Abstract
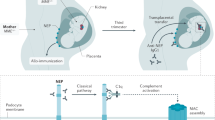
Membranous nephropathy (MN) is characterized histomorphologically by the presence of immune deposits in the subepithelial space of the glomerular filtration barrier; its clinical hallmarks are nephrotic range proteinuria with oedema. In patients with primary MN, autoimmunity is driven by circulating autoantibodies that bind to one or more antigens on the surface of glomerular podocytes. Compared with other autoimmune kidney diseases, the understanding of the pathogenesis of MN has substantially improved in the past decade, thanks to the discovery of pathogenic circulating autoantibodies against phospholipase A2 receptor 1 (PLA2R1) and thrombospondin type 1 domain-containing protein 7A (THSD7A). The subsequent identification of more proteins associated with MN, some of which are also endogenous podocyte antigens, might further advance the clinical characterization of MN, including its diagnosis, treatment and prognosis. Insights from studies in patients with MN, combined with the development of novel in vivo and in vitro experimental models, have potential to improve the management of patients with MN. Characterizing the interaction between autoimmunity and local glomerular lesions provides an opportunity to develop more specific, pathogenesis-based treatments.
Key points
-
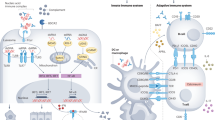
The exact pathomechanisms underlying the development of membranous nephropathy (MN) are still not fully defined, but loss of immune tolerance, genetic factors, environmental factors, complement activation, glomerular inflammation and cellular adaptive processes in the glomeruli have major roles.
-
In primary MN, circulating autoantibodies, mostly of the IgG4 subclass, bind to one or more antigens that are endogenously expressed on the surface of podocytes in glomeruli.
-
The identification of target antigens in MN allows a specific, molecular diagnosis based on staining of the target antigens in the glomeruli and detection of autoantibodies in blood.
-
Animal models have confirmed that human phospholipase A2 receptor 1 (PLA2R1) and thrombospondin type 1 domain-containing protein 7A (THSD7A) autoantibodies are pathogenic and induce disease after binding to their respective antigens.
-
MN is driven by autoimmune processes, which are the main target of immunosuppressive treatments. Measurement of anti-PLA2R1 antibodies enables assessment of immunological disease activity and informs the use of immunosuppressive treatments.
-
Potential targets for novel antigen-specific treatment options in MN include autoantibody production, antibody–antigen binding, immune-mediated podocyte injury and cell-specific glomerular pathomechanisms.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Glassock, R. J. Human idiopathic membranous nephropathy–a mystery solved? N. Engl. J. Med. 361, 81–83 (2009).
Couser, W. G. Primary membranous nephropathy. Clin. J. Am. Soc. Nephrol. 12, 983–997 (2017).
Glassock, R. J. The pathogenesis of membranous nephropathy. Curr. Opin. Nephrol. Hypertens. 21, 235–242 (2012).
Hoxha, E., von Haxthausen, F., Wiech, T. & Stahl, R. A. K. Membranous nephropathy–one morphologic pattern with different diseases. Pflügers Arch. Eur. J. Physiol. 469, 989–996 (2017).
De Vriese, A. S., Glassock, R. J., Nath, K. A., Sethi, S. & Fervenza, F. C. A proposal for a serology-based approach to membranous nephropathy. J. Am. Soc. Nephrol. 28, 421–430 (2017).
Stahl, R. A. K., Reinhard, L. & Hoxha, E. Characterization of autoantibodies in primary membranous nephropathy and their clinical significance. Expert Rev. Clin. Immunol. 15, 165–175 (2019).
Alsharhan, L. & Beck, L. H. Membranous nephropathy: core curriculum 2021. Am. J. Kidney Dis. 77, 440–453 (2021).
Beck, L. H. et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N. Engl. J. Med. 361, 11–21 (2009).
Hoxha, E. et al. Enhanced expression of the M-type phospholipase A2 receptor in glomeruli correlates with serum receptor antibodies in primary membranous nephropathy. Kidney Int. 82, 797–804 (2012).
Tomas, N. M. et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N. Engl. J. Med. 371, 2277–2287 (2014).
Hoxha, E. et al. An indirect immunofluorescence method facilitates detection of thrombospondin type 1 domain-containing 7A-specific antibodies in membranous nephropathy. J. Am. Soc. Nephrol. 28, 520–531 (2017).
Sethi, S. et al. Exostosin 1/exostosin 2-associated membranous nephropathy. J. Am. Soc. Nephrol. 30, 1123–1136 (2019).
Sethi, S. et al. Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int. 97, 163–174 (2020).
Caza, T. N. et al. NELL1 is a target antigen in malignancy-associated membranous nephropathy. Kidney Int. 99, 967–976 (2021).
Al-Rabadi, L. F. et al. Serine protease HTRA1 as a novel target antigen in primary membranous nephropathy. J. Am. Soc. Nephrol. 32, 1666–1681 (2021).
Sethi, S. et al. Semaphorin 3B-associated membranous nephropathy is a distinct type of disease predominantly present in pediatric patients. Kidney Int. 98, 1253–1264 (2020).
Sethi, S. et al. Protocadherin 7-associated membranous nephropathy. J. Am. Soc. Nephrol. 32, 1249–1261 (2021).
Caza, T. N. et al. Neural cell adhesion molecule 1 is a novel autoantigen in membranous lupus nephritis. Kidney Int. 100, 171–181 (2021).
Caza, T. N. et al. Transforming growth factor beta receptor 3 (TGFBR3)-associated membranous nephropathy. Kidney360 2, 1275–1286 (2021).
Le Quintrec, M. et al. Contactin-1 is a novel target antigen in membranous nephropathy associated with chronic inflammatory demyelinating polyneuropathy. Kidney Int. 100, 1240–1249 (2021).
Santoro, D. et al. Contactin 1, a potential new antigen target in membranous nephropathy: a case report. Am. J. Kidney Dis., https://doi.org/10.1053/j.ajkd.2021.08.025 (2021).
Reinhard, L. et al. Netrin G1 is a novel target antigen in membranous nephropathy [abstract PO1468] (American Society of Nephrology, 2021); https://www.asn-online.org/education/kidneyweek/2021/program-abstract.aspx?controlId=3607621
Sethi, S., Maddan, B. J., Nasr, S. H., Fervenza, F. C. & Haas, M. Hematopoietic stem cell transplant membranous nephropathy is associated with protocadherin FAT1 [abstract]. J. Am. Soc. Nephrol. https://doi.org/10.1681/ASN.2021111488 (2022).
Gupta, S. et al. Genetics of membranous nephropathy. Nephrol. Dial. Transpl. 33, 1493–1502 (2018).
Xie, J. et al. The genetic architecture of membranous nephropathy and its potential to improve non-invasive diagnosis. Nat. Commun. 11, 1600 (2020).
Reinhard, L., Stahl, R. A. K. & Hoxha, E. Is primary membranous nephropathy a complement mediated disease? Mol. Immunol. 128, 195–204 (2020).
Ronco, P. & Debiec, H. Molecular pathogenesis of membranous nephropathy. Annu. Rev. Pathol. Mech. Dis. 15, 287–313 (2020).
Lerner, G. B., Virmani, S., Henderson, J. M., Francis, J. M. & Beck, L. H. A conceptual framework linking immunology, pathology, and clinical features in primary membranous nephropathy. Kidney Int. 100, 289–300 (2021).
von Haxthausen, F. et al. Antigen-specific IgG subclasses in primary and malignancy-associated membranous nephropathy. Front. Immunol. 9, 3035 (2018).
Polanco, N. et al. Spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy. J. Am. Soc. Nephrol. 21, 697–704 (2010).
Fervenza, F. C. et al. Rituximab or cyclosporine in the treatment of membranous nephropathy. N. Engl. J. Med. 381, 36–46 (2019).
Scolari, F. et al. Rituximab or cyclophosphamide in the treatment of membranous nephropathy: the RI-CYCLO randomized trial. J. Am. Soc. Nephrol. 32, 972–982 (2021).
Debiec, H. et al. Early-childhood membranous nephropathy due to cationic bovine serum albumin. N. Engl. J. Med. 364, 2101–2110 (2011).
Debiec, H. et al. Allo-immune membranous nephropathy and recombinant aryl sulfatase replacement therapy: a need for tolerance induction therapy. J. Am. Soc. Nephrol. 25, 675–680 (2014).
Edgington, T. S., Glassock, R. J. & Dixon, F. J. Autologous immune complex nephritis induced with renal tubular antigen. J. Exp. Med. 127, 555–572 (1968).
Burbelo, P. D. et al. Detection of PLA2R autoantibodies before the diagnosis of membranous nephropathy. J. Am. Soc. Nephrol. 31, 208–217 (2020).
Zhao, Q. et al. Helper T cells in idiopathic membranous nephropathy. Front. Immunol. 12, 665629 (2021).
Roccatello, D. et al. New insights into immune mechanisms underlying response to rituximab in patients with membranous nephropathy: a prospective study and a review of the literature. Autoimmun. Rev. 15, 529–538 (2016).
Motavalli, R. et al. Altered Th17/Treg ratio as a possible mechanism in pathogenesis of idiopathic membranous nephropathy. Cytokine 141, 155452 (2021).
Ma, D. et al. Changes and significance of Treg and Th17 in adult patients with primary membranous nephropathy. Clin. Nephrol. 96, 155–164 (2021).
Rosenzwajg, M. et al. B- and T-cell subpopulations in patients with severe idiopathic membranous nephropathy may predict an early response to rituximab. Kidney Int. 92, 227–237 (2017).
Cantarelli, C. et al. A comprehensive phenotypic and functional immune analysis unravels circulating anti-phospholipase A2 receptor antibody secreting cells in membranous nephropathy patients. Kidney Int. Rep. 5, 1764–1776 (2020).
Shi, X. et al. Increased ratio of ICOS+/PD-1+ follicular helper T cells positively correlates with the development of human idiopathic membranous nephropathy. Clin. Exp. Pharmacol. Physiol. 43, 410–416 (2016).
Zhang, Z. et al. Higher frequencies of circulating ICOS+, IL-21+ T follicular helper cells and plasma cells in patients with new-onset membranous nephropathy. Autoimmunity 50, 458–467 (2017).
Fresquet, M. et al. Identification of a major epitope recognized by PLA2R autoantibodies in primary membranous nephropathy. J. Am. Soc. Nephrol. 26, 302–313 (2015).
Hoxha, E. et al. A mechanism for cancer-associated membranous nephropathy. N. Engl. J. Med. 374, 1995–1996 (2016).
Stahl, P. R. et al. THSD7A expression in human cancer. Genes Chromosomes Cancer 56, 314–327 (2017).
Hanset, N. et al. Podocyte antigen staining to identify distinct phenotypes and outcomes in membranous nephropathy: a retrospective multicenter cohort study. Am. J. Kidney Dis. 76, 624–635 (2020).
Zhang, Z., Gong, T., Rennke, H. G. & Hayashi, R. Duodenal schwannoma as a rare association with membranous nephropathy: a case report. Am. J. Kidney Dis. 73, 278–280 (2019).
Weinmann-Menke, J. et al. Treatment of membranous nephropathy in patients with THSD7A antibodies using immunoadsorption. Am. J. Kidney Dis. 74, 849–852 (2019).
Matsumoto, A. et al. Recurrent membranous nephropathy with a possible alteration in the etiology: a case report. BMC Nephrol. 22, 253 (2021).
Chen, M. et al. Case report: THSD7A-positive membranous nephropathy caused by tislelizumab in a lung cancer patient. Front. Immunol. 12, 619147 (2021).
Xu, X. et al. Long-term exposure to air pollution and increased risk of membranous nephropathy in China. J. Am. Soc. Nephrol. 27, 3739–3746 (2016).
van de Logt, A.-E., Fresquet, M., Wetzels, J. F. & Brenchley, P. The anti-PLA2R antibody in membranous nephropathy: what we know and what remains a decade after its discovery. Kidney Int. 96, 1292–1302 (2019).
Chen, R. et al. Fine particulate air pollution and the expression of microRNAs and circulating cytokines relevant to inflammation, coagulation, and vasoconstriction. Environ. Health Perspect. 126, 017007 (2018).
Le, Y. et al. Ambient fine particulate matter induces inflammatory responses of vascular endothelial cells through activating TLR-mediated pathway. Toxicol. Ind. Health 35, 670–678 (2019).
Cremoni, M. et al. Th17-immune response in patients with membranous nephropathy is associated with thrombosis and relapses. Front. Immunol. 11, 574997 (2020).
Liu, W. W. et al. Immunological pathogenesis of membranous nephropathy: focus on PLA2R1 and its role. Front. Immunol. 10, 1809 (2019).
Hanasaki, K. & Arita, H. Biological and pathological functions of phospholipase A2 receptor. Arch. Biochem. Biophys. 372, 215–223 (1999).
Silliman, C. C. et al. Presence of the M-type sPLA2 receptor on neutrophils and its role in elastase release and adhesion. Am. J. Physiol. Cell Physiol. 283, C1102–C1113 (2002).
Granata, F. et al. Activation of cytokine production by secreted phospholipase A2 in human lung macrophages expressing the M-type receptor. J. Immunol. 174, 464–474 (2005).
Coenen, M. J. H. et al. Phospholipase A2 receptor (PLA2R1) sequence variants in idiopathic membranous nephropathy. J. Am. Soc. Nephrol. 24, 677–683 (2013).
Stanescu, H. C. et al. Risk HLA-DQA1 and PLA2R1 alleles in idiopathic membranous nephropathy. N. Engl. J. Med. 364, 616–626 (2011).
Liu, Y.-H. et al. Association of phospholipase A2 receptor 1 polymorphisms with idiopathic membranous nephropathy in Chinese patients in Taiwan. J. Biomed. Sci. 17, 81 (2010).
Bullich, G. et al. HLA-DQA1 and PLA2R1 polymorphisms and risk of idiopathic membranous nephropathy. Clin. J. Am. Soc. Nephrol. 9, 335–343 (2014).
Ruggenenti, P. et al. Anti-phospholipase A2 receptor antibody titer predicts post-rituximab outcome of membranous nephropathy. J. Am. Soc. Nephrol. 26, 2545–2558 (2015).
Sekula, P. et al. Genetic risk variants for membranous nephropathy: extension of and association with other chronic kidney disease aetiologies. Nephrol. Dial. Transpl. 32, 325–332 (2017).
Wunnenburger, S. et al. Associations between genetic risk variants for kidney diseases and kidney disease etiology. Sci. Rep. 7, 13944 (2017).
Saeed, M., Beggs, M. L., Walker, P. D. & Larsen, C. P. PLA2R-associated membranous glomerulopathy is modulated by common variants in PLA2R1 and HLA-DQA1 genes. Genes. Immun. 15, 556–561 (2014).
Cui, Z. et al. MHC class II risk alleles and amino acid residues in idiopathic membranous nephropathy. J. Am. Soc. Nephrol. 28, 1651–1664 (2017).
Seitz-Polski, B. et al. Epitope spreading of autoantibody response to PLA2R associates with poor prognosis in membranous nephropathy. J. Am. Soc. Nephrol. 27, 1517–1533 (2016).
Seitz-Polski, B. et al. Phospholipase A2 receptor 1 epitope spreading at baseline predicts reduced likelihood of remission of membranous nephropathy. J. Am. Soc. Nephrol. 29, 401–408 (2018).
Reinhard, L. et al. Clinical relevance of domain-specific phospholipase A2 receptor 1 antibody levels in patients with membranous nephropathy. J. Am. Soc. Nephrol. 31, 197–207 (2020).
Berchtold, L. et al. HLA-D and PLA2R1 risk alleles associate with recurrent primary membranous nephropathy in kidney transplant recipients. Kidney Int. 99, 671–685 (2021).
Heymann, W., Hackel, D. B., Harwood, S., Wilson, S. G. & Hunter, J. L. Production of nephrotic syndrome in rats by Freund’s adjuvants and rat kidney suspensions. Proc. Soc. Exp. Biol. Med. 100, 660–664 (1959).
Couser, W. G., Steinmuller, D. R., Stilmant, M. M., Salant, D. J. & Lowenstein, L. M. Experimental glomerulonephritis in the isolated perfused rat kidney. J. Clin. Invest. 62, 1275–1287 (1978).
Heymann, W. III Nephrotic syndrome induced by injection of anti-kidney serum. Methods Med. Res. 5, 264–267 (1952).
Kerjaschki, D. & Farquhar, M. G. The pathogenic antigen of Heymann nephritis is a membrane glycoprotein of the renal proximal tubule brush border. Proc. Natl Acad. Sci. USA 79, 5557–5561 (1982).
Debiec, H. et al. Antenatal membranous glomerulonephritis due to anti-neutral endopeptidase antibodies. N. Engl. J. Med. 346, 2053–2060 (2002).
Tomas, N. M. et al. Autoantibodies against thrombospondin type 1 domain-containing 7A induce membranous nephropathy. J. Clin. Invest. 126, 2519–2532 (2016).
Reinhard, L. et al. Human PLA2R-antibodies induce membranous nephropathy in minipigs [abstract FR-OR31] (American Society of Nephrology, 2021); https://www.asn-online.org/education/kidneyweek/2021/program-abstract.aspx?controlId=3603987
Ancian, P., Lambeau, G., Mattéi, M.-G. & Lazdunski, M. The human 180-kDa receptor for secretory phospholipases A2. J. Biol. Chem. 270, 8963–8970 (1995).
Škoberne, A. et al. Serum with phospholipase A2 receptor autoantibodies interferes with podocyte adhesion to collagen. Eur. J. Clin. Invest. 44, 753–765 (2014).
Tomas, N. M. et al. A heterologous model of thrombospondin type 1 domain-containing 7A-associated membranous nephropathy. J. Am. Soc. Nephrol. 28, 3262–3277 (2017).
Herwig, J. et al. Thrombospondin type 1 domain-containing 7A localizes to the slit diaphragm and stabilizes membrane dynamics of fully differentiated podocytes. J. Am. Soc. Nephrol. 30, 824–839 (2019).
International Mouse Phenotyping Consortium. Thsd7a. https://www.mousephenotype.org/data/search?term=thsd7a&type=gene (accessed December 2021).
Fresquet, M. et al. Autoantigens PLA2R and THSD7A in membranous nephropathy share a common epitope motif in the N-terminal domain. J. Autoimmun. 106, 102308 (2020).
Zaghrini, C. et al. Novel ELISA for thrombospondin type 1 domain-containing 7A autoantibodies in membranous nephropathy. Kidney Int. 95, 666–679 (2019).
Kao, L., Lam, V., Waldman, M., Glassock, R. J. & Zhu, Q. Identification of the immunodominant epitope region in phospholipase A2 receptor-mediating autoantibody binding in idiopathic membranous nephropathy. J. Am. Soc. Nephrol. 26, 291–301 (2015).
Tang, H., Zhu, R., Waldman, M. & Zhu, Q. Structural determinants of the dominant conformational epitopes of phospholipase A2 receptor in primary membranous nephropathy. J. Biol. Chem. 298, 101605 (2022).
Ghiggeri, G. M. et al. Multi-autoantibody signature and clinical outcome in membranous nephropathy. Clin. J. Am. Soc. Nephrol. 15, 1762–1776 (2020).
van de Logt, A.-E. et al. Anti-PLA2R1 antibodies as prognostic biomarker in membranous nephropathy. Kidney Int. Rep. 6, 1677–1686 (2021).
Seifert, L. et al. The most N-terminal region of THSD7A is the predominant target for autoimmunity in THSD7A-associated membranous nephropathy. J. Am. Soc. Nephrol. 29, 1536–1548 (2018).
Shah, P., Tramontano, A. & Makker, S. P. Intramolecular epitope spreading in heymann nephritis. J. Am. Soc. Nephrol. 18, 3060–3066 (2007).
Salinas, G. F., Braza, F., Brouard, S., Tak, P. P. & Baeten, D. The role of B lymphocytes in the progression from autoimmunity to autoimmune disease. Clin. Immunol. 146, 34–45 (2013).
Stahl, R., Hoxha, E. & Fechner, K. PLA2R autoantibodies and recurrent membranous nephropathy after transplantation. N. Engl. J. Med. 363, 496–498 (2010).
Larsen, C. P. & Walker, P. D. Phospholipase A2 receptor (PLA2R) staining is useful in the determination of de novo versus recurrent membranous glomerulopathy. Transplantation 95, 1259–1262 (2013).
Kattah, A. et al. Anti-phospholipase A2 receptor antibodies in recurrent membranous nephropathy. Am. J. Transpl. 15, 1349–1359 (2015).
Querol, L. et al. Antibodies to contactin-1 in chronic inflammatory demyelinating polyneuropathy. Ann. Neurol. 73, 370–380 (2013).
Miura, Y. et al. Contactin 1 IgG4 associates to chronic inflammatory demyelinating polyneuropathy with sensory ataxia. Brain 138, 1484–1491 (2015).
Ravindran, A. et al. In patients with membranous lupus nephritis, exostosin-positivity and exostosin-negativity represent two different phenotypes. J. Am. Soc. Nephrol. 32, 695–706 (2021).
Salant, D. J., Belok, S., Stilmant, M. M., Darby, C. & Couser, W. G. Determinants of glomerular localization of subepithelial immune deposits: effects of altered antigen to antibody ratio, steroids, vasoactive amine antagonists, and aminonucleoside of puromycin on passive Heymann nephritis in rats. Lab. Invest. 41, 89–99 (1979).
Salant, D. J., Darby, C. & Couser, W. G. Experimental membranous glomerulonephritis in rats. Quantitative studies of glomerular immune deposit formation in isolated glomeruli and whole animals. J. Clin. Invest. 66, 71–81 (1980).
Cybulsky, A. V., Rennke, H. G., Feintzeig, I. D. & Salant, D. J. Complement-induced glomerular epithelial cell injury. Role of the membrane attack complex in rat membranous nephropathy. J. Clin. Invest. 77, 1096–1107 (1986).
Spicer, S. T. et al. Induction of passive heymann nephritis in complement component 6-deficient PVG rats. J. Immunol. 179, 172–178 (2007).
Haddad, G. et al. Altered glycosylation of IgG4 promotes lectin complement pathway activation in anti-PLA2R1-associated membranous nephropathy. J. Clin. Invest. 131, e140453 (2021).
Benzing, T. & Salant, D. Insights into glomerular filtration and albuminuria. N. Engl. J. Med. 384, 1437–1446 (2021).
Neale, T. J. et al. Proteinuria in passive Heymann nephritis is associated with lipid peroxidation and formation of adducts on type IV collagen. J. Clin. Invest. 94, 1577–1584 (1994).
Stahl, R. A. K. et al. Enhanced glomerular prostaglandin formation in experimental membranous nephropathy. Kidney Int. 31, 1126–1131 (1987).
Cybulsky, A. V., Lieberthal, W., Quigg, R. J., Rennke, H. G. & Salant, D. J. A role for thromboxane in complement-mediated glomerular injury. Am. J. Pathol. 128, 45–51 (1987).
Shankland, S. J. et al. Differential expression of transforming growth factor-β isoforms and receptors in experimental membranous nephropathy. Kidney Int. 50, 116–124 (1996).
McMillan, J. I., Riordan, J. W., Couser, W. G., Pollock, A. S. & Lovett, D. H. Characterization of a glomerular epithelial cell metalloproteinase as matrix metalloproteinase-9 with enhanced expression in a model of membranous nephropathy. J. Clin. Invest. 97, 1094–1101 (1996).
Shankland, S. J., Pippin, J. W. & Couser, W. G. Complement (C5b-9) induces glomerular epithelial cell DNA synthesis but not proliferation in vitro. Kidney Int. 56, 538–548 (1999).
Pippin, J. W. et al. DNA damage is a novel response to sublytic complement C5b-9-induced injury in podocytes. J. Clin. Invest. 111, 877–885 (2003).
Meyer-Schwesinger, C. The ubiquitin–proteasome system in kidney physiology and disease. Nat. Rev. Nephrol. 15, 393–411 (2019).
Meyer-Schwesinger, C. et al. A novel mouse model of phospholipase A2 receptor 1-associated membranous nephropathy mimics podocyte injury in patients. Kidney Int. 97, 913–919 (2020).
Hatje, F. A. et al. Tripartite separation of glomerular cell types and proteomes from reporter-free mice. J. Am. Soc. Nephrol. 32, 2175–2193 (2021).
Hoxha, E. & Stahl, R. A. K. Translational aspects of primary membranous nephropathy. Semin. Nephrol. 37, 436–446 (2017).
Kerjaschki, D. et al. Transcellular transport and membrane insertion of the C5b-9 membrane attack complex of complement by glomerular epithelial cells in experimental membranous nephropathy. J. Immunol. 143, 546–552 (1989).
Beck, L. H. et al. Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J. Am. Soc. Nephrol. 22, 1543–1550 (2011).
Wehrmann, M. et al. Long-term prognosis of chronic idiopathic membranous glomerulonephritis. An analysis of 334 cases with particular regard to tubulo-interstitial changes. Clin. Nephrol. 31, 67–76 (1989).
Horvatic, I. et al. Prognostic significance of glomerular and tubulointerstitial morphometry in idiopathic membranous nephropathy. Pathol. Res. Pract. 208, 662–667 (2012).
Chen, Y. et al. Pathological predictors of renal outcomes in nephrotic idiopathic membranous nephropathy with decreased renal function. J. Nephrol. 27, 307–316 (2014).
Mahmud, M. et al. Role of phospholipase A2 receptor 1 antibody level at diagnosis for long-term renal outcome in membranous nephropathy. PLoS ONE 14, e0221293 (2019).
Ruggenenti, P. et al. Rituximab for idiopathic membranous nephropathy: who can benefit? Clin. J. Am. Soc. Nephrol. 1, 738–748 (2006).
Ronco, P. & Debiec, H. Membranous nephropathy: current understanding of various causes in light of new target antigens. Curr. Opin. Nephrol. Hypertens. 30, 287–293 (2021).
Hoxha, E. et al. An immunofluorescence test for phospholipase-A2-receptor antibodies and its clinical usefulness in patients with membranous glomerulonephritis. Nephrol. Dial. Transplant. 26, 2526–2532 (2011).
Dähnrich, C. et al. Development of a standardized ELISA for the determination of autoantibodies against human M-type phospholipase A2 receptor in primary membranous nephropathy. Clin. Chim. Acta 421, 213–218 (2013).
Ronco, P. & Debiec, H. Membranous nephropathy: a fairy tale for immunopathologists, nephrologists and patients. Mol. Immunol. 68, 57–62 (2015).
Hofstra, J. M. et al. Antiphospholipase A2 receptor antibody titer and subclass in idiopathic membranous nephropathy. J. Am. Soc. Nephrol. 23, 1735–1743 (2012).
Kanigicherla, D. et al. Anti-PLA2R antibodies measured by ELISA predict long-term outcome in a prevalent population of patients with idiopathic membranous nephropathy. Kidney Int. 83, 940–948 (2013).
Hoxha, E. et al. Phospholipase A2 receptor autoantibodies and clinical outcome in patients with primary membranous nephropathy. J. Am. Soc. Nephrol. 25, 1357–1366 (2014).
Hoxha, E., Harendza, S., Pinnschmidt, H., Panzer, U. & Stahl, R. A. K. M-type phospholipase A2 receptor autoantibodies and renal function in patients with primary membranous nephropathy. Clin. J. Am. Soc. Nephrol. 9, 1883–1890 (2014).
Bech, A. P., Hofstra, J. M., Brenchley, P. E. & Wetzels, J. F. M. Association of anti-PLA2R antibodies with outcomes after immunosuppressive therapy in idiopathic membranous nephropathy. Clin. J. Am. Soc. Nephrol. 9, 1386–1392 (2014).
Fernández-Juárez, G. et al. The STARMEN trial indicates that alternating treatment with corticosteroids and cyclophosphamide is superior to sequential treatment with tacrolimus and rituximab in primary membranous nephropathy. Kidney Int. 99, 986–998 (2021).
Mougiakakos, D. et al. CD19-targeted CAR T cells in refractory systemic lupus erythematosus. N. Engl. J. Med. 385, 567–569 (2021).
Carambia, A. et al. Nanoparticle-based autoantigen delivery to Treg-inducing liver sinusoidal endothelial cells enables control of autoimmunity in mice. J. Hepatol. 62, 1349–1356 (2015).
Clemente-Casares, X. et al. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature 530, 434–440 (2016).
Pishesha, N. et al. Induction of antigen-specific tolerance by nanobody–antigen adducts that target class-II major histocompatibility complexes. Nat. Biomed. Eng. 5, 1389–1401 (2021).
Irani, V. et al. Molecular properties of human IgG subclasses and their implications for designing therapeutic monoclonal antibodies against infectious diseases. Mol. Immunol. 67, 171–182 (2015).
de Taeye, S. W., Rispens, T. & Vidarsson, G. The ligands for human IgG and their effector functions. Antibodies 8, 30 (2019).
Edwards, J. C. W. & Cambridge, G. B-cell targeting in rheumatoid arthritis and other autoimmune diseases. Nat. Rev. Immunol. 6, 394–403 (2006).
Acknowledgements
This work is supported by grants from the Deutsche Forschungsgemeinschaft to E.H. (Heisenberg Programme and project B1 and C1 of the SFB 1192) and R.A.K.S. (project B1 of the SFB 1192).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, made substantial contributions to discussions of the content, and wrote, reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
E.H. has received fees from Morphosys, Planegg, Germany, and Novartis, Basel, Switzerland, for advisory board activities. R.A.K.S. has received fees from Morphosys, Planegg, Germany, for advisory board activities. L.R. declares no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks B. Seitz-Polski, H. Debiec and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Epitope spreading
-
Spreading of the specificity of an immune response from an epitope to another located in the same molecule (intramolecular spreading), or in a different molecule (intermolecular spreading).
Rights and permissions
About this article
Cite this article
Hoxha, E., Reinhard, L. & Stahl, R.A.K. Membranous nephropathy: new pathogenic mechanisms and their clinical implications. Nat Rev Nephrol 18, 466–478 (2022). https://doi.org/10.1038/s41581-022-00564-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-022-00564-1
This article is cited by
-
The proteasome modulates endocytosis specifically in glomerular cells to promote kidney filtration
Nature Communications (2024)
-
Cyclophosphamide induced early remission and was superior to rituximab in idiopathic membranous nephropathy patients with high anti-PLA2R antibody levels
BMC Nephrology (2023)
-
Lesson for the clinical nephrologist: thrombospondin type-1 domain-containing protein 7A-associated membranous nephropathy and Fanconi syndrome in a patient with a squamous cell lung cancer
Journal of Nephrology (2023)
-
Diagnostik und Therapie der Membranösen Nephropathie – 2023
Wiener klinische Wochenschrift (2023)
-
Concurrent glomerular PCDH7 deposits in PLA2R-associated membranous nephropathy
CEN Case Reports (2023)