Abstract
Circulating blood is filtered across the glomerular barrier to form an ultrafiltrate of plasma in the Bowman’s space. The volume of glomerular filtration adjusted by time is defined as the glomerular filtration rate (GFR), and the total GFR is the sum of all single-nephron GFRs. Thus, when the single-nephron GFR is increased in the context of a normal number of functioning nephrons, single glomerular hyperfiltration results in ‘absolute’ hyperfiltration in the kidney. ‘Absolute’ hyperfiltration can occur in healthy people after high protein intake, during pregnancy and in patients with diabetes, obesity or autosomal-dominant polycystic kidney disease. When the number of functioning nephrons is reduced, single-nephron glomerular hyperfiltration can result in a GFR that is within or below the normal range. This ‘relative’ hyperfiltration can occur in patients with a congenitally reduced nephron number or with an acquired reduction in nephron mass consequent to surgery or kidney disease. Improved understanding of the mechanisms that underlie ‘absolute’ and ‘relative’ glomerular hyperfiltration in different clinical settings, and of whether and how the single-nephron haemodynamic and related biomechanical forces that underlie glomerular hyperfiltration promote glomerular injury, will pave the way toward the development of novel therapeutic interventions that attenuate glomerular hyperfiltration and potentially prevent or limit consequent progressive kidney injury and loss of function.
Key points
-
‘Absolute’ hyperfiltration is a supraphysiological elevation in glomerular filtration rate (GFR) that occurs when the single-nephron glomerular filtration rate (SNGFR) increases in a kidney with a normal number of functioning nephrons.
-
‘Absolute’ hyperfiltration can occur in healthy people following consumption of a high protein meal and during pregnancy as well as in patients with obesity, diabetes mellitus or autosomal-dominant polycystic kidney disease.
-
‘Relative’ hyperfiltration is an increase in SNGFR in the setting of a reduced number of functioning nephrons, which can result in a GFR that is within or below the normal range.
-
‘Relative’ hyperfiltration can occur in patients with a congenitally reduced number of nephrons and in those with an acquired reduction in kidney mass as a result of surgery or kidney disease.
-
Independent of ‘absolute’ or ‘relative’ hyperfiltration, persistent increases in the SNGFR that are associated with glomerular hypertension can eventually lead to proteinuria, glomerulosclerosis and a decline in kidney function.
-
Improved understanding of the underlying mechanisms could lead to the development of novel therapeutic interventions to attenuate glomerular hyperfiltration and protect the kidney.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Deen, W. M., Robertson, C. R. & Brenner, B. M. A model of glomerular ultrafiltration in the rat. Am. J. Physiol. 223, 1178–1183 (1972).
Pollak, M. R., Quaggin, S. E., Hoenig, M. P. & Dworkin, L. D. The glomerulus: the sphere of influence. Clin. J. Am. Soc. Nephrol. 9, 1461–1469 (2014).
Cachat, F., Combescure, C., Cauderay, M., Girardin, E. & Chehade, H. A systematic review of glomerular hyperfiltration assessment and definition in the medical literature. Clin. J. Am. Soc. Nephrol. 10, 382–389 (2015).
Porrini, E. et al. Estimated GFR: time for a critical appraisal. Nat. Rev. Nephrol. 15, 177–190 (2019).
Gaspari, F. et al. The GFR and GFR decline cannot be accurately estimated in type 2 diabetics. Kidney Int. 84, 164–173 (2013).
Denic, A. et al. Single-nephron glomerular filtration rate in healthy adults. N. Engl. J. Med. 376, 2349–2357 (2017).
Denic, A. et al. Detection and clinical patterns of nephron hypertrophy and nephrosclerosis among apparently healthy adults. Am. J. Kidney Dis. 68, 58–67 (2016).
Sharma, A., Mucino, M. J. & Ronco, C. Renal functional reserve and renal recovery after acute kidney injury. Nephron Clin. Pract. 127, 94–100 (2014).
Armenta, A., Madero, M. & Rodriguez-Iturbe, B. Functional reserve of the kidney. Clin. J. Am. Soc. Nephrol. https://doi.org/10.2215/CJN.11070821 (2021).
De Nicola, L., Keiser, J. A., Blantz, R. C. & Gabbai, F. B. Angiotensin II and renal functional reserve in rats with Goldblatt hypertension. Hypertension 19, 790–794 (1992).
Raes, A., Donckerwolcke, R., Craen, M., Hussein, M. C. & Vande Walle, J. Renal hemodynamic changes and renal functional reserve in children with type I diabetes mellitus. Pediatr. Nephrol. 22, 1903–1909 (2007).
ter Wee, P. M., Tegzess, A. M. & Donker, A. J. Renal reserve filtration capacity before and after kidney donation. J. Intern. Med. 228, 393–399 (1990).
Palsson, R. & Waikar, S. S. Renal functional reserve revisited. Adv. Chronic Kidney Dis. 25, e1–e8 (2018).
Barai, S., Gambhir, S., Prasad, N., Sharma, R. K. & Ora, M. Functional renal reserve capacity in different stages of chronic kidney disease. Nephrology 15, 350–353 (2010).
Remuzzi, A. et al. Glomerular response to hyperglycemia in human diabetic nephropathy. Am. J. Physiol. 259, F545–F552 (1990).
Brenner, B. M., Meyer, T. W. & Hostetter, T. H. Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N. Engl. J. Med. 307, 652–659 (1982).
Castellino, P., Coda, B. & DeFronzo, R. A. Effect of amino acid infusion on renal hemodynamics in humans. Am. J. Physiol. 251, F132–F140 (1986).
Hostetter, T. H. Human renal response to meat meal. Am. J. Physiol. 250, F613–F618 (1986).
Bosch, J. P., Lew, S., Glabman, S. & Lauer, A. Renal hemodynamic changes in humans. Response to protein loading in normal and diseased kidneys. Am. J. Med. 81, 809–815 (1986).
Viberti, G. et al. Effect of protein-restricted diet on renal response to a meat meal in humans. Am. J. Physiol. 253, F388–F393 (1987).
ter Wee, P. M., Rosman, J. B., van der Geest, S., Sluiter, W. J. & Donker, A. J. Renal hemodynamics during separate and combined infusion of amino acids and dopamine. Kidney Int. 29, 870–874 (1986).
Bankir, L., Roussel, R. & Bouby, N. Protein- and diabetes-induced glomerular hyperfiltration: role of glucagon, vasopressin, and urea. Am. J. Physiol. Renal Physiol. 309, F2–F23 (2015).
Mahieu, S. et al. Monosodium glutamate intake affect the function of the kidney through NMDA receptor. Life Sci. 149, 114–119 (2016).
Gabbai, F. B. The role of renal response to amino acid infusion and oral protein load in normal kidneys and kidney with acute and chronic disease. Curr. Opin. Nephrol. Hypertens. 27, 23–29 (2018).
Wei, J. et al. High-protein diet-induced glomerular hyperfiltration is dependent on neuronal nitric oxide synthase β in the macula densa via tubuloglomerular feedback response. Hypertension 74, 864–871 (2019).
Kontessis, P. et al. Renal, metabolic and hormonal responses to ingestion of animal and vegetable proteins. Kidney Int. 38, 136–144 (1990).
Wakefield, A. P., House, J. D., Ogborn, M. R., Weiler, H. A. & Aukema, H. M. A diet with 35% of energy from protein leads to kidney damage in female Sprague-Dawley rats. Br. J. Nutr. 106, 656–663 (2011).
Hostetter, T. H., Meyer, T. W., Rennke, H. G. & Brenner, B. M. Chronic effects of dietary protein in the rat with intact and reduced renal mass. Kidney Int. 30, 509–517 (1986).
Jia, Y. et al. Long-term high intake of whole proteins results in renal damage in pigs. J. Nutr. 140, 1646–1652 (2010).
Cirillo, M. et al. Protein intake and kidney function in the middle-age population: contrast between cross-sectional and longitudinal data. Nephrol. Dial. Transpl. 29, 1733–1740 (2014).
Farhadnejad, H., Asghari, G., Emamat, H., Mirmiran, P. & Azizi, F. Low-carbohydrate high-protein diet is associated with increased risk of incident chronic kidney diseases among Tehranian adults. J. Ren. Nutr. 29, 343–349 (2019).
Lew, Q.-L. J. et al. Red meat intake and risk of ESRD. J. Am. Soc. Nephrol. 28, 304–312 (2017).
Esmeijer, K., Geleijnse, J. M., de Fijter, J. W., Kromhout, D. & Hoogeveen, E. K. Dietary protein intake and kidney function decline after myocardial infarction: the Alpha Omega Cohort. Nephrol. Dial. Transpl. 35, 106–115 (2020).
Jhee, J. H. et al. High-protein diet with renal hyperfiltration is associated with rapid decline rate of renal function: a community-based prospective cohort study. Nephrol. Dial. Transpl. 35, 98–106 (2020).
Haring, B. et al. Dietary protein sources and risk for incident chronic kidney disease: results from the atherosclerosis risk in communities (ARIC) Study. J. Ren. Nutr. 27, 233–242 (2017).
Halbesma, N. et al. High protein intake associates with cardiovascular events but not with loss of renal function. J. Am. Soc. Nephrol. 20, 1797–1804 (2009).
Beasley, J. M. et al. Dietary protein intake and change in estimated GFR in the Cardiovascular Health Study. Nutrition 30, 794–799 (2014).
Friedman, A. N. et al. Comparative effects of low-carbohydrate high-protein versus low-fat diets on the kidney. Clin. J. Am. Soc. Nephrol. 7, 1103–1111 (2012).
Wycherley, T. P., Brinkworth, G. D., Clifton, P. M. & Noakes, M. Comparison of the effects of 52 weeks weight loss with either a high-protein or high-carbohydrate diet on body composition and cardiometabolic risk factors in overweight and obese males. Nutr. Diabetes 2, e40 (2012).
Li, Z. et al. Protein-enriched meal replacements do not adversely affect liver, kidney or bone density: an outpatient randomized controlled trial. Nutr. J. 9, 72 (2010).
Tay, J. et al. Long-term effects of a very low carbohydrate compared with a high carbohydrate diet on renal function in individuals with type 2 diabetes: a randomized trial. Medicine 94, e2181 (2015).
Cheung, K. L. & Lafayette, R. A. Renal physiology of pregnancy. Adv. Chronic Kidney Dis. 20, 209–214 (2013).
Baylis, C. Glomerular filtration and volume regulation in gravid animal models. Baillieres Clin. Obstet. Gynaecol. 8, 235–264 (1994).
Reckelhoff, J. F., Yokota, S. D. & Baylis, C. Renal autoregulation in midterm and late-pregnant rats. Am. J. Obstet. Gynecol. 166, 1546–1550 (1992).
Chapman, A. B. et al. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int. 54, 2056–2063 (1998).
Dunlop, W. Serial changes in renal haemodynamics during normal human pregnancy. Br. J. Obstet. Gynaecol. 88, 1–9 (1981).
Odutayo, A. & Hladunewich, M. Obstetric nephrology: renal hemodynamic and metabolic physiology in normal pregnancy. Clin. J. Am. Soc. Nephrol. 7, 2073–2080 (2012).
Roberts, M., Lindheimer, M. D. & Davison, J. M. Altered glomerular permselectivity to neutral dextrans and heteroporous membrane modeling in human pregnancy. Am. J. Physiol. 270, F338–F343 (1996).
Hladunewich, M. A. et al. The dynamics of glomerular filtration in the puerperium. Am. J. Physiol. Renal Physiol. 286, F496–F503 (2004).
Conrad, K. P. Maternal vasodilation in pregnancy: the emerging role of relaxin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R267–R275 (2011).
Lafayette, R. A. et al. Serum relaxin levels and kidney function in late pregnancy with or without preeclampsia. Clin. Nephrol. 75, 226–232 (2011).
Novak, J. et al. Reduced sensitivity of the renal circulation to angiotensin II in pregnant rats. Hypertension 30, 580–584 (1997).
Irani, R. A. & Xia, Y. Renin angiotensin signaling in normal pregnancy and preeclampsia. Semin. Nephrol. 31, 47–58 (2011).
Gant, N. F., Worley, R. J., Everett, R. B. & MacDonald, P. C. Control of vascular responsiveness during human pregnancy. Kidney Int. 18, 253–258 (1980).
AbdAlla, S., Lother, H., el Massiery, A. & Quitterer, U. Increased AT1 receptor heterodimers in preeclampsia mediate enhanced angiotensin II responsiveness. Nat. Med. 7, 1003–1009 (2001).
Gumus, I. I. et al. Does glomerular hyperfiltration in pregnancy damage the kidney in women with more parities? Int. Urol. Nephrol. 41, 927–932 (2009).
Wiles, K. S., Nelson-Piercy, C. & Bramham, K. Reproductive health and pregnancy in women with chronic kidney disease. Nat. Rev. Nephrol. 14, 165–184 (2018).
Garofalo, C. et al. A systematic review and meta-analysis suggests obesity predicts onset of chronic kidney disease in the general population. Kidney Int. 91, 1224–1235 (2017).
Hsu, C., McCulloch, C. E., Iribarren, C., Darbinian, J. & Go, A. S. Body mass index and risk for end-stage renal disease. Ann. Intern. Med. 144, 21–28 (2006).
Henegar, J. R., Bigler, S. A., Henegar, L. K., Tyagi, S. C. & Hall, J. E. Functional and structural changes in the kidney in the early stages of obesity. J. Am. Soc. Nephrol. 12, 1211–1217 (2001).
Chagnac, A. et al. Glomerular hemodynamics in severe obesity. Am. J. Physiol. Renal Physiol. 278, F817–F822 (2000).
Chagnac, A. et al. Obesity-induced glomerular hyperfiltration: its involvement in the pathogenesis of tubular sodium reabsorption. Nephrol. Dial. Transpl. 23, 3946–3952 (2008).
Wuerzner, G. et al. Marked association between obesity and glomerular hyperfiltration: a cross-sectional study in an African population. Am. J. Kidney Dis. 56, 303–312 (2010).
Brenner, B. M., Lawler, E. V. & Mackenzie, H. S. The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int. 49, 1774–1777 (1996).
Hall, J. E., do Carmo, J. M., da Silva, A. A., Wang, Z. & Hall, M. E. Obesity, kidney dysfunction and hypertension: mechanistic links. Nat. Rev. Nephrol. 15, 367–385 (2019).
Oosterhuis, N. R. et al. Extravascular renal denervation ameliorates juvenile hypertension and renal damage resulting from experimental hyperleptinemia in rats. J. Hypertens. 35, 2537–2547 (2017).
Shi, Z., Li, B. & Brooks, V. L. Role of the paraventricular nucleus of the hypothalamus in the sympathoexcitatory effects of leptin. Hypertension 66, 1034–1041 (2015).
D’Agati, V. D. et al. Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat. Rev. Nephrol. 12, 453–471 (2016).
Chagnac, A. et al. The effects of weight loss on renal function in patients with severe obesity. J. Am. Soc. Nephrol. 14, 1480–1486 (2003).
Friedman, A. N. et al. Predicting the glomerular filtration rate in bariatric surgery patients. Am. J. Nephrol. 39, 8–15 (2014).
Lieske, J. C. et al. Gastric bypass surgery and measured and estimated GFR in women. Am. J. Kidney Dis. 64, 663–665 (2014).
von Scholten, B. J. et al. Effect of large weight reductions on measured and estimated kidney function. BMC Nephrol. 18, 52 (2017).
Navarro-Díaz, M. et al. Effect of drastic weight loss after bariatric surgery on renal parameters in extremely obese patients: long-term follow-up. J. Am. Soc. Nephrol. 17, S213–S217 (2006).
Serra, A. et al. The effect of bariatric surgery on adipocytokines, renal parameters and other cardiovascular risk factors in severe and very severe obesity: 1-year follow-up. Clin. Nutr. 25, 400–408 (2006).
Ruggenenti, P. et al. Renal and systemic effects of calorie restriction in patients with type 2 diabetes with abdominal obesity: a randomized controlled trial. Diabetes 66, 75–86 (2017).
Afshinnia, F., Wilt, T. J., Duval, S., Esmaeili, A. & Ibrahim, H. N. Weight loss and proteinuria: systematic review of clinical trials and comparative cohorts. Nephrol. Dial. Transpl. 25, 1173–1183 (2010).
Li, K. et al. Effects of bariatric surgery on renal function in obese patients: a systematic review and meta analysis. PLoS One 11, e0163907 (2016).
Navaneethan, S. D. & Yehnert, H. Bariatric surgery and progression of chronic kidney disease. Surg. Obes. Relat. Dis. 5, 662–665 (2009).
Imam, T. H. et al. Estimated GFR before and after bariatric surgery in CKD. Am. J. Kidney Dis. 69, 380–388 (2017).
Morales, E. et al. Renoprotective role of bariatric surgery in patients with established chronic kidney disease. Clin. Kidney J. 14, 2037–2046 (2021).
Praga, M. et al. Effects of body-weight loss and captopril treatment on proteinuria associated with obesity. Nephron 70, 35–41 (1995).
Morales, E., Valero, M. A., León, M., Hernández, E. & Praga, M. Beneficial effects of weight loss in overweight patients with chronic proteinuric nephropathies. Am. J. Kidney Dis. 41, 319–327 (2003).
Navaneethan, S. D. et al. Bariatric surgery, kidney function, insulin resistance, and adipokines in patients with decreased GFR: a cohort study. Am. J. Kidney Dis. 65, 345–347 (2015).
Favre, G., Schiavo, L., Lemoine, S., Esnault, V. L. M. & Iannelli, A. Longitudinal assessment of renal function in native kidney after bariatric surgery. Surg. Obes. Relat. Dis. 14, 1411–1418 (2018).
López-Martínez, M. et al. The estimation of GFR and the adjustment for BSA in overweight and obesity: a dreadful combination of two errors. Int. J. Obes. 44, 1129–1140 (2020).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–612 (2009).
Levey, A. S. et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 130, 461–470 (1999).
Inker, L. A. et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 367, 20–29 (2012).
Tamboli, R. A. et al. Body composition and energy metabolism following Roux-en-Y gastric bypass surgery. Obesity 18, 1718–1724 (2010).
Chew-Harris, J. S. C., Florkowski, C. M., George, P. M., Elmslie, J. L. & Endre, Z. H. The relative effects of fat versus muscle mass on cystatin C and estimates of renal function in healthy young men. Ann. Clin. Biochem. 50, 39–46 (2013).
Chang, A. R. et al. Performance of glomerular filtration rate estimating equations before and after bariatric surgery. Kidney Med. 2, 699–706.e1 (2020).
Delanaye, P., Radermecker, R. P., Rorive, M., Depas, G. & Krzesinski, J. M. Indexing glomerular filtration rate for body surface area in obese patients is misleading: concept and example. Nephrol. Dial. Transpl. 20, 2024–2028 (2005).
Friedman, A. N. et al. Effect of bariatric surgery on CKD risk. J. Am. Soc. Nephrol. 29, 1289–1300 (2018).
Mallamaci, F. et al. ACE inhibition is renoprotective among obese patients with proteinuria. J. Am. Soc. Nephrol. 22, 1122–1128 (2011).
Zingerman, B. et al. Effect of acetazolamide on obesity-induced glomerular hyperfiltration: a randomized controlled trial. PLoS ONE 10, e0137163 (2015).
Saran, R. et al. US renal data system 2019 annual data report: epidemiology of kidney disease in the United States. Am. J. Kidney Dis. 75, A6–A7 (2020).
Tonneijck, L. et al. Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J. Am. Soc. Nephrol. 28, 1023–1039 (2017).
Hostetter, T. H., Troy, J. L. & Brenner, B. M. Glomerular hemodynamics in experimental diabetes mellitus. Kidney Int. 19, 410–415 (1981).
Zatz, R., Meyer, T. W., Rennke, H. G. & Brenner, B. M. Predominance of hemodynamic rather than metabolic factors in the pathogenesis of diabetic glomerulopathy. Proc. Natl Acad. Sci. USA 82, 5963–5967 (1985).
Sasson, A. N. & Cherney, D. Z. Renal hyperfiltration related to diabetes mellitus and obesity in human disease. World J. Diabetes 3, 1–6 (2012).
Sochett, E. B. et al. Impact of renin angiotensin system modulation on the hyperfiltration state in type 1 diabetes. J. Am. Soc. Nephrol. 17, 1703–1709 (2006).
Lovshin, J. A. et al. Renin-angiotensin-aldosterone system activation in long-standing type 1 diabetes. JCI Insight 3, 96968 (2018).
Chiarelli, F. et al. Increased circulating nitric oxide in young patients with type 1 diabetes and persistent microalbuminuria: relation to glomerular hyperfiltration. Diabetes 49, 1258–1263 (2000).
Cherney, D. Z. I. et al. Renal hyperfiltration is a determinant of endothelial function responses to cyclooxygenase 2 inhibition in type 1 diabetes. Diabetes Care 33, 1344–1346 (2010).
Vallon, V. & Thomson, S. C. The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat. Rev. Nephrol. 16, 317–336 (2020).
Cherney, D. Z. I., Scholey, J. W. & Miller, J. A. Insights into the regulation of renal hemodynamic function in diabetic mellitus. Curr. Diabetes Rev. 4, 280–290 (2008).
Mogensen, C. E. & Andersen, M. J. Increased kidney size and glomerular filtration rate in untreated juvenile diabetes: normalization by insulin-treatment. Diabetologia 11, 221–224 (1975).
Wiseman, M. J., Saunders, A. J., Keen, H. & Viberti, G. Effect of blood glucose control on increased glomerular filtration rate and kidney size in insulin-dependent diabetes. N. Engl. J. Med. 312, 617–621 (1985).
De Cosmo, S. et al. Glucose-induced changes in renal haemodynamics in proteinuric type 1 (insulin-dependent) diabetic patients: inhibition by acetylsalicilic acid infusion. Diabetologia 36, 622–627 (1993).
Magee, G. M. et al. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia 52, 691–697 (2009).
Ruggenenti, P. et al. Glomerular hyperfiltration and renal disease progression in type 2 diabetes. Diabetes Care 35, 2061–2068 (2012).
Bjornstad, P. et al. Rapid GFR decline is associated with renal hyperfiltration and impaired GFR in adults with Type 1 diabetes. Nephrol. Dial. Transpl. 30, 1706–1711 (2015).
Ficociello, L. H. et al. Renal hyperfiltration and the development of microalbuminuria in type 1 diabetes. Diabetes Care 32, 889–893 (2009).
Thomas, M. C. et al. Hyperfiltration in type 1 diabetes: does it exist and does it matter for nephropathy? Diabetologia 55, 1505–1513 (2012).
Molitch, M. E. et al. Early glomerular hyperfiltration and long-term kidney outcomes in type 1 diabetes: The DCCT/EDIC experience. Clin. J. Am. Soc. Nephrol. 14, 854–861 (2019).
Pugliese, G. et al. Diabetic kidney disease: New clinical and therapeutic issues. Joint position statement of the Italian Diabetes Society and the Italian Society of Nephrology on ‘The natural history of diabetic kidney disease and treatment of hyperglycemia in patients with type 2 diabetes and impaired renal function’. Nutr. Metab. Cardiovasc. Dis. 29, 1127–1150 (2019).
Porrini, E. et al. Non-proteinuric pathways in loss of renal function in patients with type 2 diabetes. Lancet Diabetes Endocrinol. 3, 382–391 (2015).
Kramer, H. J., Nguyen, Q. D., Curhan, G. & Hsu, C.-Y. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA 289, 3273–3277 (2003).
Thomas, M. C. et al. Nonalbuminuric renal impairment in type 2 diabetic patients and in the general population (national evaluation of the frequency of renal impairment co-existing with NIDDM [NEFRON] 11). Diabetes Care 32, 1497–1502 (2009).
Thomas, M. C. et al. Diabetic kidney disease. Nat. Rev. Dis. Prim. 1, 15018 (2015).
Remuzzi, G., Schieppati, A. & Ruggenenti, P. Clinical practice. Nephropathy in patients with type 2 diabetes. N. Engl. J. Med. 346, 1145–1151 (2002).
Alicic, R. Z., Rooney, M. T. & Tuttle, K. R. Diabetic kidney disease: challenges, progress, and possibilities. Clin. J. Am. Soc. Nephrol. 12, 2032–2045 (2017).
Lastra, G., Syed, S., Kurukulasuriya, L. R., Manrique, C. & Sowers, J. R. Type 2 diabetes mellitus and hypertension: an update. Endocrinol. Metab. Clin. North. Am. 43, 103–122 (2014).
Mennuni, S. et al. Hypertension and kidneys: unraveling complex molecular mechanisms underlying hypertensive renal damage. J. Hum. Hypertens. 28, 74–79 (2014).
Lemley, K. V. A basis for accelerated progression of diabetic nephropathy in Pima Indians. Kidney Int. Suppl. https://doi.org/10.1046/j.1523-1755.63.s83.9.x (2003).
Carrara, F. et al. Increased pre-glomerular resistance and kidney hypoperfusion may sustain accelerated GFR decline in hypertensive, type 2 diabetics with normal and high normal albuminuria. Nephrol. Dial. Transpl. 33, 317 (2018).
Lewis, E. J., Hunsicker, L. G., Bain, R. P. & Rohde, R. D. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N. Engl. J. Med. 329, 1456–1462 (1993).
Brenner, B. M. et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 345, 861–869 (2001).
Zatz, R. et al. Prevention of diabetic glomerulopathy by pharmacological amelioration of glomerular capillary hypertension. J. Clin. Invest. 77, 1925–1930 (1986).
Benigni, A., Gagliardini, E. & Remuzzi, G. Changes in glomerular perm-selectivity induced by angiotensin II imply podocyte dysfunction and slit diaphragm protein rearrangement. Semin. Nephrol. 24, 131–140 (2004).
Remuzzi, A. et al. Short- and long-term effect of angiotensin II receptor blockade in rats with experimental diabetes. J. Am. Soc. Nephrol. 4, 40–49 (1993).
Gagliardini, E. et al. Unlike each drug alone, lisinopril if combined with avosentan promotes regression of renal lesions in experimental diabetes. Am. J. Physiol. Renal Physiol. 297, F1448–F1456 (2009).
Perkovic, V. et al. Canagliflozin and renal outcomes in Type 2 diabetes and nephropathy. N. Engl. J. Med. 380, 2295–2306 (2019).
Wanner, C. et al. Empagliflozin and progression of kidney disease in Type 2 Diabetes. N. Engl. J. Med. 375, 323–334 (2016).
Neal, B. et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 377, 644–657 (2017).
Wiviott, S. D. et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 380, 347–357 (2019).
Heerspink, H. J. L. et al. Dapagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 383, 1436–1446 (2020).
Ruggenenti, P. et al. Nephrotic-range proteinuria in type 2 diabetes: effects of empagliflozin on kidney disease progression and clinical outcomes. EClinicalMedicine 43, 101240 (2022).
Cherney, D. Z. I. et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 129, 587–597 (2014).
Kidokoro, K. et al. Evaluation of glomerular hemodynamic function by empagliflozin in diabetic mice using in vivo imaging. Circulation 140, 303–315 (2019).
van Bommel, E. J. M. et al. The renal hemodynamic effects of the SGLT2 inhibitor dapagliflozin are caused by post-glomerular vasodilatation rather than pre-glomerular vasoconstriction in metformin-treated patients with type 2 diabetes in the randomized, double-blind RED trial. Kidney Int. 97, 202–212 (2020).
Ott, C., Kannenkeril, D., Jung, S. & Schnieder, R. Combination therapy of empagliflozin and linagliptin vs. metformin and insulin glargine on intra- and renal hemodynamics in type 2 diabetes [abstract SA-OR083] (ASN Kidney Week, 2019).
Thomson, S. C. & Vallon, V. Effects of SGLT2 inhibitor and dietary NaCl on glomerular hemodynamics assessed by micropuncture in diabetic rats. Am. J. Physiol. Renal Physiol. 320, F761–F771 (2021).
Zhao, Y. et al. Effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on serum uric acid level: a meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 20, 458–462 (2018).
DeFronzo, R. A., Reeves, W. B. & Awad, A. S. Pathophysiology of diabetic kidney disease: impact of SGLT2 inhibitors. Nat. Rev. Nephrol. 17, 319–334 (2021).
Grantham, J. J. Clinical practice. Autosomal dominant polycystic kidney disease. N. Engl. J. Med. 359, 1477–1485 (2008).
Spithoven, E. M. et al. Analysis of data from the ERA-EDTA Registry indicates that conventional treatments for chronic kidney disease do not reduce the need for renal replacement therapy in autosomal dominant polycystic kidney disease. Kidney Int. 86, 1244–1252 (2014).
Grantham, J. J., Chapman, A. B. & Torres, V. E. Volume progression in autosomal dominant polycystic kidney disease: the major factor determining clinical outcomes. Clin. J. Am. Soc. Nephrol. 1, 148–157 (2006).
Grantham, J. J., Mulamalla, S. & Swenson-Fields, K. I. Why kidneys fail in autosomal dominant polycystic kidney disease. Nat. Rev. Nephrol. 7, 556–566 (2011).
Franz, K. A. & Reubi, F. C. Rate of functional deterioration in polycystic kidney disease. Kidney Int. 23, 526–529 (1983).
Chapman, A. B. et al. Renal structure in early autosomal-dominant polycystic kidney disease (ADPKD): the consortium for radiologic imaging studies of polycystic kidney disease (CRISP) cohort. Kidney Int. 64, 1035–1045 (2003).
Grantham, J. J. et al. Volume progression in polycystic kidney disease. N. Engl. J. Med. 354, 2122–2130 (2006).
Fick-Brosnahan, G. M., Belz, M. M., McFann, K. K., Johnson, A. M. & Schrier, R. W. Relationship between renal volume growth and renal function in autosomal dominant polycystic kidney disease: a longitudinal study. Am. J. Kidney Dis. 39, 1127–1134 (2002).
King, B. F., Reed, J. E., Bergstralh, E. J., Sheedy, P. F. & Torres, V. E. Quantification and longitudinal trends of kidney, renal cyst, and renal parenchyma volumes in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 11, 1505–1511 (2000).
Meijer, E. et al. Early renal abnormalities in autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 5, 1091–1098 (2010).
Torres, V. E. et al. Magnetic resonance measurements of renal blood flow and disease progression in autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 2, 112–120 (2007).
Wong, H., Vivian, L., Weiler, G. & Filler, G. Patients with autosomal dominant polycystic kidney disease hyperfiltrate early in their disease. Am. J. Kidney Dis. 43, 624–628 (2004).
Helal, I. et al. Glomerular hyperfiltration and renal progression in children with autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 6, 2439–2443 (2011).
Messchendorp, A. L. et al. Kidney function reserve capacity in early and later stage autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 13, 1680–1692 (2018).
Gentile, G., Mastroluca, D., Perna, A., Remuzzi, G. & Ruggenenti, P. Glomerular hyperfiltration is a common risk factor for accelerated GFR decline in young adults with autosomal polycystic kidney disease (ADPKD) (American Society of Nephrology (ASN) Kidney Week 2014).
Caroli, A. et al. Effect of longacting somatostatin analogue on kidney and cyst growth in autosomal dominant polycystic kidney disease (ALADIN): a randomised, placebo-controlled, multicentre trial. Lancet 382, 1485–1495 (2013).
Brouhard, B. H., LaGrone, L. F., Richards, G. E. & Travis, L. B. Somatostatin limits rise in glomerular filtration rate after a protein meal. J. Pediatr. 110, 729–734 (1987).
Vora, J. et al. Renal response to intravenous somatostatin in insulin-dependent diabetic patients and normal subjects. J. Clin. Endocrinol. Metab. 64, 975–979 (1987).
Ginès, A. et al. Effects of somatostatin on renal function in cirrhosis. Gastroenterology 103, 1868–1874 (1992).
Colao, A. et al. A 12-month phase 3 study of pasireotide in Cushing’s disease. N. Engl. J. Med. 366, 914–924 (2012).
Remuzzi, G., Perico, N., Macia, M. & Ruggenenti, P. The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int. Suppl. https://doi.org/10.1111/j.1523-1755.2005.09911.x (2005).
Chapman, A. B., Johnson, A., Gabow, P. A. & Schrier, R. W. The renin-angiotensin-aldosterone system and autosomal dominant polycystic kidney disease. N. Engl. J. Med. 323, 1091–1096 (1990).
Loghman-Adham, M., Soto, C. E., Inagami, T. & Cassis, L. The intrarenal renin-angiotensin system in autosomal dominant polycystic kidney disease. Am. J. Physiol. Renal Physiol. 287, F775–F788 (2004).
Cadnapaphornchai, M. A., McFann, K., Strain, J. D., Masoumi, A. & Schrier, R. W. Prospective change in renal volume and function in children with ADPKD. Clin. J. Am. Soc. Nephrol. 4, 820–829 (2009).
Schrier, R. W. et al. Blood pressure in early autosomal dominant polycystic kidney disease. N. Engl. J. Med. 371, 2255–2266 (2014).
van Gastel, M. D. A. & Torres, V. E. Polycystic kidney disease and the vasopressin pathway. Ann. Nutr. Metab. 70, 43–50 (2017).
Bankir, L., Bouby, N. & Ritz, E. Vasopressin: a novel target for the prevention and retardation of kidney disease? Nat. Rev. Nephrol. 9, 223–239 (2013).
Irazabal, M. V. et al. Short-term effects of tolvaptan on renal function and volume in patients with autosomal dominant polycystic kidney disease. Kidney Int. 80, 295–301 (2011).
Boertien, W. E. et al. Short-term renal hemodynamic effects of tolvaptan in subjects with autosomal dominant polycystic kidney disease at various stages of chronic kidney disease. Kidney Int. 84, 1278–1286 (2013).
Takahashi, N. et al. Vasopressin stimulates Cl- transport in ascending thin limb of Henle’s loop in hamster. J. Clin. Invest. 95, 1623–1627 (1995).
Mutig, K. et al. Vasopressin V2 receptor expression along rat, mouse, and human renal epithelia with focus on TAL. Am. J. Physiol. Renal Physiol. 293, F1166–F1177 (2007).
Gabow, P. A. et al. The clinical utility of renal concentrating capacity in polycystic kidney disease. Kidney Int. 35, 675–680 (1989).
Torres, V. E., Wilson, D. M., Offord, K. P., Burnett, J. C. & Romero, J. C. Natriuretic response to volume expansion in polycystic kidney disease. Mayo Clin. Proc. 64, 509–515 (1989).
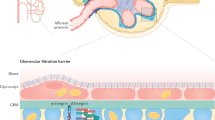
Deen, W. M., Lazzara, M. J. & Myers, B. D. Structural determinants of glomerular permeability. Am. J. Physiol. Renal Physiol. 281, F579–F596 (2001).
Dane, M. J. C. et al. Glomerular endothelial surface layer acts as a barrier against albumin filtration. Am. J. Pathol. 182, 1532–1540 (2013).
Kawachi, H. et al. Role of podocyte slit diaphragm as a filtration barrier. Nephrology 11, 274–281 (2006).
Tryggvason, K. & Wartiovaara, J. Molecular basis of glomerular permselectivity. Curr. Opin. Nephrol. Hypertens. 10, 543–549 (2001).
Srivastava, T. et al. Hyperfiltration-mediated injury in the remaining kidney of a transplant donor. Transplantation 102, 1624–1635 (2018).
Chagnac, A., Zingerman, B., Rozen-Zvi, B. & Herman-Edelstein, M. Consequences of glomerular hyperfiltration: the role of physical forces in the pathogenesis of chronic kidney disease in diabetes and obesity. Nephron 143, 38–42 (2019).
Kriz, W. & Endlich, K. Podocytes and disease: introduction. Semin. Nephrol. 32, 305–306 (2012).
Mundel, P. & Shankland, S. J. Podocyte biology and response to injury. J. Am. Soc. Nephrol. 13, 3005–3015 (2002).
Kriz, W., Gretz, N. & Lemley, K. V. Progression of glomerular diseases: is the podocyte the culprit? Kidney Int. 54, 687–697 (1998).
Fries, J. W., Sandstrom, D. J., Meyer, T. W. & Rennke, H. G. Glomerular hypertrophy and epithelial cell injury modulate progressive glomerulosclerosis in the rat. Lab. Invest. 60, 205–218 (1989).
Kriz, W. & Lemley, K. V. The role of the podocyte in glomerulosclerosis. Curr. Opin. Nephrol. Hypertens. 8, 489–497 (1999).
Pabst, R. & Sterzel, R. B. Cell renewal of glomerular cell types in normal rats. An autoradiographic analysis. Kidney Int. 24, 626–631 (1983).
Lemley, K. V. Mechanical challenges to the glomerulus and podocyte loss: evolution of a paradigm. Pflugers Arch. 469, 959–963 (2017).
Gagliardini, E., Conti, S., Benigni, A., Remuzzi, G. & Remuzzi, A. Imaging of the porous ultrastructure of the glomerular epithelial filtration slit. J. Am. Soc. Nephrol. 21, 2081–2089 (2010).
Rice, W. L. et al. High resolution helium ion scanning microscopy of the rat kidney. PLoS One 8, e57051 (2013).
Tsuji, K. et al. Re-characterization of the glomerulopathy in CD2AP deficient mice by high-resolution helium ion scanning microscopy. Sci. Rep. 7, 8321 (2017).
Rodewald, R. & Karnovsky, M. J. Porous substructure of the glomerular slit diaphragm in the rat and mouse. J. Cell Biol. 60, 423–433 (1974).
Butt, L. et al. A molecular mechanism explaining albuminuria in kidney disease. Nat. Metab. 2, 461–474 (2020).
Kriz, W. & Lemley, K. V. Mechanical challenges to the glomerular filtration barrier: adaptations and pathway to sclerosis. Pediatr. Nephrol. 32, 405–417 (2017).
Benzing, T. & Salant, D. Insights into glomerular filtration and albuminuria. N. Engl. J. Med. 384, 1437–1446 (2021).
Neal, C. R. et al. Glomerular filtration into the subpodocyte space is highly restricted under physiological perfusion conditions. Am. J. Physiol. Renal Physiol. 293, F1787–F1798 (2007).
Remuzzi, A., Puntorieri, S., Mazzoleni, A. & Remuzzi, G. Sex related differences in glomerular ultrafiltration and proteinuria in Munich-Wistar rats. Kidney Int. 34, 481–486 (1988).
Remuzzi, A. et al. Role of ultrastructural determinants of glomerular permeability in ultrafiltration function loss. JCI Insight 5, 137249 (2020).
Fall, B. et al. Urinary podocyte loss is increased in patients with Fabry disease and correlates with clinical severity of Fabry nephropathy. PLoS One 11, e0168346 (2016).
Mella, A. et al. Detection of urinary podocytes by flow cytometry in idiopathic membranous nephropathy. Sci. Rep. 10, 16362 (2020).
Vogelmann, S. U., Nelson, W. J., Myers, B. D. & Lemley, K. V. Urinary excretion of viable podocytes in health and renal disease. Am. J. Physiol. Renal Physiol. 285, F40–F48 (2003).
Hayslett, J. P. Functional adaptation to reduction in renal mass. Physiol. Rev. 59, 137–164 (1979).
Deen, W. M., Maddox, D. A., Robertson, C. R. & Brenner, B. M. Dynamics of glomerular ultrafiltration in the rat. VII. Response to reduced renal mass. Am. J. Physiol. 227, 556–562 (1974).
Kaufman, J. M., Siegel, N. J. & Hayslett, J. P. Functional and hemodynamic adaptation to progressive renal ablation. Circ. Res. 36, 286–293 (1975).
Hostetter, T. H., Rennke, H. G. & Brenner, B. M. Compensatory renal hemodynamic injury: a final common pathway of residual nephron destruction. Am. J. Kidney Dis. 1, 310–314 (1982).
Morrison, A. B. Experimental chronic renal insufficiency. Methods Achiev. Exp. Pathol. 1, 455–475 (1966).
Purkerson, M. L., Hoffsten, P. E. & Klahr, S. Pathogenesis of the glomerulopathy associated with renal infarction in rats. Kidney Int. 9, 407–417 (1976).
Shea, S. M., Raskova, J. & Morrison, A. B. A stereologic study of glomerular hypertrophy in the subtotally nephrectomized rat. Am. J. Pathol. 90, 201–210 (1978).
Shimamura, T. & Morrison, A. B. A progressive glomerulosclerosis occurring in partial five-sixths nephrectomized rats. Am. J. Pathol. 79, 95–106 (1975).
Hostetter, T. H., Olson, J. L., Rennke, H. G., Venkatachalam, M. A. & Brenner, B. M. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am. J. Physiol. 241, F85–F93 (1981).
Anderson, S., Meyer, T. W., Rennke, H. G. & Brenner, B. M. Control of glomerular hypertension limits glomerular injury in rats with reduced renal mass. J. Clin. Invest. 76, 612–619 (1985).
Remuzzi, A., Puntorieri, S., Battaglia, C., Bertani, T. & Remuzzi, G. Angiotensin converting enzyme inhibition ameliorates glomerular filtration of macromolecules and water and lessens glomerular injury in the rat. J. Clin. Invest. 85, 541–549 (1990).
Wachtel, L. W., Cole, L. J. & Rosen, V. J. X-ray-induced glomerulosclerosis in rats: modification of lesion by food restriction, uninephrectomy, and age. J. Gerontol. 21, 442–448 (1966).
Teodoru, C. V., Saifer, A. & Frankel, H. Conditioning factors influencing evolution of experimental glomerulonephritis in rabbits. Am. J. Physiol. 196, 457–460 (1959).
Velosa, J. A., Glasser, R. J., Nevins, T. E. & Michael, A. F. Experimental model of focal sclerosis. II. Correlation with immunopathologic changes, macromolecular kinetics, and polyanion loss. Lab. Invest. 36, 527–534 (1977).
Beyer, M. M., Steinberg, A. D., Nicastri, A. D. & Friedman, E. A. Unilateral nephrectomy: effect on survival in NZB/NZW mice. Science 198, 511–513 (1977).
Schreuder, M. F., Westland, R. & van Wijk, J. A. E. Unilateral multicystic dysplastic kidney: a meta-analysis of observational studies on the incidence, associated urinary tract malformations and the contralateral kidney. Nephrol. Dial. Transpl. 24, 1810–1818 (2009).
Westland, R., Schreuder, M. F., Ket, J. C. F. & van Wijk, J. A. E. Unilateral renal agenesis: a systematic review on associated anomalies and renal injury. Nephrol. Dial. Transpl. 28, 1844–1855 (2013).
van Vuuren, S. H. et al. Compensatory enlargement of a solitary functioning kidney during fetal development. Ultrasound Obstet. Gynecol. 40, 665–668 (2012).
Abou Jaoudé, P. et al. Congenital versus acquired solitary kidney: is the difference relevant? Nephrol. Dial. Transpl. 26, 2188–2194 (2011).
Westland, R., Kurvers, R. A. J., van Wijk, J. A. E. & Schreuder, M. F. Risk factors for renal injury in children with a solitary functioning kidney. Pediatrics 131, e478–e485 (2013).
Sanna-Cherchi, S. et al. Renal outcome in patients with congenital anomalies of the kidney and urinary tract. Kidney Int. 76, 528–533 (2009).
Abitbol, C. L. & Moxey-Mims, M. Chronic kidney disease: Low birth weight and the global burden of kidney disease. Nat. Rev. Nephrol. 12, 199–200 (2016).
Luyckx, V. A. & Brenner, B. M. Clinical consequences of developmental programming of low nephron number. Anat. Rec. 303, 2613–2631 (2020).
Ojo, A. Addressing the global burden of chronic kidney disease through clinical and translational research. Trans. Am. Clin. Climatol. Assoc. 125, 229–246 (2014).
Luyckx, V. A. & Brenner, B. M. Birth weight, malnutrition and kidney-associated outcomes–a global concern. Nat. Rev. Nephrol. 11, 135–149 (2015).
Seymour, A. E. Glomerular disease in whites versus Australian aboriginals: ‘last of a race in ruin’ (G.K. Chesterton). Nephrol. Dial. Transpl. 10, 769–770 (1995).
Thomas, M. Deprivation and dialysis: pathways to kidney failure in Australian Aborigines. Adv. Chronic Kidney Dis. 12, 84–87 (2005).
Hoy, W. E. et al. The influence of birthweight, past poststreptococcal glomerulonephritis and current body mass index on levels of albuminuria in young adults: the multideterminant model of renal disease in a remote Australian Aboriginal population with high rates of renal disease and renal failure. Nephrol. Dial. Transpl. 31, 971–977 (2016).
Hoy, W. E. et al. The multidimensional nature of renal disease: rates and associations of albuminuria in an Australian Aboriginal community. Kidney Int. 54, 1296–1304 (1998).
Stengel, B., Tarver-Carr, M. E., Powe, N. R., Eberhardt, M. S. & Brancati, F. L. Lifestyle factors, obesity and the risk of chronic kidney disease. Epidemiology 14, 479–487 (2003).
Nenov, V. D., Taal, M. W., Sakharova, O. V. & Brenner, B. M. Multi-hit nature of chronic renal disease. Curr. Opin. Nephrol. Hypertens. 9, 85–97 (2000).
Lenihan, C. R. et al. Longitudinal study of living kidney donor glomerular dynamics after nephrectomy. J. Clin. Invest. 125, 1311–1318 (2015).
Kasiske, B. L. et al. A prospective controlled study of metabolic and physiologic effects of kidney donation suggests that donors retain stable kidney function over the first nine years. Kidney Int. 98, 168–175 (2020).
Gill, J. S. & Tonelli, M. Understanding rare adverse outcomes following living kidney donation. JAMA 311, 577–579 (2014).
Steiner, R. GFR-related risks for kidney donors are here to stay, but what are they? Am. J. Transpl. 18, 2612 (2018).
Muzaale, A. D. et al. Risk of end-stage renal disease following live kidney donation. JAMA 311, 579–586 (2014).
Mjøen, G. et al. Long-term risks for kidney donors. Kidney Int. 86, 162–167 (2014).
Anjum, S. et al. Patterns of end-stage renal disease caused by diabetes, hypertension, and glomerulonephritis in live kidney donors. Am. J. Transpl. 16, 3540–3547 (2016).
Li, L. et al. Risk of chronic kidney disease after cancer nephrectomy. Nat. Rev. Nephrol. 10, 135–145 (2014).
Timsit, M.-O. et al. Kidney function following nephrectomy: similitude and discrepancies between kidney cancer and living donation. Urol. Oncol. 30, 482–486 (2012).
Miller, A. J., Kiberd, B. A., Alwayn, I. P., Odutayo, A. & Tennankore, K. K. Donor-recipient weight and sex mismatch and the risk of graft loss in renal transplantation. Clin. J. Am. Soc. Nephrol. 12, 669–676 (2017).
Giral, M. et al. Kidney and recipient weight incompatibility reduces long-term graft survival. J. Am. Soc. Nephrol. 21, 1022–1029 (2010).
Kasiske, B. L., Snyder, J. J. & Gilbertson, D. Inadequate donor size in cadaver kidney transplantation. J. Am. Soc. Nephrol. 13, 2152–2159 (2002).
el-Agroudy, A. E. et al. Effect of donor/recipient body weight mismatch on patient and graft outcome in living-donor kidney transplantation. Am. J. Nephrol. 23, 294–299 (2003).
Ghafari, A., Etemadi, J. & Ardalan, M. Impact of donor/recipient body weight mismatch on allograft outcome in renal transplant recipients. Transpl. Proc. 40, 135–136 (2008).
Miles, A. M. et al. The effect of kidney size on cadaveric renal allograft outcome. Transplantation 61, 894–897 (1996).
Brenner, B. M., Cohen, R. A. & Milford, E. L. In renal transplantation, one size may not fit all. J. Am. Soc. Nephrol. 3, 162–169 (1992).
Brenner, B. M. & Milford, E. L. Nephron underdosing: a programmed cause of chronic renal allograft failure. Am. J. Kidney Dis. 21, 66–72 (1993).
GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 395, 709–733 (2020).
Ruggenenti, P., Schieppati, A. & Remuzzi, G. Progression, remission, regression of chronic renal diseases. Lancet 357, 1601–1608 (2001).
Ruggenenti, P. et al. Role of remission clinics in the longitudinal treatment of CKD. J. Am. Soc. Nephrol. 19, 1213–1224 (2008).
Brøchner-Mortensen, J., Rickers, H. & Balslev, I. Renal function and body composition before and after intestinal bypass operation in obese patients. Scand. J. Clin. Lab. Invest. 40, 695–702 (1980).
Clerte, M. et al. The measured glomerular filtration rate (mGFR) before and 6 months after bariatric surgery: a pilot study. Nephrol. Ther. 13, 160–167 (2017).
Chuah, L. L. et al. Measurement of glomerular filtration rate in patients undergoing obesity surgery. BMC Nephrol. 19, 383 (2018).
Acknowledgements
The authors wish to thank Kerstin Mierke (Istituto di Ricerche Farmacologiche Mario Negri IRCCS, Italy) for her help with English language editing of the manuscript before submission.
Author information
Authors and Affiliations
Contributions
M.C. and N.P. researched the data for the article. M.C., N.P., P.R., A.R. and G.R. wrote the text and reviewed or edited the manuscript before submission. All authors made substantial contributions to discussions of the content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks M. Praga, H. Trujillo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Microalbuminuria
-
A moderate increase in albuminuria indicating incipient nephropathy.
- Macroalbuminuria
-
A severe increase in albuminuria characteristic of overt nephropathy.
Rights and permissions
About this article
Cite this article
Cortinovis, M., Perico, N., Ruggenenti, P. et al. Glomerular hyperfiltration. Nat Rev Nephrol 18, 435–451 (2022). https://doi.org/10.1038/s41581-022-00559-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-022-00559-y
This article is cited by
-
Threshold-modifying effect of the systemic inflammatory response index on kidney function decline in hypertensive patients
European Journal of Medical Research (2024)
-
Application of nanotechnology in the treatment of glomerulonephritis: current status and future perspectives
Journal of Nanobiotechnology (2024)
-
Sirtuins in kidney health and disease
Nature Reviews Nephrology (2024)
-
Development of a formula for estimated glomerular filtration rate in pregnant women from physiological hyperfiltration of serum creatinine
Scientific Reports (2024)
-
The incidence and outcome of hypertension at diagnosis in children with a kidney tumor
Pediatric Nephrology (2024)