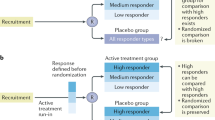
Abstract
Patient involvement in clinical trial design can facilitate the recruitment and retention of participants as well as potentially increase the uptake of the tested intervention and the impact of the findings on patient outcomes. Despite these benefits, patients still have very limited involvement in designing and conducting trials in nephrology. Many trials do not address research questions and outcomes that are important to patients, including patient-reported outcomes that reflect how patients feel and function. This limitation can undermine the relevance, reliability and value of trial-based evidence for decision-making in clinical practice and health policy. However, efforts to involve patients with kidney disease are increasing across all stages of the trial process from priority setting, to study design (including selection of outcomes and approaches to improve participant recruitment and retention) and dissemination and implementation of the findings. Harnessing the patient voice in designing trials can ensure that efforts and resources are directed towards patient-centred trials that address the needs, concerns and priorities of patients living with kidney disease with the aim of achieving transformative improvements in care and outcomes.
Key points
-
Patients have limited involvement in designing and conducting trials and are not routinely given positions of agency and power in their organizational and governance structure.
-
The involvement of patients in the design of clinical trials can help to improve participant recruitment and retention and increase the uptake and impact of the findings.
-
Patients can be involved in research priority setting, study design (including participant recruitment and retention and selection of outcomes, including patient-reported outcomes), as well as the dissemination and implementation of trial findings.
-
Resources need to be allocated to provide the administrative, educational, financial and logistical support required to support the meaningful involvement of patients across all stages of the trial.
-
Patient-centred trials that are designed and conducted with patient involvement can provide the evidence needed for patients to make informed decisions that reflect their desired health outcomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Guyatt, G. H. et al. Users’ Guides to the Medical Literature: XXV. Evidence-based medicine: principles for applying the Users’ Guides to patient care. Evidence-based Medicine Working Group. JAMA 284, 1290–1296 (2000).
Banerjee, D. et al. International perspectives on patient involvement in clinical trials in nephrology. Kidney Int. 98, 566–571 (2020).
Cochrane Collaboration. Cochrane Kidney and Transplant Register of Studies. Cochrane http://kidneyandtransplant.cochrane.org/cochrane-kidney-and-transplant-specialised-register (2018).
Tallon, D., Chard, J. & Dieppe, P. Relation between agendas of the research community and the research consumer. Lancet 355, 2037–2040 (2000).
Wicks, P., Richards, T., Denegri, S. & Godlee, F. Patients’ roles and rights in research. BMJ 362, k3193 (2018).
Ju, A. et al. Patient-led identification and prioritization of exercise interventions for fatigue on dialysis: a workshop report. Clin. Kidney J. 14, 831–839 (2020).
Chong, L. S. H. et al. Range and heterogeneity of outcomes in randomized trials of pediatric chronic kidney disease. J. Pediatr. 186, 110–117 (2017).
Sautenet, B. et al. Range and variability of outcomes reported in randomized trials conducted in patients with polycystic kidney disease: a systematic review. Am. J. Kidney Dis. 76, 213–223 (2020).
Sautenet, B. et al. Range and consistency of outcomes reported in randomized trials conducted in kidney transplant recipients: a systematic review. transplantation. Transplantation 102, 2065–2071 (2018).
Sautenet, B. et al. Scope and consistency of outcomes reported in randomized trials conducted in adults receiving hemodialysis: a systematic review. Am. J. Kidney Dis. 72, 62–74 (2018).
National Institute for Health and Care Research. UK Standards for Public Involvement (NIHR, 2019).
South, A. et al. Models and impact of patient and public involvement in studies carried out by the Medical Research Council Clinical Trials Unit at University College London: findings from ten case studies. Trials 17, 376 (2016).
Frank, L., Basch, E. & Selby, J. V. The PCORI perspective on patient-centered outcomes research. JAMA 312, 1513–1514 (2014).
PCORI. PCORI Methodology Standards. (Patient-Centered Outcomes Research Institute, Washington DC, US, 2018).
James Lind Alliance. The James Lind Alliance guidebook version 10 (JLA, 2018).
Williamson, P. R. et al. The COMET Handbook: version 1.0. Trials 18, 280 (2017).
Calvert, M. et al. Guidelines for inclusion of patient-reported outcomes in clinical trial protocols: the SPIRIT-PRO extension. JAMA 319, 483–494 (2018).
Calvert, M. et al. SPIRIT-PRO Extension explanation and elaboration: guidelines for inclusion of patient-reported outcomes in protocols of clinical trials. BMJ Open 11, 2045105 (2021).
Cruz Rivera, S. et al. ‘Give Us The Tools!’: development of knowledge transfer tools to support the involvement of patient partners in the development of clinical trial protocols with patient-reported outcomes (PROs), in accordance with SPIRIT-PRO Extension. BMJ Open 11, e046450 (2021).
Turner, G., Aiyegbusi, O. L., Price, G., Skrybant, M. & Calvert, M. Moving beyond project-specific patient and public involvement in research. J. R. Soc. Med. 113, 16–23 (2020).
Murdoch, A. et al. Re-envisioning the Canadian Nephrology Trials Network: a Can-SOLVE-CKD stakeholder meeting of patient partners and researchers. Can. J. Kidney Health Dis. 8, 20543581211030396 (2021).
Kidney Research UK. UK Renal Trials Network. Kidney Research UK https://kidneyresearchuk.org/research/research-networks/uk-renal-trials-network/ (2021).
Baigent, C. et al. Challenges in conducting clinical trials in nephrology: conclusions from a Kidney Disease-Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2017, 297–305 (2017).
Jun, M. et al. Assessing the extent to which current clinical research is consistent with patient priorities: a scoping review using a case study in patients on or nearing dialysis. Can. J. Kidney Health Dis. 2, 35 (2015).
Manns, B. et al. Setting research priorities for patients on or nearing dialysis. Clin. J. Am. Soc. Nephrol. 9, 1813–1821 (2014).
Tong, A. et al. Research priorities in CKD: report of a national workshop conducted in Australia. Am. J. Kidney Dis. 66, 212–222 (2015).
Mc Laughlin, L., Spence, S. & Noyes, J. Identifying integrated health services and social care research priorities in kidney disease in Wales: research prioritisation exercise. BMJ Open 10, e036872 (2020).
Schipper, K. & Abma, T. A. Coping, family and mastery: top priorities for social science research by patients with chronic kidney disease. Nephrol. Dial. Transpl. 26, 3189–3195 (2011).
Knight, S. R. et al. Defining priorities for future research: results of the UK kidney transplant priority setting partnership. PLoS ONE 11, e0162136 (2016).
Tong, A. et al. Reporting guideline for priority setting of health research (REPRISE). BMC Med. Res. Methodol. 19, 243 (2019).
Browne, T. et al. Engaging patients and family members to design and implement patient-centered kidney disease research. Res. Involv. Engagem. 6, 66 (2020).
Moore, G. F. et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 350, h1258 (2015).
Stevenson, J. et al. Targeted, structured text messaging to improve dietary and lifestyle behaviours for people on maintenance haemodialysis (KIDNEYTEXT): study protocol for a randomised controlled trial. BMJ Open 9, e023545 (2019).
Guha, C. et al. Patient needs and priorities for patient navigator programmes in chronic kidney disease: a workshop report. BMJ Open 10, e040617 (2020).
Ameling, J. M. et al. Development of a decision aid to inform patients’ and families’ renal replacement therapy selection decisions. BMC Med. Inf. Decis. Mak. 12, 140 (2012).
Hedayati, S. S. et al. Rationale and design of a trial of sertraline vs. cognitive behavioral therapy for end-stage renal disease patients with depression (ASCEND). Contemp. Clin. Trials 47, 1–11 (2016).
Singh, J. A. et al. Individualized decision aid for diverse women with lupus nephritis (IDEA-WON): a randomized controlled trial. PLoS Med. 16, e1002800 (2019).
Singh, J. A. et al. Barriers to medication decision making in women with lupus nephritis: a formative study using nominal group technique. J. Rheumatol. 42, 1616–1623 (2015).
Singh, J. A., Qu, H., Yazdany, J., Chatham, W. & Shewchuk, R. Minorities with lupus nephritis and medications: a study of facilitators to medication decision-making. Arthritis Res. Ther. 17, 367 (2015).
Brunsdon, D. et al. What are the most important unanswered research questions in trial retention? A James Lind Alliance Priority Setting Partnership: the PRioRiTy II (Prioritising Retention in Randomised Trials) study. Trials 20, 593 (2019).
Natale, P. et al. Transparency, trust and minimizing burden to increase recruitment and retention in trials: a systematic review. J. Clin. Epidemiol. 134, 35–51 (2021).
LaPlante, A. et al. Enrollment, retention, and strategies for including disadvantaged populations in randomized controlled trials: a systematic review protocol. Syst. Rev. 10, 233 (2021).
Rogers, W. A. Evidence based medicine and justice: a framework for looking at the impact of EBM upon vulnerable or disadvantaged groups. J. Med. Ethics 30, 141–145 (2004).
Heiat, A., Gross, C. P. & Krumholz, H. M. Representation of the elderly, women, and minorities in heart failure clinical trials. Arch. Intern. Med. 162, 1682–1688 (2002).
Crocker, J. C. et al. Impact of patient and public involvement on enrolment and retention in clinical trials: systematic review and meta-analysis. BMJ 363, k4738 (2018).
Hurst, F. P. et al. Stimulating patient engagement in medical device development in kidney disease: a report of a kidney health initiative workshop. Am. J. Kidney Dis. 70, 561–569 (2017).
Cukor, D. et al. Patient and other stakeholder engagement in patient-centered outcomes research institute funded studies of patients with kidney diseases. Clin. J. Am. Soc. Nephrol. 11, 1703–1712 (2016).
Ishida, J. H. et al. Understanding and overcoming the challenges related to cardiovascular trials involving patients with kidney disease. Clin. J. Am. Soc. Nephrol. 16, 1435–1444 (2021).
Natale, P. et al. Recruitment and retention in clinical trials in chronic kidney disease: report from national workshops with patients, caregivers and health professionals. Nephrol. Dial. Transpl. 35, 755–764 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02481206 (2021).
Byrne, J., Khunti, K., Stone, M., Farooqi, A. & Carr, S. Feasibility of a structured group education session to improve self-management of blood pressure in people with chronic kidney disease: an open randomised pilot trial. BMJ Open 1, e000381 (2011).
Hemkens, L. G. How routinely collected data for randomized trials provide long-term randomized real-world evidence. JAMA Netw. Open 1, e186014 (2018).
Witham, M. D. et al. Developing a roadmap to improve trial delivery for under-served groups: results from a UK multi-stakeholder process. Trials 21, 694 (2020).
UyBico, S. J., Pavel, S. & Gross, C. P. Recruiting vulnerable populations into research: a systematic review of recruitment interventions. J. Gen. Intern. Med. 22, 852–863 (2007).
Joseph, P. D., Caldwell, P. H., Tong, A., Hanson, C. S. & Craig, J. C. Stakeholder views of clinical trials in low and middle income countries: a systematic review. Pediatrics 137, e20152800 (2016).
Welch, V. A. et al. CONSORT-Equity 2017 extension and elaboration for better reporting of health equity in randomised trials. BMJ 359, j5085 (2017).
Cochrane Collaboration. Cochrane Methods Equity: PROGRESS-Plus. Cochrane https://methods.cochrane.org/equity/projects/evidence-equity/progress-plus (2022).
Yudkin, J. S., Lipska, K. J. & Montori, V. M. The idolatry of the surrogate. BMJ 343, d7995 (2011).
Gandhi, G. Y. et al. Patient-important outcomes in registered diabetes trials. JAMA 299, 2543–2549 (2008).
Ioannidis, J. P. et al. Increasing value and reducing waste in research design, conduct, and analysis. Lancet 383, 166–175 (2014).
Nair, D. & Wilson, F. P. Patient-reported outcome measures for adults with kidney disease: current measures, ongoing initiatives, and future opportunities for incorporation into patient-centered kidney care. Am. J. Kidney Dis. 76, 791–802 (2019).
US Food and Drug Administration. Clinical outcome assessment (COA): glossary of terms (FDA, 2021).
Byrne, M. et al. A core outcomes set for clinical trials of interventions for young adults with type 1 diabetes: an international, multi-perspective Delphi consensus study. Trials 18, 602 (2017).
van Rijssen, L. B. et al. Core Set of Patient-Reported Outcomes in Pancreatic Cancer (COPRAC): an international Delphi study among patients and health care providers. Ann. Surg. 270, 158–164 (2019).
Maxwell, L. J. et al. Core domain set selection according to OMERACT filter 2.1: the OMERACT methodology. J. Rheumatol. 46, 1014–1020 (2019).
Himmelfarb, J., Vanholder, R., Mehrotra, R. & Tonelli, M. The current and future landscape of dialysis. Nat. Rev. Nephrol. 16, 573–585 (2020).
Tong, A. et al. Implementing core outcomes in kidney disease: report of the Standardized Outcomes in Nephrology (SONG) implementation workshop. Kidney Int. 94, 1053–1068 (2018).
Manera, K. E. et al. Establishing a core outcome set for peritoneal dialysis: report of the SONG-PD (Standardized Outcomes in Nephrology-Peritoneal Dialysis) consensus workshop. Am. J. Kidney Dis. 75, 404–412 (2020).
Hanson, C. S. et al. Establishing core outcome domains in pediatric kidney disease: report of the Standardized Outcomes in Nephrology-Children and Adolescents (SONG-KIDS) Consensus Workshops. Kidney Int. 98, 553–565 (2020).
Cho, Y. et al. Establishing a core outcome set for autosomal dominant polycystic kidney disease: report of the standardized outcomes in nephrology-polycystic kidney disease (SONG-PKD) Consensus Workshop. Am. J. Kidney Dis. 77, 255–263 (2021).
Ju, A. et al. Validation of a core patient-reported outcome measure for fatigue in patients receiving hemodialysis. Clin. J. Am. Soc. Nephrol. 15, 1614–1621 (2020).
Ju, A. et al. Establishing a core outcome measure for fatigue in patients on hemodialysis: a standardized outcomes in nephrology-hemodialysis (SONG-HD) Consensus Workshop Report. Am. J. Kidney Dis. 72, 104–112 (2018).
Tong, A. et al. Toward establishing core outcome domains for trials in kidney transplantation: report of the standardized outcomes in nephrology-kidney transplantation consensus workshops. Transplantation 101, 1887–1896 (2017).
Selewski, D. T. et al. Patient-reported outcomes in glomerular disease. Clin. J. Am. Soc. Nephrol. 12, 140–148 (2017).
Flythe, J. E. et al. Toward patient-centered innovation: a conceptual framework for patient-reported outcome measures for transformative kidney replacement devices. Clin. J. Am. Soc. Nephrol. 15, 1522–1530 (2020).
Mokkink, L. B. et al. The COSMIN checklist for evaluating the methodological quality of studies on measurement properties: a clarification of its content. BMC Med. Res. Methodol. 10, 22 (2010).
Chong, K. & Unruh, M. Why does quality of life remain an under-investigated issue in chronic kidney disease and why is it rarely set as an outcome measure in trials in this population? Nephrol. Dial. Transpl. 32, ii47–ii52 (2017).
Ward, J. M., Getchell, L. & Garg, A. X. Patient and caregiver involvement in a multicentre clustered hemodialysis trial. Can. Med. Assoc. J. 190, S32–S33 (2018).
World Medical Association. Declaration of Helsinki: medical research involving human subjects. WMA http://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (2021).
Schroter, S., Price, A., Malički, M., Richards, T. & Clarke, M. Frequency and format of clinical trial results dissemination to patients: a survey of authors of trials indexed in PubMed. BMJ Open 9, e032701 (2019).
Barnes, A. & Patrick, S. Lay summaries of clinical study results: an overview. Pharm. Med. 33, 261–268 (2019).
European Commission. Recommendations of the expert group on clinical trials for the implementation of Regulation (EU) No 536/2014 on clinical trials on medicinal products for human use (European Commission, 2021).
Multi-Regional Clinical Trials Center of Brigham and Women’s Hospital and Harvard. Return of aggregate results to participants principles (MRCT, 2021).
Roberts, S. et al. Process evaluation of a cluster-randomised trial testing a pressure ulcer prevention care bundle: a mixed-methods study. Implement. Sci. 12, 18 (2017).
Deverka, P. A. et al. A new framework for patient engagement in cancer clinical trials cooperative group studies. J. Natl Cancer Inst. 110, 553–559 (2018).
Milliner, D. S. et al. End points for clinical trials in primary hyperoxaluria. Clin. J. Am. Soc. Nephrol. 15, 1056–1065 (2020).
Gutman T. et al. Consumer involvement in published chronic kidney disease research: a systematic review. Clin. J. Am. Soc. Nephrol. (in the press).
Greenwood, K. et al. The impact of patient and public involvement in the SlowMo study: reflections on peer innovation. Health Expect. 25, 191–202 (2022).
Schilling, I. et al. Patient involvement in clinical trials: motivation and expectations differ between patients and researchers involved in a trial on urinary tract infections. Res. Involv. Engagem. 5, 15 (2019).
Duffett, L. Patient engagement: what partnering with patient in research is all about. Thromb. Res. 150, 113–120 (2017).
Linde, P. G. et al. Overcoming barriers in kidney health-forging a platform for innovation. J Am. Soc. Nephrol. 27, 1902–1910 (2016).
Boutin, M. et al. Culture and process change as a priority for patient engagement in medicines development. Ther. Innov. Regul. Sci. 51, 29–38 (2017).
Mader, L. B., Harris, T., Kläger, S., Wilkinson, I. B. & Hiemstra, T. F. Inverting the patient involvement paradigm: defining patient led research. Res. Involv. Engagem. 4, 21 (2018).
NHS Cambridge University Hospital. Cambridge patient led research hub. NHS https://www.cuh.nhs.uk/our-research/get-involved/patient-led-research/ (2022).
Staniszewska, S. et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ 358, j3453 (2017).
Yu, R., Hanley, B., Denegri, S., Ahmed, J. & McNally, N. J. Evaluation of a patient and public involvement training programme for researchers at a large biomedical research centre in the UK. BMJ Open 11, e047995 (2021).
Kelly, S. et al. Dementia priority setting partnership with the James Lind Alliance: using patient and public involvement and the evidence base to inform the research agenda. Age Ageing 44, 985–993 (2015).
Seeralan, T. et al. Patient involvement in developing a patient-targeted feedback intervention after depression screening in primary care within the randomized controlled trial GET.FEEDBACK.GP. Health Expect. 24, 95–112 (2021).
Ellis, J. et al. Considerations in developing and delivering a nonpharmacological intervention for symptom management in lung cancer: the views of patients and informal caregivers. J. Pain Symptom Manage. 44, 831–842 (2012).
Poleshuck, E. et al. Using patient engagement in the design and rationale of a trial for women with depression in obstetrics and gynecology practices. Contemp. Clin. Trials 43, 83–92 (2015).
Maloney, C. et al. Patient perspectives on participation in the ENABLE II randomized controlled trial of a concurrent oncology palliative care intervention: benefits and burdens. Palliat. Med. 27, 375–383 (2013).
Lovell, N., Etkind, S. N., Bajwah, S., Maddocks, M. & Higginson, I. J. What influenced people with chronic or refractory breathlessness and advanced disease to take part and remain in a drug trial? A qualitative study. Trials 21, 215 (2020).
Arriens, C. et al. Lupus patient decisions about clinical trial participation: a qualitative evaluation of perceptions, facilitators and barriers. Lupus Sci. Med. 7, e000360 (2020).
Harman, N. L. et al. Selecting Core Outcomes for Randomised Effectiveness trials In Type 2 diabetes (SCORE-IT): a patient and healthcare professional consensus on a core outcome set for type 2 diabetes. BMJ Open Diabetes Res. Care 7, e000700 (2019).
Hepprich, M., Donath, M. Y. & Hemkens, L. G. Patient involvement to inform the design of a clinical trial in postbariatric hypoglycaemia. BMC Med. Res. Methodol. 20, 290 (2020).
den Bakker, C. M. et al. Electronic health program to empower patients in returning to normal activities after colorectal surgical procedures: mixed-methods process evaluation alongside a randomized controlled trial. J. Med. Internet Res. 21, e10674 (2019).
Gutman, T. et al. Principles and strategies for involving patients in research in chronic kidney disease: report from national workshops. Nephrol. Dial. Transpl. 35, 1595–1594 (2020).
Coulman, K. D. et al. Understanding and optimising patient and public involvement in trial oversight: an ethnographic study of eight clinical trials. Trials 21, 543 (2020).
Author information
Authors and Affiliations
Contributions
A.T. researched the data for the article. All authors made substantial contributions to discussion of the content, writing the text and review or editing of the article before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks Melanie Calvert, who co-reviewed with Nicola Anderson, Istvan Mucsi and John Peipert, for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Research priority setting
-
Refers to activities that involve identifying, prioritizing and establishing consensus on research topics or questions that are important to stakeholders.
- Process evaluations
-
Evaluations that are conducted as part of a trial to explore the process of implementing the intervention. They typically involve ascertaining the trial participants’ perspectives on the intervention and may include investigating contextual factors that affect the intervention and the acceptability of the intervention.
- Choice experiments
-
A survey approach that is used to assess participant preferences.
- Patient navigator
-
A person (usually trained non-medical personnel) who assists patients in accessing and navigating the health-care system. They may facilitate access to care and care management in various ways, including through communication, education, care coordination and advocacy.
- Nominal group technique study
-
A structured method that is used to identify and prioritize ideas generated by a group of participants.
- Data linkage
-
The process of identifying, matching and combining records that correspond to the same person, usually from multiple data sets. For example, linked administrative data may be used in clinical trials.
- Social marketing
-
In the context of health, social marketing involves creating, communicating and delivering information using patient-centred and science-based strategies that are designed to influence health behaviour.
- Patient-reported outcomes
-
Outcomes that are directly reported by the patient without interpretation by a clinician or anyone else, and usually reflect how the patient feels and functions, for example, quality of life, symptoms (pain, fatigue) and functioning.
- Core outcomes
-
Consensus-based minimum sets of outcomes to be reported in all trials in a specific health or medical area.
- Content validity
-
The extent to which a patient-reported outcome measure adequately reflects the construct to be measured.
- Construct validity
-
The degree to which the scores of a patient-reported outcome instrument are consistent with the hypothesis based on the assumption that the instrument measures the intended construct.
- Criterion validity
-
The extent to which the scores of a patient-reported outcome measure are an adequate reflection of a ‘gold standard.’
- Patient-caregiver partner
-
A patient or informal caregiver involved in a research study as an equal partner with other members of the research team.
Rights and permissions
About this article
Cite this article
Tong, A., Scholes-Robertson, N., Hawley, C. et al. Patient-centred clinical trial design. Nat Rev Nephrol 18, 514–523 (2022). https://doi.org/10.1038/s41581-022-00585-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-022-00585-w
This article is cited by
-
How can we support research participants who stop taking part? Communications guidance developed through public-researcher collaboration
Research Involvement and Engagement (2024)
-
Delivering Digital Health Solutions that Patients Need: A Call to Action
Therapeutic Innovation & Regulatory Science (2024)