Abstract
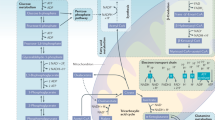
Kidney tubular epithelial cells (TECs) have a crucial role in the damage and repair response to acute and chronic injury. To adequately respond to constant changes in the environment, TECs have considerable bioenergetic needs, which are supported by metabolic pathways. Although little is known about TEC metabolism, a number of ground-breaking studies have shown that defective glucose metabolism or fatty acid oxidation in the kidney has a key role in the response to kidney injury. Imbalanced use of these metabolic pathways can predispose TECs to apoptosis and dedifferentiation, and contribute to lipotoxicity and kidney injury. The accumulation of lipids and aberrant metabolic adaptations of TECs during kidney disease can also be driven by receptors of the innate immune system. Similar to their actions in innate immune cells, pattern recognition receptors regulate the metabolic rewiring of TECs, causing cellular dysfunction and lipid accumulation. TECs should therefore be considered a specialized cell type — like cells of the innate immune system — that is subject to regulation by immunometabolism. Targeting energy metabolism in TECs could represent a strategy for metabolically reprogramming the kidney and promoting kidney repair.
Key points
-
Kidney tubular epithelial cells (TECs) have a high energy demand, relying primarily on fatty acid oxidation as an energy source.
-
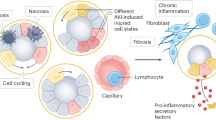
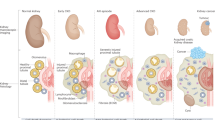
The metabolic and immune profile of TECs is affected by injury; further alterations occur with disease progression or repair processes.
-
Following exposure to cellular stress, TECs rewire their metabolism, resulting in an accumulation of lipids and lipotoxicity.
-
Activation of immunometabolic processes influence the response of TECs to stress; innate immune sensors act to sense immunological changes in the intracellular and extracellular environment but also regulate the metabolic needs, responses and phenotype of TECs.
-
Targeting (immuno)metabolism through, for example, the targeting of innate immune sensors and their metabolic responses may represent a novel strategy for treating or preventing kidney disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wang, Z. M. et al. Specific metabolic rates of major organs and tissues across adulthood: evaluation by mechanistic model of resting energy expenditure. Am. J. Clin. Nutr. 92, 1369–1377 (2010).
Kang, H. M. et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 21, 37–46 (2015).
Ji, R. et al. The Warburg effect promotes mitochondrial injury regulated by uncoupling protein-2 in septic acute kidney injury. Shock 55, 640–648 (2021).
Stokman, G. et al. NLRX1 dampens oxidative stress and apoptosis in tissue injury via control of mitochondrial activity. J. Exp. Med. 214, 2405–2420 (2017).
Rampanelli, E. et al. Metabolic injury-induced NLRP3 inflammasome activation dampens phospholipid degradation. Sci. Rep. 7, 2861 (2017).
Chou, W. C., Rampanelli, E., Li, X. & Ting, J. P. Y. Impact of intracellular innate immune receptors on immunometabolism. Cell. Mol. Immunol. 19, 337–351 (2021).
O’Neill, L. A. J., Kishton, R. J. & Rathmell, J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 16, 553–565 (2016).
Dionne, M. S. Immune-metabolic interaction in Drosophila. Fly 8, 75 (2014).
Oliva, R. & Quibod, I. L. Immunity and starvation: new opportunities to elevate disease resistance in crops. Curr. Opin. Plant. Biol. 38, 84–91 (2017).
Hotamisligil, G. S. & Erbay, E. Nutrient sensing and inflammation in metabolic diseases. Nat. Rev. Immunol. 8, 923–934 (2008).
Schroder, K., Zhou, R. & Tschopp, J. The NLRP3 inflammasome: a sensor for metabolic danger? Science 327, 296–300 (2010).
Tammaro, A., Kers, J., Scantlebery, A. M. L. & Florquin, S. Metabolic flexibility and innate immunity in renal ischemia reperfusion injury: the fine balance between adaptive repair and tissue degeneration. Front. Immunol. 11, 1346 (2020).
Bhargava, P. & Schnellmann, R. G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 13, 629–646 (2017).
Mandel, L. J. Metabolic substrates, cellular energy production, and the regulation of proximal tubular transport. Annu. Rev. Physiol. 47, 85–101 (1985).
Dickman, K. G. & Mandel, L. J. Differential effects of respiratory inhibitors on glycolysis in proximal tubules. Am. J. Physiol. Renal Fluid Electrolyte Physiol. 258, F1608-15 (1990).
Jang, H. S., Noh, M. R., Kim, J. & Padanilam, B. J. Defective mitochondrial fatty acid oxidation and lipotoxicity in kidney diseases. Front. Med. 7, 65 (2020).
Tang, C. et al. Mitochondrial quality control in kidney injury and repair. Nat. Rev. Nephrol. 17, 299–318 (2020).
Wang, H., Zhang, S. & Guo, J. Lipotoxic proximal tubular injury: a primary event in diabetic kidney disease. Front. Med. 8, 751529 (2021).
Malek, M. & Nematbakhsh, M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J. Renal Inj. Prev. 4, 20 (2015).
Weinberg, J. M., Venkatachalam, M. A., Roeser, N. F. & Nissim, I. Mitochondrial dysfunction during hypoxia/reoxygenation and its correction by anaerobic metabolism of citric acid cycle intermediates. Proc. Natl Acad. Sci. USA 97, 2826–2831 (2000).
Zager, R. A., Johnson, A. C. M. & Becker, K. Renal cortical pyruvate depletion during AKI. J. Am. Soc. Nephrol. 25, 998–1012 (2014).
Yan, L.-J. Folic acid-induced animal model of kidney disease. Animal Model. Exp. Med. 4, 329–342 (2021).
Shen, Y. et al. Tubule-derived lactate is required for fibroblast activation in acute kidney injury. Am. J. Physiol. Renal Physiol. 318, F689–F701 (2020).
Legouis, D. et al. Altered proximal tubular cell glucose metabolism during acute kidney injury is associated with mortality. Nat. Metab. 2, 732–743 (2020).
Scantlebery, A. M. L. et al. The dysregulation of metabolic pathways and induction of the pentose phosphate pathway in renal ischaemia–reperfusion injury. J. Pathol. 253, 404–414 (2021).
Ash, S. R. & Cuppage, F. E. Shift toward anaerobic glycolysis in the regenerating rat kidney. Am. J. Pathol. 60, 385–402 (1970).
Smith, J. A., Jay Stallons, L. & Schnellmann, R. G. Renal cortical hexokinase and pentose phosphate pathway activation through the EGFR/Akt signaling pathway in endotoxin-induced acute kidney injury. Am. J. Physiol. Renal Physiol. 307, 435–444 (2014).
Bensaad, K. et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell 126, 107–120 (2006).
Kim, J., Devalaraja-Narashimha, K. & Padanilam, B. J. TIGAR regulates glycolysis in ischemic kidney proximal tubules. Am. J. Physiol. Renal Physiol. 308, F298–F308 (2015).
Kishi, S. et al. Meclizine preconditioning protects the kidney against ischemia-reperfusion injury. EBioMedicine 2, 1090–1101 (2015).
Lan, R. et al. Mitochondrial pathology and glycolytic shift during proximal tubule atrophy after ischemic AKI. J. Am. Soc. Nephrol. 27, 3356–3367 (2016).
Zarjou, A. & Agarwal, A. Sepsis and acute kidney injury. J. Am. Soc. Nephrol. 22, 999–1006 (2011).
Li, Y. et al. Evolution of altered tubular metabolism and mitochondrial function in sepsis-associated acute kidney injury. Am. J. Physiol. Renal Physiol. 319, F229–F244 (2020).
Tran, M. et al. PGC-1α promotes recovery after acute kidney injury during systemic inflammation in mice. J. Clin. Invest. 121, 4003–4014 (2011).
Chouchani, E. T. et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515, 431–435 (2014).
Demine, S., Renard, P. & Arnould, T. Mitochondrial uncoupling: a key controller of biological processes in physiology and diseases. Cells 8, 795 (2019).
Xiong, W. et al. Relieving lipid accumulation through UCP1 suppresses the progression of acute kidney injury by promoting the AMPK/ULK1/autophagy pathway. Theranostics 11, 4637 (2021).
Tan, C. et al. Inhibition of aerobic glycolysis alleviates sepsis‑induced acute kidney injury by promoting lactate/Sirtuin 3/AMPK‑regulated autophagy. Int. J. Mol. Med. 47, 1–1 (2021).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Liu, M., Quek, L.-E., Sultani, G. & Turner, N. Epithelial-mesenchymal transition induction is associated with augmented glucose uptake and lactate production in pancreatic ductal adenocarcinoma. Cancer Metab. 4, 19 (2016).
Naito, M., Bomsztyk, K. & Zager, R. A. Endotoxin mediates recruitment of RNA polymerase II to target genes in acute renal failure. J. Am. Soc. Nephrol. 19, 1321–1330 (2008).
Hato, T. et al. Two-photon intravital fluorescence lifetime imaging of the kidney reveals cell-type specific metabolic signatures. J. Am. Soc. Nephrol. 28, 2420–2430 (2017).
Netea, M. G. et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 20, 375–388 (2020).
Rao, S. et al. Early lipid changes in acute kidney injury using SWATH lipidomics coupled with MALDI tissue imaging. Am. J. Physiol. Renal Physiol. 310, F1136–F1147 (2016).
Liu, Y. et al. Metabolomic changes and protective effect of L-carnitine in rat kidney ischemia/reperfusion injury. Kidney Blood Press. Res. 35, 373–381 (2012).
Tran, M. T. et al. PGC1α drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature 531, 528–532 (2016).
Wakino, S., Hasegawa, K. & Itoh, H. Sirtuin and metabolic kidney disease. Kidney Int. 88, 691–698 (2015).
Tran, M. T. et al. PGC1α-dependent NAD biosynthesis links oxidative metabolism to renal protection. Nature 531, 528 (2016).
Ozkok, A. & Edelstein, C. L. Pathophysiology of cisplatin-induced acute kidney injury. Biomed. Res. Int. 2014, 967826 (2014).
Zager, R. A., Johnson, A. C. M. & Hanson, S. Y. Renal tubular triglyercide accumulation following endotoxic, toxic, and ischemic injury. Kidney Int. 67, 111–121 (2005).
Johnson, A. C., Stahl, A. & Zager, R. A. Triglyceride accumulation in injured renal tubular cells: alterations in both synthetic and catabolic pathways. Kidney Int. 67, 2196–2209 (2005).
Zager, R. A., Johnson, A. C. & Becker, K. Acute unilateral ischemic renal injury induces progressive renal inflammation, lipid accumulation, histone modification, and ‘end-stage’ kidney disease. Am. J. Physiol. Renal Physiol. 301, F1334–F1345 (2011).
Zhang, X., Agborbesong, E. & Li, X. The role of mitochondria in acute kidney injury and chronic kidney disease and its therapeutic potential. Int. J. Mol. Sci. 22, 11253 (2021).
Todorović, Z. et al. Lipidomics provides new insight into pathogenesis and therapeutic targets of the ischemia–reperfusion injury. Int. J. Mol. Sci. 22, 2798 (2021).
Ke, Q. et al. UCP2-induced hypoxia promotes lipid accumulation and tubulointerstitial fibrosis during ischemic kidney injury. Cell Death Dis. 11, 26 (2020).
Sheng, L. & Zhuang, S. New insights into the role and mechanism of partial epithelial-mesenchymal transition in kidney fibrosis. Front. Physiol. 11, 1190 (2020).
Chang-Panesso, M. et al. FOXM1 drives proximal tubule proliferation during repair from acute ischemic kidney injury. J. Clin. Invest. 129, 5501–5517 (2019).
Lemos, D. R. et al. Interleukin-1 β activates a MYC-dependent metabolic switch in kidney stromal cells necessary for progressive tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 29, 1690–1705 (2018).
Louis, K. & Hertig, A. How tubular epithelial cells dictate the rate of renal fibrogenesis? World J. Nephrol. 4, 367 (2015).
Dhillon, P. et al. The nuclear receptor ESRRA protects from kidney disease by coupling metabolism and differentiation. Cell Metab. 33, 379–394.e8 (2021).
Zhang, G., Darshi, M. & Sharma, K. The Warburg effect in diabetic kidney disease. Semin. Nephrol. 38, 111–120 (2018).
Houtkooper, R. H. et al. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature 497, 451–457 (2013).
Declèves, A. E. et al. Regulation of lipid accumulation by AMK-activated kinase in high fat diet-induced kidney injury. Kidney Int. 85, 611–623 (2014).
Shackelford, D. B. & Shaw, R. J. The LKB1–AMPK pathway: metabolism and growth control in tumour suppression. Nat. Rev. Cancer 9, 563–575 (2009).
Han, S. H. et al. Deletion of Lkb1 in renal tubular epithelial cells leads to CKD by altering metabolism. J. Am. Soc. Nephrol. 27, 439–453 (2016).
Chevalier, R. L. Molecular and cellular pathophysiology of obstructive nephropathy. Pediatr. Nephrol. 13, 612–619 (1999).
Yan, Q. et al. Autophagy activation contributes to lipid accumulation in tubular epithelial cells during kidney fibrosis. Cell Death Discov. 4, 2 (2018).
Herman-Edelstein, M., Scherzer, P., Tobar, A., Levi, M. & Gafter, U. Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J. Lipid Res. 55, 561 (2014).
Khan, S. et al. Fatty acid transport protein-2 regulates glycemic control and diabetic kidney disease progression. JCI Insight 5, e136845 (2020).
Rampanelli, E. et al. Excessive dietary lipid intake provokes an acquired form of lysosomal lipid storage disease in the kidney. J. Pathol. 246, 470–484 (2018).
Prasad, G. V. R. Metabolic syndrome and chronic kidney disease: current status and future directions. World J. Nephrol. 3, 210 (2014).
Ohshima, T. et al. α-Galactosidase A deficient mice: a model of Fabry disease. Proc. Natl Acad. Sci. USA 94, 2540–2544 (1997).
Schmitz, G. & Muller, G. Structure and function of lamellar bodies, lipid-protein complexes involved in storage and secretion of cellular lipids. J. Lipid Res. 32, 1539–1570 (1991).
Platt, F. M., Boland, B. & van der Spoel, A. C. Lysosomal storage disorders: the cellular impact of lysosomal dysfunction. J. Cell Biol. 199, 723–734 (2012).
Takabatake, Y., Yamamoto, T. & Isaka, Y. Stagnation of autophagy: a novel mechanism of renal lipotoxicity. Autophagy 13, 775–776 (2017).
Nakamura, S. et al. LC3 lipidation is essential for TFEB activation during the lysosomal damage response to kidney injury. Nat. Cell Biol. 22, 1252–1263 (2020).
Spampanato, C. et al. Transcription factor EB (TFEB) is a new therapeutic target for Pompe disease. EMBO Mol. Med. 5, 691 (2013).
Chen, L. et al. Fasting-induced hormonal regulation of lysosomal function. Cell Res. 27, 748–763 (2017).
Settembre, C. & Ballabio, A. Lysosome: regulator of lipid degradation pathways. Trends Cell Biol. 24, 743–750 (2014).
Yamamoto, T. et al. High-fat diet-induced lysosomal dysfunction and impaired autophagic flux contribute to lipotoxicity in the kidney. J. Am. Soc. Nephrol. 28, 1534–1551 (2017).
Piccinini, A. M. & Midwood, K. S. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010, 153 (2010).
Leemans, J. C., Kors, L., Anders, H. J. & Florquin, S. Pattern recognition receptors and the inflammasome in kidney disease. Nat. Rev. Nephrol. 10, 398–414 (2014).
Triantafilou, M., Miyake, K., Golenbock, D. T. & Triantafilou, K. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J. Cell Sci. 115, 2603–2611 (2002).
O’Neill, L. A. J. When signaling pathways collide: positive and negative regulation of toll-like receptor signal transduction. Immunity 29, 12–20 (2008).
Leemans, J. C. et al. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J. Clin. Invest. 115, 2894–2903 (2005).
Wu, H. et al. TLR4 activation mediates kidney ischemia/reperfusion injury. J. Clin. Invest. 117, 2847–2859 (2007).
Pulskens, W. P. et al. Toll-like receptor-4 coordinates the innate immune response of the kidney to renal ischemia/reperfusion injury. PLoS ONE 3, e3596 (2008).
Reilly, M. et al. Randomized, double-blind, placebo-controlled, dose-escalating phase I, healthy subjects study of intravenous OPN-305, a humanized anti-TLR2 antibody. Clin. Pharmacol. Ther. 94, 593–600 (2013).
Kulkarni, O. P. et al. Toll-like receptor 4-induced IL-22 accelerates kidney regeneration. J. Am. Soc. Nephrol. 25, 978–989 (2014).
Pulskens, W. P. et al. TLR4 promotes fibrosis but attenuates tubular damage in progressive renal injury. J. Am. Soc. Nephrol. 21, 1299–1308 (2010).
Leemans, J. C. et al. The role of toll-like receptor 2 in inflammation and fibrosis during progressive renal injury. PLoS ONE 4, e5704 (2009).
Everts, B. et al. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKɛ supports the anabolic demands of dendritic cell activation. Nat. Immunol. 15, 323–332 (2014).
Kelly, B. & O’Neill, L. A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 25, 771–784 (2015).
Krawczyk, C. M. et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood 115, 4742–4749 (2010).
Feingold, K. R., Wang, Y., Moser, A., Shigenaga, J. K. & Grunfeld, C. LPS decreases fatty acid oxidation and nuclear hormone receptors in the kidney. J. Lipid Res. 49, 2179 (2008).
Küper, C., Beck, F.-X. & Neuhofer, W. Toll-like receptor 4 activates NF-κB and MAP kinase pathways to regulate expression of proinflammatory COX-2 in renal medullary collecting duct cells. Am. J. Physiol. Renal Physiol. 302, 38–46 (2012).
Brooks Robey, R. et al. Regulation of mesangial cell hexokinase activity and expression by heparin-binding epidermal growth factor-like growth factor: epidermal growth factors and phorbol esters increase glucose metabolism via a common mechanism involving classic mitogen-activated protein kinase pathway activation and induction of hexokinase II expression. J. Biol. Chem. 277, 14370–14378 (2002).
Kim, S. Y. et al. Hypoxic stress up-regulates the expression of Toll-like receptor 4 in macrophages via hypoxia-inducible factor. Immunology 129, 516 (2010).
Semenza, G. L. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology 24, 97–106 (2009).
Conde, E. et al. Hypoxia inducible factor 1-alpha (HIF-1 alpha) is induced during reperfusion after renal ischemia and is critical for proximal tubule cell survival. PLoS ONE 7, e33258 (2012).
West, X. Z. et al. Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands. Nature 467, 972 (2010).
Binder, C. J., Papac-Milicevic, N. & Witztum, J. L. Innate sensing of oxidation-specific epitopes in health and disease. Nat. Rev. Immunol. 16, 485–497 (2016).
Gounarides, J. et al. Lack of involvement of CEP adducts in TLR activation and in angiogenesis. PLoS ONE 9, e111472 (2014).
Lin, M. et al. Toll-like receptor 4 promotes tubular inflammation in diabetic nephropathy. J. Am. Soc. Nephrol. 23, 86–102 (2012).
Lin, M. et al. The TLR4 antagonist CRX-526 protects against advanced diabetic nephropathy. Kidney Int. 83, 887–900 (2013).
Devaraj, S. et al. Knockout of Toll-like receptor-2 attenuates both the proinflammatory state of diabetes and incipient diabetic nephropathy. Arterioscler. Thromb. Vasc. Biol. 31, 1796–1804 (2011).
Jheng, H. F. et al. Albumin stimulates renal tubular inflammation through an HSP70-TLR4 axis in mice with early diabetic nephropathy. Dis. Model. Mech. 8, 1311–1321 (2015).
Cha, J. J. et al. Renal protective effects of toll-like receptor 4 signaling blockade in type 2 diabetic mice. Endocrinology 154, 2144–2155 (2013).
Shi, H. et al. TLR4 links innate immunity and fatty acid–induced insulin resistance. J. Clin. Invest. 116, 3015–3025 (2006).
Pierre, N. et al. Toll-like receptor 4 knockout mice are protected against endoplasmic reticulum stress induced by a high-fat diet. PLoS ONE 8, e65061 (2013).
Min, H. S. et al. Effects of toll-like receptor antagonist 4,5-dihydro-3-phenyl-5-isoxasole acetic acid on the progression of kidney disease in mice on a high-fat diet. Kidney Res. Clin. Pract. 33, 33–44 (2014).
Kuwabara, T. et al. Exacerbation of diabetic nephropathy by hyperlipidaemia is mediated by toll-like receptor 4 in mice. Diabetologia 55, 2256–2266 (2012).
Xu, X. H. et al. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation 104, 3103–3108 (2001).
Rutledge, J. C., Ng, K. F., Aung, H. H. & Wilson, D. W. Role of triglyceride-rich lipoproteins in diabetic nephropathy. Nat. Rev. Nephrol. 6, 361–370 (2010).
Leemans, J. C., Cassel, S. L. & Sutterwala, F. S. Sensing damage by the NLRP3 inflammasome. Immunol. Rev. 243, 152–162 (2011).
Muñoz-Planillo, R. et al. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38, 1142–1153 (2013).
Iyer, S. S. et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc. Natl Acad. Sci. USA 106, 20388–20393 (2009).
Vilaysane, A. et al. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J. Am. Soc. Nephrol. 21, 1732–1744 (2010).
Pulskens, W. P. et al. Nlrp3 prevents early renal interstitial edema and vascular permeability in unilateral ureteral obstruction. PLoS ONE 9, e85775 (2014).
Kim, H. J. et al. NLRP3 inflammasome knockout mice are protected against ischemic but not cisplatin-induced acute kidney injury. J. Pharmacol. Exp. Ther. 346, 465–472 (2013).
Bakker, P. J. et al. A tissue-specific role for Nlrp3 in tubular epithelial repair after renal ischemia/reperfusion. Am. J. Pathol. 184, 2013–2022 (2014).
Qiu, Y. Y. & Tang, L. Q. Roles of the NLRP3 inflammasome in the pathogenesis of diabetic nephropathy. Pharmacol. Res. 114, 251–264 (2016).
Hou, Y. et al. CD36 promotes NLRP3 inflammasome activation via the mtROS pathway in renal tubular epithelial cells of diabetic kidneys. Cell Death Dis. 126, 1–16 (2021).
Lu, M. et al. Curcumin ameliorates diabetic nephropathy by suppressing NLRP3 inflammasome signaling. Biomed. Res. Int. 2017, 1516985 (2017).
Sun, Z. et al. Artesunate ameliorates high glucose-induced rat glomerular mesangial cell injury by suppressing the TLR4/NF-κB/NLRP3 inflammasome pathway. Chem. Biol. Interact. 293, 11–19 (2018).
Han, Y. et al. Reactive oxygen species promote tubular injury in diabetic nephropathy: the role of the mitochondrial ros-txnip-nlrp3 biological axis. Redox Biol. 16, 32–46 (2018).
Próchnicki, T. & Latz, E. Inflammasomes on the crossroads of innate immune recognition and metabolic control. Cell Metab. 26, 71–93 (2017).
Braga, T. T. et al. Soluble uric acid activates the NLRP3 inflammasome. Sci. Rep. 71, 1–14 (2017).
Rajamäki, K. et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS ONE 5, e11765 (2010).
Shi, G. et al. Inflammasomes induced by 7-ketocholesterol and other stimuli in RPE and in bone marrow–derived cells differ markedly in their production of IL-1β and IL-18. Invest. Ophthalmol. Vis. Sci. 56, 1658 (2015).
Chalkiadaki, A. & Guarente, L. High-fat diet triggers inflammation-induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell Metab. 16, 180–188 (2012).
Calle, P., Torrico, S., Muñoz, A. & Hotter, G. CPT1a downregulation protects against cholesterol-induced fibrosis in tubular epithelial cells by downregulating TGFβ-1 and inflammasome. Biochem. Biophys. Res. Commun. 517, 715–721 (2019).
Xia, X. et al. NLRX1 negatively regulates TLR-induced NF-κB signaling by targeting TRAF6 and IKK. Immunity 34, 843–853 (2011).
Guo, H. et al. NLRX1 sequesters STING to negatively regulate the interferon response, thereby facilitating the replication of HIV-1 and DNA viruses. Cell Host Microbe 19, 515–528 (2016).
Moore, C. B. et al. NLRX1 is a regulator of mitochondrial antiviral immunity. Nature 451, 573–577 (2008).
Lei, Y. et al. The mitochondrial proteins NLRX1 and TUFM form a complex that regulates type I interferon and autophagy. Immunity 36, 933–946 (2012).
Arnoult, D. et al. An N-terminal addressing sequence targets NLRX1 to the mitochondrial matrix. J. Cell Sci. 122, 3161 (2009).
Imbeault, E., Mahvelati, T. M., Braun, R., Gris, P. & Gris, D. Nlrx1 regulates neuronal cell death. Mol. Brain 7, 90 (2014).
Jaworska, J. et al. NLRX1 prevents mitochondrial induced apoptosis and enhances macrophage antiviral immunity by interacting with influenza virus PB1-F2 protein. Proc. Natl Acad. Sci. USA 111, E2110–E2119 (2014).
Koblansky, A. A. et al. The innate immune receptor NLRX1 functions as a tumor suppressor by reducing colon tumorigenesis and key tumor-promoting signals. Cell Rep. 14, 2562–2575 (2016).
Tattoli, I. et al. NLRX1 acts as an epithelial-intrinsic tumor suppressor through the modulation of TNF-mediated proliferation. Cell Rep. 14, 2576–2586 (2016).
Soares, F. et al. The mitochondrial protein NLRX1 controls the balance between extrinsic and intrinsic apoptosis. J. Biol. Chem. 289, 19317–19330 (2014).
Kang, M.-J. et al. Suppression of NLRX1 in chronic obstructive pulmonary disease. J. Clin. Invest. 125, 2458–2462 (2015).
Li, H., Zhang, S., Li, F. & Qin, L. NLRX1 attenuates apoptosis and inflammatory responses in myocardial ischemia by inhibiting MAVS-dependent NLRP3 inflammasome activation. Mol. Immunol. 76, 90–97 (2016).
Hong, M., Yoon, Sil & Wilson, I. A. Structure and functional characterization of the RNA-binding element of the NLRX1 innate immune modulator. Immunity 36, 337–347 (2012).
Lu, P. et al. Modeling-enabled characterization of novel NLRX1 ligands. PLoS ONE 10, e0145420 (2015).
Singh, K. et al. NLRX1 acts as tumor suppressor by regulating TNF-α induced apoptosis and metabolism in cancer cells. Biochim. Biophys. Acta 1853, 1073–1086 (2015).
Leber, A. et al. NLRX1 regulates effector and metabolic functions of CD4+ T cells. J. Immunol. 198, 2260–2268 (2017).
Kors, L. et al. Deletion of NLRX1 increases fatty acid metabolism and prevents diet-induced hepatic steatosis and metabolic syndrome. Biochim. Biophys. Acta Mol. Basis Dis. 1864, 1883–1895 (2018).
Costford, S. R. et al. Male mice lacking NLRX1 are partially protected from high-fat diet–induced hyperglycemia. J. Endocr. Soc. 2, 336 (2018).
Terao, Y. et al. Phospholipase A2 is activated in the kidney, but not in the liver during ischemia-reperfusion. Res. Commun. Mol. Pathol. Pharmacol. 96, 277–289 (1997).
Vallés, P. G., Lorenzo, A. G., Bocanegra, V. & Vallés, R. Acute kidney injury: what part do toll-like receptors play? Int. J. Nephrol. Renovasc. Dis. 7, 241 (2014).
Smith, R. L., Soeters, M. R., Wüst, R. C. I. & Houtkooper, R. H. Metabolic flexibility as an adaptation to energy resources and requirements in health and disease. Endocr. Rev. 39, 489–517 (2018).
Andreux, P. A., Houtkooper, R. H. & Auwerx, J. Pharmacological approaches to restore mitochondrial function. Nat. Rev. Drug Discov. 12, 465–483 (2013).
Kang, H. M. et al. Defective fatty acid oxidation in renal tubular epithelial cells plays a key role in kidney fibrosis development. Nat. Med. 21, 37 (2015).
Sivarajah, A. et al. Agonists of peroxisome-proliferator activated receptor-gamma reduce renal ischemia/reperfusion injury. Am. J. Nephrol. 23, 267–276 (2003).
Zapata-P Erez, R., Wanders, R. J. A., Van Karnebeek, C. D. M. & Houtkooper, R. H. NAD+ homeostasis in human health and disease. EMBO Mol. Med. 13, e13943 (2021).
Zheng, M. et al. Nicotinamide reduces renal interstitial fibrosis by suppressing tubular injury and inflammation. J. Cell. Mol. Med. 23, 3995–4004 (2019).
Zapata-Pérez, R. et al. Reduced nicotinamide mononucleotide is a new and potent NAD+ precursor in mammalian cells and mice. FASEB J. 35, 1–17 (2021).
Giroud-Gerbetant, J. et al. A reduced form of nicotinamide riboside defines a new path for NAD+ biosynthesis and acts as an orally bioavailable NAD+ precursor. Mol. Metab. 30, 192–202 (2019).
Lee, S. Y. et al. PGC1 α activators mitigate diabetic tubulopathy by improving mitochondrial dynamics and quality control. J. Diabetes Res. 2017, 6483572 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03831464 (2022).
Howitz, K. T. et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425, 191–196 (2003).
Kitada, M. & Koya, D. Renal protective effects of resveratrol. Oxid. Med. Cell. Longev. 2013, 568093 (2013).
Andreux, P. A. et al. The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nat. Metab. 1, 595–603 (2019).
Zou, D. et al. Oral delivery of nanoparticle urolithin A normalizes cellular stress and improves survival in mouse model of cisplatin-induced AKI. Am. J. Physiol. Renal Physiol. 317, F1255–F1264 (2019).
Faivre, A., Verissimo, T., Auwerx, H., Legouis, D. & de Seigneux, S. Tubular cell glucose metabolism shift during acute and chronic injuries. Front. Med. 8, 2085 (2021).
Shirakawa, K. & Sano, M. Sodium-glucose co-transporter 2 inhibitors correct metabolic maladaptation of proximal tubular epithelial cells in high-glucose conditions. Int. J. Mol. Sci. 21, 7676 (2020).
Liu, X. et al. Empagliflozin improves diabetic renal tubular injury by alleviating mitochondrial fission via AMPK/SP1/PGAM5 pathway. Metabolism 111, 154334 (2020).
Bessho, R. et al. Hypoxia-inducible factor-1α is the therapeutic target of the SGLT2 inhibitor for diabetic nephropathy. Sci. Rep. 9, 1–12 (2019).
Farhadi, P., Yarani, R., Dokaneheifard, S. & Mansouri, K. The emerging role of targeting cancer metabolism for cancer therapy. Tumor Biol. 42, 1–18 (2020).
Festa, B. P. et al. Impaired autophagy bridges lysosomal storage disease and epithelial dysfunction in the kidney. Nat. Commun. 9, 1–17 (2018).
Zhang, W., Li, X., Wang, S., Chen, Y. & Liu, H. Regulation of TFEB activity and its potential as a therapeutic target against kidney diseases. Cell Death Discov. 6, 32 (2020).
Irazoqui, J. E. Key roles of MiT transcription factors in innate immunity and inflammation. Trends Immunol. 41, 157–171 (2020).
Author information
Authors and Affiliations
Contributions
All authors contributed substantially to discussion of the content, writing of the article, and reviewing and/or editing of the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks Hans Joachim Anders, Niels Olsen Saraiva Câmara and Theodoros Eleftheriadis for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Lipids
-
A ubiquitous group of compounds that are insoluble in water and essential for energy metabolism, cell membrane integrity, cell signalling and general homeostasis.
- Complex lipids
-
Unlike simple lipids, which contain a maximum of two types of chemical moieties (e.g. fatty acids and glycerol), complex lipids contain three or more chemical moieties (e.g. fatty acids, glycerol and a phosphate group).
- Long-chain fatty acids
-
Fatty acids containing more than 12 carbon atoms and are an important energy source in the kidney.
- Pathogen-associated molecular patterns
-
(PAMPs). Molecules that are released upon pathogen infection and activate the pattern recognition receptors of the innate immune response.
- Damage-associated molecular patterns
-
(DAMPs). Molecules that are released upon damage and activate the pattern recognition receptors of the innate immune response.
- Trained immunity
-
The concept that, like the adaptive immune system, the innate immune response can also have an immune memory.
- Lipotoxicity
-
The accumulation of lipids in tissues that is harmful for the function and structure of the cells. For instance, it can reduce energy metabolism.
- Perilipin 2
-
A protein that is important for lipid droplet metabolism and is involved in the storage of lipids.
- Mitochondrial antiviral-signalling proteins
-
Proteins that are located in the inner membrane of the mitochondria, peroxisomes and endoplasmic reticulum that are essential for the antiviral innate immune response.
- Translation–elongation factors
-
Proteins that have important roles during protein synthesis, especially in the elongation cycle at the ribosome.
- Dynamin-1-like protein
-
A protein involved in mitochondrial fission, which is important for mitochondrial function.
Rights and permissions
About this article
Cite this article
van der Rijt, S., Leemans, J.C., Florquin, S. et al. Immunometabolic rewiring of tubular epithelial cells in kidney disease. Nat Rev Nephrol 18, 588–603 (2022). https://doi.org/10.1038/s41581-022-00592-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-022-00592-x
This article is cited by
-
Acetyl-CoA synthetase 2 promotes diabetic renal tubular injury in mice by rewiring fatty acid metabolism through SIRT1/ChREBP pathway
Acta Pharmacologica Sinica (2024)
-
Mitochondrial oxidative damage reprograms lipid metabolism of renal tubular epithelial cells in the diabetic kidney
Cellular and Molecular Life Sciences (2024)
-
N6-methyladenosine methylation in kidney injury
Clinical Epigenetics (2023)
-
Inhibition of PFKP in renal tubular epithelial cell restrains TGF-β induced glycolysis and renal fibrosis
Cell Death & Disease (2023)
-
Palmitoyltransferase DHHC9 and acyl protein thioesterase APT1 modulate renal fibrosis through regulating β-catenin palmitoylation
Nature Communications (2023)