cells involvement in viral immune control of chronic human herpesvirus 8 infection. J. Immunol. 180, 3417–3425 (2008).
Wu, Y. et al. Human γδ T cells: a lymphoid lineage cell capable of professional phagocytosis. J. Immunol. 183, 5622–5629 (2009).
Nikolic, B., Cooke, D. T., Zhao, G. & Sykes, M. Both γ/δ T cells and NK cells inhibit the engraftment of xenogeneic rat bone marrow cells in mice. J. Immunol. 166, 1398–1404 (2001).
Hering, B. J. et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat. Med. 12, 301–303 (2006).
Cardona, K. et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat. Med. 12, 304–306 (2006).
Moses, R. D., Pierson, R. N. III, Winn, H. J. & Auchincloss, H. J. Xenogeneic proliferation and lymphokine production are dependent on CD4+helper T cells and self antigen-presenting cells in the mouse. J. Exp. Med. 172, 567–575 (1990).
Moses, R. D., Winn, H. J. & Auchincloss, H. Jr. Evidence that multiple defects in cell-surface molecule interactions across species differences are responsible for diminished xenogeneic T cell responses. Transplantation 53, 203–209 (1992).
Zhao, Y. et al. Maturation and function of mouse T cells with a transgeneic TCR positively selected by highly disparate xenogeneic porcine MHC. Cell. Mol. Biol. 47, 217–228 (2000).
Zhao, Y. et al. Positive and negative selection of functional mouse CD4 cells by porcine MHC in pig thymus grafts. J. Immunol. 159, 2100–2107 (1997).
Zhao, Y., Swenson, K., Sergio, J. J. & Sykes, M. Pig MHC mediates positive selection of mouse CD4+ T cells with a mouse MHC-restricted TCR in pig thymus grafts. J. Immunol. 161, 1320–1326 (1998).
Shimizu, I., Fudaba, Y., Shimizu, A., Yang, Y. G. & Sykes, M. Comparison of human T cell repertoire generated in xenogeneic porcine and human thymus grafts. Transplantation 86, 601–610 (2008).
Dorling, A., Lombardi, G., Binns, R. & Lechler, R. I. Detection of primary direct and indirect human anti-porcine T cell responses using a porcine dendritic cell population. Eur. J. Immunol. 26, 1378–1387 (1996).
Yamada, K., Sachs, D. H. & DerSimonian, H. Human anti-porcine xenogeneic T cell response. Evidence for allelic specificity of mixed leukocyte reaction and for both direct and indirect pathways of recognition. J. Immunol. 155, 5249–5256 (1995).
Rollins, S. A. et al. Evidence that activation of human T cells by porcine endothelium involves direct recognition of porcine SLA and costimulation by porcine ligands for LFA-1 and CD2. Transplantation 57, 1709–1716 (1994).
Yamada, K., Sachs, D. H. & DerSimonian, H. Direct and indirect recognition of pig class II antigens by human T cells. Transplant. Proc. 27, 258–259 (1995).
Godwin, J. W., D’Apice, A. J. & Cowan, P. J. Characterization of pig intercellular adhesion molecule-2 and its interaction with human LFA-1. Am. J. Transplant. 4, 515–525 (2004).
Murray, A. G., Khodadoust, M. M., Pober, J. S. & Bothwell, A. L. Porcine aortic endothelial cells activate human T cells: direct presentation of MHC antigens and costimulation by ligands for human CD2 and CD28. Immunity 1, 57–63 (1994).
Zheng, L., Ben, L. H., Pober, J. S. & Bothwell, A. L. Porcine endothelial cells, unlike human endothelial cells, can be killed by human CTL via Fas ligand and cannot be protected by Bcl-2. J. Immunol. 169, 6850–6855 (2002).
Yi, S., Feng, X., Wang, Y., Kay, T. W. & O’Connell, P. J. CD4+ cells play a major role in xenogeneic human anti-pig cytotoxicity through the Fas/Fas ligand lytic pathway. Transplantation 67, 435–443 (1999).
Sultan, P. et al. Pig but not human interferon-γ initiates human cell-mediated rejection of pig tissue in vivo. Proc. Natl Acad. Sci. Usa. 94, 8767–8772 (1997).
Hering, B. J. & Walawalkar, N. Pig-to-nonhuman primate islet xenotransplantation. Transpl. Immunol. 21, 81–86 (2009).
Buhler, L. et al. Persistence of indirect but not direct T cell xenoresponses in baboon recipients of pig cell and organ transplants. Am. J. Transplant. 16, 1917–1922 (2016).
Yamada, K. et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of α1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat. Med. 11, 32–34 (2005).
Davila, E. et al. T-cell responses during pig-to-primate xenotransplantation. Xenotransplantation 13, 31–40 (2006).
Lee, R. S. et al. Blockade of CD28-B7, but not CD40-CD154, prevents costimulation of allogeneic porcine and xenogeneic human anti-porcine T cell responses. J. Immunol. 164, 3434–3444 (2000).
Wu, G. et al. Co-stimulation blockade targeting CD154 and CD28/B7 modulates the induced antibody response after a pig-to-baboon cardiac xenograft. Xenotransplantation 12, 197–208 (2005).
Brenner, P. M. et al. Orthotopic cardiac xenotransplantation of GalKO/hCD46/hTM-transgenic pig hearts into baboons (40 days survival) using a CD40mAb or CD40L-Ab costimulation blockade. Xenotransplantation 24, 29 (2017).
Mohiuddin, M. M. et al. One-year heterotopic cardiac xenograft survival in a pig to baboon model. Am. J. Transplant. 14, 488–489 (2014).
Iwase, H. et al. Pig kidney graft survival in a baboon for 136 days: longest life-supporting organ graft survival to date. Xenotransplantation 22, 302–309 (2015).
Cardona, K. et al. Engraftment of adult porcine islet xenografts in diabetic nonhuman primates through targeting of costimulation pathways. Am. J. Transplant. 7, 2260–2268 (2007).
Mohiuddin, M. M. et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nat. Commun. 7, 11138 (2016).
Jin, X. et al. Enhanced suppression of the xenogeneic T-cell response in vitro by xenoantigen stimulated and expanded regulatory T cells. Transplantation 97, 30–38 (2014).
Yi, S. et al. Adoptive transfer with in vitro expanded human regulatory T cells protects against porcine islet xenograft rejection via interleukin-10 in humanized mice. Diabetes 61, 1180–1191 (2012).
Fu, Y. et al. In vitro suppression of xenoimmune-mediated macrophage activation by human CD4+CD25+regulatory T cells. Transplantation 86, 865–874 (2008).
Stern, J. K. et al. Extended pig skin graft survival in baboons receiving Hcd47 Tg/Galt-KO pig PBSC plus host Treg infusion. Xenotransplantation 24, 9 (2017).
Shin, J. S. et al. Failure of transplantation tolerance induction by autologous regulatory T cells in the pig-to-non-human primate islet xenotransplantation model. Xenotransplantation 23, 300–309 (2016).
Layton, D. S. et al. Differential cytokine expression and regulation of human anti-pig xenogeneic responses by modified porcine dendritic cells. Xenotransplantation 15, 257–267 (2008).
Madelon, N., Puga Yung, G. L. & Seebach, J. D. Human anti-pig NK cell and CD8+ T-cell responses in the presence of regulatory dendritic cells. Xenotransplantation 23, 479–489 (2016).
Ciubotariu, R. et al. Specific suppression of human CD4+ Th cell responses to pig MHC antigens by CD8+CD28− regulatory T cells. J. Immunol. 161, 5193–5202 (1998).
Niemann, H. & Rath, D. Progress in reproductive biotechnology in swine. Theriogenology 56, 1291–1304 (2001).
Buhler, L., Friedman, T., Iacomini, J. & Cooper, D. K. Xenotransplantation — state of the art — update 1999. Front. Biosci. 4, D416–D432 (1999).
Kozlowski, T. et al. Apheresis and column absorption for specific removal of Gal-α-1,3 Gal natural antibodies in a pig-to-baboon model. Transplant. Proc. 29, 961 (1997).
Kuwaki, K. et al. Heart transplantation in baboons using α1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat. Med. 11, 29–31 (2005).
Hsu, P. D., Lander, E. S. & Zhang, F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262–1278 (2014).
Estrada, J. L. et al. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/beta4GalNT2 genes. Xenotransplantation 22, 194–202 (2015).
Wong, B. S. et al. Allosensitization does not increase the risk of xenoreactivity to α1,3-galactosyltransferase gene-knockout miniature swine in patients on transplantation waiting lists. Transplantation 82, 314–319 (2006).
Hara, H. et al. Allosensitized humans are at no greater risk of humoral rejection of GT-KO pig organs than other humans. Xenotransplantation 13, 357–365 (2006).
Cooper, D. K., Tseng, Y. L. & Saidman, S. L. Alloantibody and xenoantibody cross-reactivity in transplantation. Transplantation 77, 1–5 (2004).
Chong, A. S.-F. et al. Delayed xenograft rejection in the concordant hamster heart into Lewis rat model. Transplantation 62, 90–96 (1996).
Lin, Y., Sobis, H., Vandeputte, M. & Waer, M. Mechanism of leflunomide-induced prevention of xenoantibody formation and xenograft rejection in the hamster to rat heart transplantation model. Transplant. Proc. 27, 305–306 (1995).
Lundvig, D. M., Immenschuh, S. & Wagener, F. A. Heme oxygenase, inflammation, and fibrosis: the good, the bad, and the ugly? Front. Pharmacol. 3, 81 (2012).
Mohiuddin, M. M. et al. Progressive genetic modifications of porcine cardiac xenografts extend survival to 9 months. Xenotransplantation 29, e12744 (2022).
Niemann, H. & Petersen, B. The production of multi-transgenic pigs: update and perspectives for xenotransplantation. Transgenic Res. 25, 361–374 (2016).
Singh, A. K. et al. Cardiac xenografts show reduced survival in the absence of transgenic human thrombomodulin expression in donor pigs. Xenotransplantation 26, e12465 (2018).
Connolly, M. R. et al. Humanized von Willebrand factor reduces platelet sequestration in ex vivo and in vivo xenotransplant models. Xenotransplantation 28, e12712 (2021).
Lee, S. J. et al. Seven years of experiences of preclinical experiments of Xeno-Heart transplantation of pig to non-human primate (Cynomolgus Monkey). Transplant. Proc. 50, 1167–1171 (2018).
Fischer, K. et al. Efficient production of multi-modified pigs for xenotransplantation by ‘combineering’, gene stacking and gene editing. Sci. Rep. 6, 29081 (2016).
Ma, D. et al. Kidney transplantation from triple-knockout pigs expressing multiple human proteins in cynomolgus macaques. Am. J. Transplant. 22, 46–57 (2022).
Kim, S. C. et al. Long-term survival of pig-to-rhesus macaque renal xenografts is dependent on CD4 T cell depletion. Am. J. Transplant. 19, 2174–2185 (2019).
Adams, A. B. et al. Xenoantigen deletion and chemical immunosuppression can prolong renal xenograft survival. Ann. Surg. 268, 564–573 (2018).
Tsuyuki, S., Kono, M. & Bloom, E. T. Cloning and potential utility of porcine Fas ligand: overexpression in porcine endothelial cells protects them from attack by human cytolytic cells. Xenotransplantation 9, 410–421 (2002).
Bahr, A. et al. Ubiquitous LEA29Y expression blocks T cell co-stimulation but permits sexual reproduction in genetically modified pigs. PLoS One 11, e0155676 (2016).
Nottle, M. B. et al. Targeted insertion of an anti-CD2 monoclonal antibody transgene into the GGTA1 locus in pigs using FokI-dCas9. Sci. Rep. 7, 8383 (2017).
Vabres, B. et al. hCTLA4-Ig transgene expression in keratocytes modulates rejection of corneal xenografts in a pig to non-human primate anterior lamellar keratoplasty model. Xenotransplantation 21, 431–443 (2014).
Klymiuk, N. et al. Xenografted islet cell clusters from INSLEA29Y transgenic pigs rescue diabetes and prevent immune rejection in humanized mice. Diabetes 61, 1527–1532 (2012).
Fischer, K. et al. Viable pigs after simultaneous inactivation of porcine MHC class I and three xenoreactive antigen genes GGTA1, CMAH and B4GALNT2. Xenotransplantation 27, e12560 (2019).
Puga Yung, G. et al. Release of pig leukocytes and reduced human NK cell recruitment during ex vivo perfusion of HLA-E/human CD46 double-transgenic pig limbs with human blood. Xenotransplantation 25, 12357 (2018).
Shah, J. A. et al. A bridge to somewhere: 25-day survival after pig-to-baboon liver xenotransplantation. Ann. Surg. 263, 1069–1071 (2016).
Markmann, J. F. et al. Executive summary of IPITA-TTS opinion leaders report on the future of beta-cell replacement. Transplantation 100, e25–e31 (2016).
Graham, M. L. et al. Clinically available immunosuppression averts rejection but not systemic inflammation after porcine islet xenotransplant in cynomolgus macaques. Am. J. Transplant. 22, 745–760 (2022).
Bottino, R. et al. Pig-to-monkey islet xenotransplantation using multi-transgenic pigs. Am. J. Transplant. 14, 2275–2287 (2014).
Dor, F. J., Cheng, J., Alt, A., Cooper, D. K. & Schuurman, H. J. Galα1,3 Gal expression on porcine pancreatic islets, testis, spleen, and thymus. Xenotransplantation 11, 101–106 (2004).
Ozmen, L. et al. Inhibition of thrombin abrogates the instant blood-mediated inflammatory reaction triggered by isolated human islets: possible application of the thrombin inhibitor melagatran in clinical islet transplantation. Diabetes 51, 1779–1784 (2002).
Barton, F. B. et al. Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care 35, 1436–1445 (2012).
Cooper, D. K. et al. Progress in clinical encapsulated islet xenotransplantation. Transplantation 100, 2301–2308 (2016).
Morozov, V. A. et al. No PERV transmission during a clinical trial of pig islet cell transplantation. Virus Res. 227, 34–40 (2017).
Wynyard, S., Nathu, D., Garkavenko, O., Denner, J. & Elliott, R. Microbiological safety of the first clinical pig islet xenotransplantation trial in New Zealand. Xenotransplantation 21, 309–323 (2014).
Garkavenko, O. et al. The first clinical xenotransplantation trial in New Zealand: efficacy and safety. Xenotransplantation 19, 6 (2012).
Singh, A. et al. Long-term tolerance of islet allografts in nonhuman primates induced by apoptotic donor leukocytes. Nat. Commun. 10, 3495 (2019).
Wang, S. et al. Transient B-cell depletion combined with apoptotic donor splenocytes induces xeno-specific T- and B-cell tolerance to islet xenografts. Diabetes 62, 3143–3150 (2013).
Higginbotham, L. et al. Pre-transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model. Xenotransplantation 22, 221–230 (2015).
Kim, S. C. et al. Fc-Silent anti-CD154 domain antibody effectively prevents nonhuman primate renal allograft rejection. Am. J. Transplant. 17, 1182–1192 (2017).
Cooper, D. K. C. et al. Selection of patients for initial clinical trials of solid organ xenotransplantation. Transplantation 101, 1551–1558 (2017).
Cooper, D. K. C. et al. Xenotransplantation — the current status and prospects. Br. Med. Bull. 125, 5–14 (2018).
Montgomery, R. A. et al. Results of two cases of pig-to-human kidney xenotransplantation. N. Engl. J. Med. 386, 1889–1898 (2022).
Porrett, P. M. et al. First clinical-grade porcine kidney xenotransplant using a human decedent model. Am. J. Transplant. 22, 1037–1053 (2022).
Langin, M. et al. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature 564, 430–433 (2018).
Goerlich, C. E. et al. The growth of xenotransplanted hearts can be reduced with growth hormone receptor knockout pig donors. J. Thorac. Cardiovasc. Surg. https://doi.org/10.1016/j.jtcvs.2021.07.051 (2021).
Riedel, E. O. et al. Functional changes of the liver in the absence of growth hormone (GH) action — proteomic and metabolomic insights from a GH receptor deficient pig model. Mol. Metab. 36, 100978 (2020).
Griffith, B. P. et al. Genetically modified porcine-to-human cardiac xenotransplantation. N. Eng. J. Med. 387, 35–44 (2022).
Sykes, M. Hematopoietic cell transplantation for tolerance induction: animal models to clinical trials. Transplantation 87, 309–316 (2009).
Sykes, M. IXA honorary member lecture, 2017: the long and winding road to tolerance. Xenotransplantation 25, e12419 (2018).
Waffarn, E. E. et al. Mixed xenogeneic porcine chimerism tolerizes human anti-pig natural antibody-producing cells in a humanized mouse model. Xenotransplantation 28, e12691 (2021).
Li, H. W. et al. Impact of mixed xenogeneic porcine hematopoietic chimerism on human NK cell recognition in a humanized mouse model. Am. J. Transplant. 17, 353–364 (2017).
Sharabi, Y., Aksentijevich, I., Sundt, T. M. III, Sachs, D. H. & Sykes, M. Specific tolerance induction across a xenogeneic barrier: production of mixed rat/mouse lymphohematopoietic chimeras using a nonlethal preparative regimen. J. Exp. Med. 172, 195–202 (1990).
Sharabi, Y. & Sachs, D. H. Mixed chimerism and permanent specific transplantation tolerance induced by a non-lethal preparative regimen. J. Exp. Med. 169, 493–502 (1989).
Lee, L. A., Sergio, J. J. & Sykes, M. Natural killer cells weakly resist engraftment of allogeneic long-term multilineage-repopulating hematopoietic stem cells. Transplantation 61, 125–132 (1996).
Tomita, Y., Lee, L. A. & Sykes, M. Engraftment of rat bone marrow and its role in negative selection of murine T cells in mice conditioned with a modified non-myeloablative regimen. Xenotransplantation 1, 109–117 (1994).
Lee, L. A., Sergio, J. J. & Sykes, M. Evidence for non-immune mechanisms in the loss of hematopoietic chimerism in rat-mouse mixed xenogeneic chimeras. Xenotransplantation 2, 57–66 (1995).
Aksentijevich, I., Sachs, D. H., Sharabi, Y., Sundt, T. M. I. & Sykes, M. Humoral tolerance in mixed xenogeneic chimeras prepared by a non-myeloablative conditioning regimen. Transplant. Proc. 23, 880–882 (1991).
Kawahara, T., Rodriguez-Barbosa, J. I., Zhao, Y., Zhao, G. & Sykes, M. Global unresponsiveness as a mechanism of natural killer cell tolerance in mixed xenogeneic chimeras. Am. J. Transplant. 7, 2090–2097 (2007).
Gritsch, H. A. et al. The importance of non-immune factors in reconstitution by discordant xenogeneic hematopoietic cells. Transplantation 57, 906–917 (1994).
Chen, A. M. et al. Porcine stem cell engraftment and seeding of murine thymus with class II+ cells in mice expressing porcine cytokines: toward tolerance induction across discordant xenogeneic barriers. Transplantation 69, 2484–2490 (2000).
Aksentijevich, I., Sachs, D. H. & Sykes, M. Natural antibodies can inhibit bone marrow engraftment in the rat–mouse species combination. J. Immunol. 147, 4140–4146 (1991).
Aksentijevich, I., Sachs, D. H. & Sykes, M. Humoral tolerance in xenogeneic BMT recipients conditioned with a non-myeloablative regimen. Transplantation 53, 1108–1114 (1992).
Aksentijevich, I., Sachs, D. H. & Sykes, M. Normal mouse serum contains natural antibody against bone marrow cells of a concordant xenogeneic species. J. Immunol. 147, 79–85 (1991).
Lee, L. A., Sergio, J. J., Sachs, D. H. & Sykes, M. Mechanism of tolerance in mixed xenogeneic chimeras prepared with a non-myeloablative conditioning regimen. Transplant. Proc. 26, 1197–1198 (1994).
Thall, A. D. Generation of α1,3Galactosyltransferase deficient mice. Subcell. Biochem. 32, 259–279 (1999).
Yang, Y.-G. et al. Tolerization of anti-galα1-3 gal natural antibody-forming B cells by induction of mixed chimerism. J. Exp. Med. 187, 1335–1342 (1998).
Ohdan, H., Yang, Y.-G., Shimizu, A., Swenson, K. G. & Sykes, M. Mixed bone marrow chimerism induced without lethal conditioning prevents T cell and anti-Galα1,3Gal-mediated graft rejection. J. Clin. Invest. 104, 281–290 (1999).
Ohdan, H., Yang, Y.-G., Swenson, K. G., Kitamura, H. & Sykes, M. T cell and B cell tolerance to galα1,3gal-expressing heart xenografts is achieved in α1,3-galactosyltransferase-deficient mice by non-myeloablative induction of mixed chimerism. Transplantation 71, 1532–1542 (2001).
Ohdan, H., Swenson, K. G., Kitamura, H., Yang, Y.-G. & Sykes, M. Tolerization of Galα1,3Gal-reactive B cells in presensitized α1,3-galactosyltransferase-deficient mice by nonmyeloablative induction of mixed chimerism. Xenotransplantation 8, 227–238 (2002).
Nikolic, B. et al. Role of intrathymic rat class II cells in maintaining deletional tolerance in xenogeneic rat–mouse bone marrow chimeras. Transplantation 65, 1216–1224 (1998).
Lan, P., Tonomura, N., Shimizu, A., Wang, S. & Yang, Y. G. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood 108, 487–492 (2006).
Lan, P. et al. Induction of human T cell tolerance to porcine xenoantigens through mixed hematopoietic chimerism. Blood 103, 3964–3969 (2004).
Watanabe, H. et al. Intra-bone bone marrow transplantation from hCD47 transgenic pigs to baboons prolongs chimerism to >60 days and promotes increased porcine lung transplant survival. Xenotransplantation 27, e12552 (2020).
Kawahara, T., Ohdan, H., Zhao, G., Yang, Y.-G. & Sykes, M. Peritoneal cavity B cells are precursors of splenic IgM natural antibody-producing cells. J. Immunol. 171, 5406–5414 (2003).
Ohdan, H. et al. Mac-1-negative B-1b phenotype of natural antibody-producing cells, including those responding to Galα1,3 Gal epitopes in α1,3-galactosyltransferase deficient mice. J. Immunol. 165, 5518–5529 (2000).
Griffin, D. O., Holodick, N. E. & Rothstein, T. L. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+CD27+CD43+CD70−. J. Exp. Med. 208, 67–80 (2011).
Griffin, D. O., Holodick, N. E. & Rothstein, T. L. Human B1 cells are CD3−: a reply to “A human equivalent of mouse B-1 cells?” and “The nature of circulating CD27+CD43+B cells”. J. Exp. Med. 208, 2566–2569 (2011).
Griffin, D. O. & Rothstein, T. L. A small CD11b+ human B1 cell subpopulation stimulates T cells and is expanded in lupus. J. Exp. Med. 208, 2591–2598 (2011).
Griffin, D. O. & Rothstein, T. L. Human “orchestrator” CD11b+ B1 cells spontaneously secrete IL-10 and regulate T cell activity. Mol. Med. 18, 1003–1008 (2012).
Reynaud, C. A. & Weill, J. C. Gene profiling of CD11b+ and CD11b− B1 cell subsets reveals potential cell sorting artifacts. J. Exp. Med. 209, 433–434 (2012).
Xu, Y. et al. Characterization of anti-Gal antibody-producing cells of baboons and humans. Transplantation 81, 940–948 (2006).
Kawahara, T., Shimizu, I., Ohdan, H., Zhao, G. & Sykes, M. Differing mechanisms of early and late B cell tolerance induced by mixed chimerism. Am. J. Transplant. 5, 2821–2829 (2005).
Shimizu, I. et al. B-cell extrinsic CR1/CR2 promotes natural antibody production and tolerance induction of anti-α-Gal-producing B-1 cells. Blood 109, 1773–1781 (2006).
Bardwell, P. D., Ohdan, H. & Sykes, M. B cell tolerance and xenotransplantation. Curr. Op. Organ. Transplant. 10, 252–258 (2005).
Abe, M. et al. Elimination of porcine hemopoietic cells by macrophages in mice. J. Immunol. 168, 621–628 (2002).
Wang, H. & Yang, Y. G. Innate cellular immunity and xenotransplantation. Curr. Opin. Organ. Transplant. 17, 162–167 (2012).
Wang, H. et al. Attenuation of phagocytosis of xenogeneic cells by manipulating CD47. Blood 109, 836–842 (2007).
Ide, K. et al. Role for CD47-SIRPα signaling in xenograft rejection by macrophages. Proc. Natl Acad. Sci. USA 104, 5062–5066 (2007).
Wang, H., Wu, X., Wang, Y., Oldenborg, P. A. & Yang, Y. G. CD47 is required for suppression of allograft rejection by donor-specific transfusion. J. Immunol. 184, 3401–3407 (2010).
Navarro-Alvarez, N. & Yang, Y. G. Lack of CD47 on donor hepatocytes promotes innate immune cell activation and graft rejection: a potential barrier to hepatocyte xenotransplantation. Am. J. Transplant. 11, 107–108 (2011).
Wang, Y., Wang, H., Bronson, R., Fu, Y. & Yang, Y. G. Rapid dendritic cell activation and resistance to allotolerance induction in anti-CD154-treated mice receiving CD47-deficient donor-specific transfusion. Cell Transpl. 23, 355–363 (2013).
Ide, K. et al. Role for CD47-SIRPα signaling in xenograft rejection by macrophages. Proc. Natl Acad. Sci. USA 104, 5062–5066 (2007).
Wong, A. S. et al. Polymorphism in the innate immune receptor SIRPα controls CD47 binding and autoimmunity in the nonobese diabetic mouse. J. Immunol. 193, 4833–4844 (2014).
Tena, A. et al. Transgenic expression of human CD47 markedly increases engraftment in a murine model of pig-to-human hematopoietic cell transplantation. Am. J. Transplant. 14, 2713–2722 (2014).
Wang, C. et al. Human CD47 expression permits survival of porcine cells in immunodeficient mice that express SIRPα capable of binding to human CD47. Cell Transpl. 20, 1915–1920 (2011).
Wang, H. et al. Lack of CD47 on nonhematopoietic cells induces split macrophage tolerance to CD47null cells. Proc. Natl Acad. Sci. USA 104, 13744–13749 (2007).
Tena, A. A. et al. Prolonged survival of pig skin on baboons after administration of pig cells expressing human CD47. Transplantation 101, 316–321 (2017).
Tasaki, M. et al. High incidence of xenogenic bone marrow engraftment in pig-to-baboon intra-bone bone marrow transplantation. Am. J. Transplant. 15, 974–983 (2015).
Zhao, Y., Ohdan, H., Manilay, J. O. & Sykes, M. NK cell tolerance in mixed allogeneic chimeras. J. Immunol. 170, 5398–5405 (2003).
Sasaki, H., Xu, X. C., Smith, D. M., Howard, T. & Mohanakumar, T. HLA-G expression protects porcine endothelial cells against natural killer cell-mediated xenogeneic cytotoxicity. Transplantation 67, 31–37 (1999).
Torgersen, K. M. et al. Major histocompatibility complex class I-independent killing of xenogenic targets by rat allospecific natural killer cells. Transplantation 63, 119–123 (1997).
Forte, P. et al. HLA-G inhibits rolling adhesion of activated human NK cells on porcine endothelial cells. J. Immunol. 167, 6002–6008 (2001).
Silver, E. T., Lavender, K. J., Gong, D. E., Hazes, B. & Kane, K. P. Allelic variation in the ectodomain of the inhibitory Ly-49G2 receptor alters its specificity for allogeneic and xenogeneic ligands. J. Immunol. 169, 4752–4760 (2002).
Forte, P., Baumann, B. C., Weiss, E. H. & Seebach, J. D. HLA-E expression on porcine cells: protection from human NK cytotoxicity depends on peptide loading. Am. J. Transplant. 5, 2085–2093 (2005).
Benoit, L. A., Shannon, J., Chamberlain, J. W. & Miller, R. G. Influence of xenogeneic β2-microglobulin on functional recognition of H-2Kb by the NK cell inhibitory receptor Ly49C. J. Immunol. 175, 3542–3553 (2005).
Nakamura, M. C. et al. Natural killing of xenogeneic cells mediated by the mouse Ly-49D receptor. J. Immunol. 163, 4694–4700 (1999).
Tran, P. D. et al. Porcine cells express more than one functional ligand for the human lymphocyte activating receptor NKG2D. Xenotransplantation 15, 321–332 (2008).
Lilienfeld, B. G., Garcia-Borges, C., Crew, M. D. & Seebach, J. D. Porcine UL16-binding protein 1 expressed on the surface of endothelial cells triggers human NK cytotoxicity through NKG2D. J. Immunol. 177, 2146–2152 (2006).
Forte, P., Lilienfeld, B. G., Baumann, B. C. & Seebach, J. D. Human NK cytotoxicity against porcine cells is triggered by NKp44 and NKG2D. J. Immunol. 175, 5463–5470 (2005).
Clark, E. A. & Sturge, J. C. Phylogeny of NK cell reactivity against human and nonhuman primate lymphoblastoid cell lines: evolving and conserved target antigens. J. Immunol. 126, 969–974 (1981).
Coudert, J. D., Scarpellino, L., Gros, F., Vivier, E. & Held, W. Sustained NKG2D engagement induces cross-tolerance of multiple distinct NK cell activation pathways. Blood 111, 3571–3578 (2008).
Tripathy, S. K. et al. Continuous engagement of a self-specific activation receptor induces NK cell tolerance. J. Exp. Med. 205, 1829–1841 (2008).
del Rio, M. L., Seebach, J. D., Fernandez-Renedo, C. & Rodriguez-Barbosa, J. I. ITIM-dependent negative signaling pathways for the control of cell-mediated xenogeneic immune responses. Xenotransplantation 20, 397–406 (2013).
Lee, L. A. et al. Specific tolerance across a discordant xenogeneic transplantation barrier. Proc. Natl Acad. Sci. USA 91, 10864–10867 (1994).
Zhao, Y. et al. Skin graft tolerance across a discordant xenogeneic barrier. Nat. Med. 2, 1211–1216 (1996).
Zhao, Y. et al. Despite efficient intrathymic negative selection of host-reactive T cells, autoimmune disease may develop in porcine thymus-grafted athymic mice: evidence for failure of regulatory mechanisms suppressing autoimmunity. Transplantation 75, 1832–1840 (2002).
Rodriguez-Barbosa, J. I. et al. Enhanced CD4 reconstitution by grafting neonatal porcine tissue in alternative locations is associated with donor-specific tolerance and suppression of pre-existing xenoreactive T cells. Transplantation 72, 1223–1231 (2001).
Zhao, Y. et al. Immune restoration by fetal pig thymus grafts in T cell-depleted, thymectomized mice. J. Immunol. 158, 1641–1649 (1997).
Nikolic, B. et al. Normal development in porcine thymus grafts and specific tolerance of human T cells to porcine donor MHC. J. Immunol. 162, 3402–3407 (1999).
Kalscheuer, H. O. et al. Xenograft tolerance and immune function of human T cell developing in pig thymus xenografts. J. Immunol. 192, 3442–3450 (2014).
Kalscheuer, H. et al. A model for personalized in vivo analysis of human immune responsiveness. Sci. Transl. Med. 4, 125ra130 (2012).
Wu, A. et al. Xenogeneic thymus transplantation in a pig-to-baboon model. Transplantation 75, 282–291 (2003).
Zhao, Y. et al. The critical role of mouse CD4+ cells in the rejection of highly disparate xenogeneic pig thymus grafts. Xenotransplant 7, 129–137 (2000).
deBlecourt, M. World’s first heart transplant, cultured thymus implantation performed at Duke. Milestone could eliminate need for anti-rejection medications., https://www.dukehealth.org/blog/worlds-first-heart-transplant-cultured-thymus-implantation-performed-duke (2022).
Auchincloss, H. Jr, Mayer, T., Ghobrial, R. & Winn, H. J. T-cell subsets, bm mutants, and the mechanisms of allogeneic skin graft rejection. Immunol. Res. 8, 149–164 (1989).
Yamada, K. et al. Thymic transplantation in miniature swine. I. Development and function of the “thymokidney”. Transplantation 68, 1684–1692 (1999).
Yamada, K. et al. Thymic transplantation in miniature swine. II. Induction of tolerance by transplantation of composite thymokidneys to thymectomized recipients. J. Immunol. 164, 3079–3086 (2000).
Yamada, K. et al. Thymic transplantation in miniature swine: III. Induction of tolerance by transplantation of composite thymokidneys across fully major histocompatibility complex-mismatched barriers. Transplantation 76, 530–536 (2003).
Kamano, C. et al. Vascularized thymic lobe transplantation in miniature swine: thymopoiesis and tolerance induction across fully MHC-mismatched barriers. Proc. Natl Acad. Sci. USA 101, 3827–3832 (2004).
Nobori, S. et al. Long-term acceptance of fully allogeneic cardiac grafts by cotransplantation of vascularized thymus in miniature swine. Transplantation 81, 26–35 (2006).
Barth, R. N. et al. Xenogeneic thymokidney and thymic tissue transplantation in a pig-to-baboon model: I. Evidence for pig-specific T-cell unresponsiveness. Transplantation 75, 1615–1624 (2003).
Griesemer, A. D. et al. Results of gal-knockout porcine thymokidney xenografts. Am. J. Transplant. 9, 2669–2678 (2009).
Takeuchi, K. et al. Expression of human CD47 in pig glomeruli prevents proteinuria and prolongs graft survival following pig-to-baboon xenotransplantation. Xenotransplantation 28, e12708 (2021).
Tanabe, T. et al. Role of intrinsic (Graft) versus extrinsic (Host) factors in the growth of transplanted organs following allogeneic and xenogeneic transplantation. Am. J. Transplant. 17, 1778–1790 (2017).
Khosravi Maharlooei, M. H. et al. Generation of human/pig hybrid thymus to achieve immune tolerance to pig antigens with optimal immune function. Xenotransplantation 24, 15 (2017).
Fudaba, Y. et al. Abnormal regulatory and effector T cell function predispose to autoimmunity following xenogeneic thymic transplantation. J. Immunol. 181, 7649–7659 (2008).
Gras-Pena, R. et al. Human stem cell-derived thymic epithelial cells enhance human T cell development in a xenogeneic thymus. J. Allergy Clin. Immunol. 149, 1755–1771 (2021).
Sachs, D. H. The pig as a potential xenograft donor. Vet. Immunol. Immunopathol. 43, 185–191 (1994).
Sachs, D. H. in Swine as Models in Biomedical Research (eds Swindle, M. M., Moody, D. C. & Phillips, L. D.) Ch. 1, 3–15 (Iowa State University Press, 1992).
Mezrich, J. D. et al. Histocompatible miniature swine: an inbred large-animal model. Transplantation 75, 904–907 (2003).
Patience, C., Takeuchi, Y. & Weiss, R. A. Infection of human cells by an endogenous retrovirus of pigs. Nat. Med. 3, 282–286 (1997).
Fishman, J. A. Infectious disease risks in xenotransplantation. Am. J. Transplant. 18, 1857–1864 (2018).
Wynyard, S., Garkavenko, O. & Elliot, R. Multiplex high resolution melting assay for estimation of Porcine Endogenous Retrovirus (PERV) relative gene dosage in pigs and detection of PERV infection in xenograft recipients. J. Virol. Methods 175, 95–100 (2011).
Denner, J. Why was PERV not transmitted during preclinical and clinical xenotransplantation trials and after inoculation of animals? Retrovirology 15, 28 (2018).
Cooper, D. K. C. et al. Regulation of clinical xenotransplantation-time for a reappraisal. Transplantation 101, 1766–1769 (2017).
Dieckhoff, B. et al. Knockdown of porcine endogenous retrovirus (PERV) expression by PERV-specific shRNA in transgenic pigs. Xenotransplantation 15, 36–45 (2008).
Yang, L. et al. Genome-wide inactivation of porcine endogenous retroviruses (PERVs). Science 350, 1101–1104 (2015).
Niu, D. et al. Inactivation of porcine endogenous retrovirus in pigs using CRISPR-Cas9. Science 357, 1303–1307 (2017).
Schaefer, K. A. et al. Unexpected mutations after CRISPR-Cas9 editing in vivo. Nat. Methods 14, 547–548 (2017).
Nellore, A. & Fishman, J. A. Donor-derived infections and infectious risk in xenotransplantation and allotransplantation. Xenotransplantation 25, e12423 (2018).
Ison, M. G. The epidemiology and prevention of donor-derived infections. Adv. Chronic Kidney Dis. 16, 234–241 (2009).
Food and Drug Administration. Source animal, product, preclinical, and clinical issues regarding the use of xenotransplantation products in humans: guidance for industry. (2016).
Bloom, E. T. National policies for xenotransplantation in the USA. Xenotransplantation 14, 345–346 (2007).
World Health Organization. Second WHO global consultation on regulatory requirements for xenotransplantation clinical trials, October 17–19 2011, WHO, Geneva, Switzerland. https://apps.who.int/iris/handle/10665/341817 (2011).
Hering, B. J. et al. First update of the International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes — executive summary. Xenotransplantation 23, 3–13 (2016).
Navarro-Alvarez, N. et al. The effects of exogenous administration of human coagulation factors following pig-to-baboon liver xenotransplantation. Am. J. Transplant. 16, 1715–1725 (2016).
Shah, J. A. et al. Prolonged survival following pig-to-primate liver xenotransplantation utilizing exogenous coagulation factors and costimulation blockade. Am. J. Transplant. 17, 2178–2185 (2017).
Graham, M. L., Bellin, M. D., Papas, K. K., Hering, B. J. & Schuurman, H. J. Species incompatibilities in the pig-to-macaque islet xenotransplant model affect transplant outcome: a comparison with allotransplantation. Xenotransplantation 18, 328–342 (2011).
Hansen-Estruch, C., Porrett, P. M., Kumar, V. & Locke, J. E. The science of xenotransplantation for nephrologists. Curr. Opin. Nephrol. Hypertens. 31, 387–393 (2022).
Cooper, D. K. C. et al. Effects of cyclosporine and antibody adsorption on pig cardiac xenograft survival in the baboon. J. Heart Transplant. 7, 238–246 (1988).
Cozzi, E. et al. in Xenotransplantation: The Transplantation of Organs and Tissues Between Species (eds Cooper, D.K.C., et al) Ch. 49, 665–682 (Springer-Verlag, 1997).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors receive sponsored research support from Choironex, a fully-owned subsidiary of Nephro Health.
Peer review
Peer review information
Nature Reviews Nephrology thanks Devin Eckhoff, Jayme Locke and Eckhard Wolf for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sykes, M., Sachs, D.H. Progress in xenotransplantation: overcoming immune barriers. Nat Rev Nephrol 18, 745–761 (2022). https://doi.org/10.1038/s41581-022-00624-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-022-00624-6
This article is cited by
-
Spatiotemporal immune atlas of a clinical-grade gene-edited pig-to-human kidney xenotransplant
Nature Communications (2024)
-
Replacing renal function using bioengineered tissues
Nature Reviews Bioengineering (2023)