Abstract
Kidney lifespan is a patient-oriented outcome that provides much needed context for understanding chronic kidney disease (CKD). Nephron endowment, age-associated decline in nephron number, kidney injury history and the intrinsic capacity of nephrons to adapt to haemodynamic and metabolic overload vary widely within the population. Defining percentiles of kidney function might therefore help to predict individual kidney lifespan and distinguish healthy ageing from progressive forms of CKD. In response to nephron loss, the remaining nephrons undergo functional and structural adaptations to meet the ongoing haemodynamic and metabolic demands of the organism. When these changes are no longer sufficient to maintain kidney cell homeostasis, remnant nephron demise occurs and CKD progression ensues. An individual’s trajectory of glomerular filtration rate and albuminuria reflects the extent of nephron loss and adaptation of the remaining nephrons. Nephron overload represents the final common pathway of CKD progression and is largely independent of upstream disease mechanisms. Thus, interventions that efficiently attenuate nephron overload in early disease stages can protect remnant kidney cells and nephrons, and delay CKD progression. This Review provides a conceptual framework for individualized diagnosis, monitoring and treatment of CKD with the goal of maximizing kidney lifespan.
Key points
-
The current chronic kidney disease (CKD) classification is useful from the perspective of epidemiology, public health care and advocacy. Kidney lifespan is a more individualized, patient-oriented outcome that takes into account linear and non-linear declines in estimated glomerular filtration rate (eGFR) for the prediction of individual prognoses.
-
Population percentiles of eGFR acknowledge its age-specific spectrum and, along with individualized eGFR slopes, could help distinguish between healthy kidney ageing and progressive CKD. Percentiles are population-specific and should help identify patients at risk of CKD, as well as improve patient prognosis and management.
-
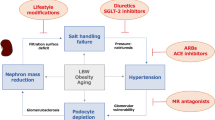
Adaptation to haemodynamic and metabolic overload is observed in the remaining nephrons in CKD but not in physiological kidney ageing. Adaptation is first evidenced by nephron hypertrophy and later by albuminuria.
-
Haemodynamic stress promotes podocyte loss directly and metabolic stress is a key driver of loss of tubular epithelial cells. Both types of stress can lead to secondary focal segmental glomerulosclerosis, tubular atrophy and interstitial fibrosis.
-
Progressive nephron loss reduces kidney lifespan. Dual blockade of the renin–angiotensin–aldosterone system and of sodium–glucose co-transporter 2 is very potent in both alleviating mechanisms of stress and prolonging kidney lifespan, and hence CKD has become a treatable disease.
-
Prolonging kidney lifespan with novel combination therapies is effective in patients with glomerular forms of CKD. Broad implementation of this approach requires effort at all levels, including improving our ability to assess and predict individual kidney lifespan, implementing remnant nephron overload as a pathophysiological concept and a treatment target, and raising awareness that CKD is a treatable disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kidney Disease: Improving Global Outcomes CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 3, 1–150 (2013).
Benghanem Gharbi, M. et al. Chronic kidney disease, hypertension, diabetes, and obesity in the adult population of Morocco: how to avoid “over”- and “under”-diagnosis of CKD. Kidney Int. 89, 1363–1371 (2016).
Jonsson, A. J., Lund, S. H., Eriksen, B. O., Palsson, R. & Indridason, O. S. The prevalence of chronic kidney disease in Iceland according to KDIGO criteria and age-adapted estimated glomerular filtration rate thresholds. Kidney Int. 98, 1286–1295 (2020).
Hill, N. R. et al. Global prevalence of chronic kidney disease–a systematic review and meta-analysis. PLoS ONE 11, e0158765 (2016).
Matsushita, K. et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 3, 514–525 (2015).
Figurek, A., Luyckx, V. A. & Mueller, T. F. A systematic review of renal functional reserve in adult living kidney donors. Kidney Int. Rep. 5, 448–458 (2020).
Zelmer, J. L. The economic burden of end-stage renal disease in Canada. Kidney Int. 72, 1122–1129 (2007).
Levey, A. S., Stevens, L. A. & Hostetter, T. Automatic reporting of estimated glomerular filtration rate–just what the doctor ordered. Clin. Chem. 52, 2188–2193 (2006).
Delanaye, P., Cavalier, E. & Pottel, H. Serum creatinine: not so simple! Nephron 136, 302–308 (2017).
Porrini, E. et al. Estimated GFR: time for a critical appraisal. Nat. Rev. Nephrol. 15, 177–190 (2019).
Bello, A. K. et al. Quality of chronic kidney disease management in Canadian primary care. JAMA Netw. Open 2, e1910704 (2019).
Hallan, S. I. et al. Age and association of kidney measures with mortality and end-stage renal disease. JAMA 308, 2349–2360 (2012).
Delanaye, P. et al. CKD: a call for an age-adapted definition. J. Am. Soc. Nephrol. 30, 1785–1805 (2019).
Denic, A., Glassock, R. J. & Rule, A. D. Structural and functional changes with the aging kidney. Adv. Chronic Kidney Dis. 23, 19–28 (2016).
Welch, H. G., Schwartz, L. M. & Woloshin, S. Overdiagnosed: Making People Sick in the Pursuit of Health (Beacon Press, 2011).
Liu, P. et al. Accounting for age in the definition of chronic kidney disease. JAMA Intern. Med. 181, 1359–1366 (2021).
Daugirdas, J. T., Meyer, K., Greene, T., Butler, R. S. & Poggio, E. D. Scaling of measured glomerular filtration rate in kidney donor candidates by anthropometric estimates of body surface area, body water, metabolic rate, or liver size. Clin. J. Am. Soc. Nephrol. 4, 1575–1583 (2009).
Denic, A. et al. Single-nephron glomerular filtration rate in healthy adults. N. Engl. J. Med. 376, 2349–2357 (2017).
Khan, S., Loi, V. & Rosner, M. H. Drug-induced kidney injury in the elderly. Drugs Aging 34, 729–741 (2017).
Eriksen, B. O. et al. Blood pressure and age-related GFR decline in the general population. BMC Nephrol. 18, 77 (2017).
Palsson, R. & Waikar, S. S. Renal functional reserve revisited. Adv. Chronic Kidney Dis. 25, e1–e8 (2018).
Hughson, M., Farris, A. B. III, Douglas-Denton, R., Hoy, W. E. & Bertram, J. F. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int. 63, 2113–2122 (2003).
Luyckx, V. A. et al. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet 382, 273–283 (2013).
Harer, M. W., Charlton, J. R., Tipple, T. E. & Reidy, K. J. Preterm birth and neonatal acute kidney injury: implications on adolescent and adult outcomes. J. Perinatol. 40, 1286–1295 (2020).
Crump, C., Sundquist, J., Winkleby, M. A. & Sundquist, K. Preterm birth and risk of chronic kidney disease from childhood into mid-adulthood: national cohort study. BMJ 365, l1346 (2019).
Ruggajo, P. et al. Familial factors, low birth weight, and development of ESRD: a nationwide registry study. Am. J. Kidney Dis. 67, 601–608 (2016).
Low Birth Weight and Nephron Number Working Group The impact of kidney development on the life course: a consensus document for action. Nephron 136, 3–49 (2017).
Abitbol, C. L. & Ingelfinger, J. R. Nephron mass and cardiovascular and renal disease risks. Semin. Nephrol. 29, 445–454 (2009).
Wiles, K. et al. The impact of chronic kidney disease stages 3-5 on pregnancy outcomes. Nephrol. Dialysis Transpl. https://doi.org/10.1093/ndt/gfaa247 (2020).
Coca, S. G., Singanamala, S. & Parikh, C. R. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 81, 442–448 (2012).
Kellum, J. A. et al. Acute kidney injury. Nat. Rev. Dis. Prim. 7, 52 (2021).
Newsome, B. B. et al. Long-term risk of mortality and end-stage renal disease among the elderly after small increases in serum creatinine level during hospitalization for acute myocardial infarction. Arch. Intern. Med. 168, 609–616 (2008).
Murphy, D. et al. Trends in prevalence of chronic kidney disease in the United States. Ann. Intern. Med. 165, 473–481 (2016).
Gregg, E. W. et al. Changes in diabetes-related complications in the United States, 1990-2010. N. Engl. J. Med. 370, 1514–1523 (2014).
Wakasugi, M., Kazama, J. J. & Narita, I. Secular trends in end-stage kidney disease requiring renal replacement therapy in Japan: Japanese Society of Dialysis Therapy Registry data from 1983 to 2016. Nephrology 25, 172–178 (2020).
Romagnani, P. et al. Chronic kidney disease. Nat. Rev. Dis. Prim. 3, 17088 (2017).
Brenner, B. M., Garcia, D. L. & Anderson, S. Glomeruli and blood pressure. Less of one, more the other? Am. J. Hypertens. 1, 335–347 (1988).
Freedman, B. I., Limou, S., Ma, L. & Kopp, J. B. APOL1-associated nephropathy: a key contributor to racial disparities in CKD. Am. J. Kidney Dis. 72, S8–S16 (2018).
Luyckx, V. A. et al. Sustainable development goals relevant to kidney health: an update on progress. Nat. Rev. Nephrol. 17, 15–32 (2021).
Hostetter, T. H., Olson, J. L., Rennke, H. G., Venkatachalam, M. A. & Brenner, B. M. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. J. Am. Soc. Nephrol. 12, 1315–1325 (2001).
Giral, M. et al. Kidney and recipient weight incompatibility reduces long-term graft survival. J. Am. Soc. Nephrol. 21, 1022–1029 (2010).
Al-Sehli, R. et al. What should the serum creatinine be after transplantation? An approach to integrate donor and recipient information to assess posttransplant kidney function. Transplant 99, 1960–1967 (2015).
Diao, J. A. et al. In search of a better equation–performance and equity in estimates of kidney function. N. Engl. J. Med. 384, 396–399 (2021).
Inker, L. A. et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N. Engl. J. Med. 385, 1737–1749 (2021).
Peralta, C. A. et al. Racial and ethnic differences in kidney function decline among persons without chronic kidney disease. J. Am. Soc. Nephrol. 22, 1327–1334 (2011).
Glassock, R., Denic, A. & Rule, A. D. In Brenner and Rector’s The Kidney 11th edn, Ch. 22 (ed. Yu, A. et al.) 710–730 (Elsevier, 2019).
Baba, M. et al. Longitudinal study of the decline in renal function in healthy subjects. PLoS ONE 10, e0129036 (2015).
Carlström, M., Wilcox, C. S. & Arendshorst, W. J. Renal autoregulation in health and disease. Physiol. Rev. 95, 405–511 (2015).
Anders, H. J., Davis, J. M. & Thurau, K. Nephron protection in diabetic kidney disease. N. Engl. J. Med. 375, 2096–2098 (2016).
Vallon, V. The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annu. Rev. Med. 66, 255–270 (2015).
Firsov, D. & Bonny, O. Circadian rhythms and the kidney. Nat. Rev. Nephrol. 14, 626–635 (2018).
Bosch, J. P. et al. Renal functional reserve in humans. Effect of protein intake on glomerular filtration rate. Am. J. Med. 75, 943–950 (1983).
Kriz, W. & Lemley, K. V. A potential role for mechanical forces in the detachment of podocytes and the progression of CKD. J. Am. Soc. Nephrol. 26, 258–269 (2015).
Ichikawa, I., Hoyer, J. R., Seiler, M. W. & Brenner, B. M. Mechanism of glomerulotubular balance in the setting of heterogeneous glomerular injury. Preservation of a close functional linkage between individual nephrons and surrounding microvasculature. J. Clin. Invest. 69, 185–198 (1982).
Rule, A. D. et al. The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann. Intern. Med. 152, 561–567 (2010).
Pontzer, H. et al. Daily energy expenditure through the human life course. Science 373, 808–812 (2021).
Locke, J. E. et al. Obesity increases the risk of end-stage renal disease among living kidney donors. Kidney Int. 91, 699–703 (2017).
Mueller, T. F. & Luyckx, V. A. The natural history of residual renal function in transplant donors. J. Am. Soc. Nephrol. 23, 1462–1466 (2012).
Lenihan, C. R. et al. Longitudinal study of living kidney donor glomerular dynamics after nephrectomy. J. Clin. Invest. 125, 1311–1318 (2015).
Strieder, T. et al. Effects of perfusion pressures on podocyte loss in the isolated perfused mouse kidney. Cell. Physiol. Biochem. 55, 1–12 (2021).
Hughson, M. D., Hoy, W. E., Douglas-Denton, R. N., Zimanyi, M. A. & Bertram, J. F. Towards a definition of glomerulomegaly: clinical-pathological and methodological considerations. Nephrol. Dialysis Transpl. 26, 2202–2208 (2011).
Liapis, H., Romagnani, P. & Anders, H. J. New insights into the pathology of podocyte loss: mitotic catastrophe. Am. J. Pathol. 183, 1364–1374 (2013).
Hodgin, J. B. et al. Glomerular aging and focal global glomerulosclerosis: a podometric perspective. J. Am. Soc. Nephrol. 26, 3162–3178 (2015).
Kopp, J. B. et al. Podocytopathies. Nat. Rev. Dis. Prim. 6, 68 (2020).
Benz, K. et al. Early glomerular alterations in genetically determined low nephron number. Am. J. Physiol. Ren. Physiol. 300, F521–F530 (2011).
Butt, L. et al. A molecular mechanism explaining albuminuria in kidney disease. Nat. Metab. 2, 461–474 (2020).
Fine, L. G. & Norman, J. Cellular events in renal hypertrophy. Annu. Rev. Physiol. 51, 19–32 (1989).
Fine, L. G., Schlondorff, D., Trizna, W., Gilbert, R. M. & Bricker, N. S. Functional profile of the isolated uremic nephron. Impaired water permeability and adenylate cyclase responsiveness of the cortical collecting tubule to vasopressin. J. Clin. Invest. 61, 1519–1527 (1978).
Denic, A. et al. Clinical and pathology findings associate consistently with larger glomerular volume. J. Am. Soc. Nephrol. 29, 1960–1969 (2018).
Menn-Josephy, H. et al. Renal interstitial fibrosis: an imperfect predictor of kidney disease progression in some patient cohorts. Am. J. Nephrol. 44, 289–299 (2016).
Abedini, A. et al. Urinary single-cell profiling captures the cellular diversity of the kidney. J. Am. Soc. Nephrol. 32, 614–627 (2021).
Kriz, W. & Lemley, K. V. Potential relevance of shear stress for slit diaphragm and podocyte function. Kidney Int. 91, 1283–1286 (2017).
Ryu, M., Mulay, S. R., Miosge, N., Gross, O. & Anders, H. J. Tumour necrosis factor-α drives Alport glomerulosclerosis in mice by promoting podocyte apoptosis. J. Pathol. 226, 120–131 (2012).
Tao, J., Polumbo, C., Reidy, K., Sweetwyne, M. & Susztak, K. A multicolor podocyte reporter highlights heterogeneous podocyte changes in focal segmental glomerulosclerosis. Kidney Int. 85, 972–980 (2014).
Wickman, L. et al. Urine podocyte mRNAs, proteinuria, and progression in human glomerular diseases. J. Am. Soc. Nephrol. 24, 2081–2095 (2013).
Ruggenenti, P., Cravedi, P. & Remuzzi, G. Mechanisms and treatment of CKD. J. Am. Soc. Nephrol. 23, 1917–1928 (2012).
Ma, Q., Steiger, S. & Anders, H. J. Sodium glucose transporter-2 inhibition has no renoprotective effects on non-diabetic chronic kidney disease. Physiol. Rep. 5, e13228 (2017).
De Chiara, L., Lazzeri, E. & Romagnani, P. Tubular epithelial cell polyploidy is essential to survive AKI but it contributes to CKD progression. Nephrol. Dialysis Transpl. Assoc. 36, i29–i31 (2021).
Câmara, N. O., Iseki, K., Kramer, H., Liu, Z. H. & Sharma, K. Kidney disease and obesity: epidemiology, mechanisms and treatment. Nat. Rev. Nephrol. 13, 181–190 (2017).
Helal, I., Fick-Brosnahan, G. M., Reed-Gitomer, B. & Schrier, R. W. Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat. Rev. Nephrol. 8, 293–300 (2012).
Tuttle, K. R. et al. Effect of strict glycemic control on renal hemodynamic response to amino acids and renal enlargement in insulin-dependent diabetes mellitus. N. Engl. J. Med. 324, 1626–1632 (1991).
Anders, H. J., Huber, T. B., Isermann, B. & Schiffer, M. CKD in diabetes: diabetic kidney disease versus nondiabetic kidney disease. Nat. Rev. Nephrol. 14, 361–377 (2018).
Brenner, B. M., Meyer, T. W. & Hostetter, T. H. Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N. Engl. J. Med. 307, 652–659 (1982).
Hummel, D. et al. Dihydropyridine calcium antagonists are associated with increased albuminuria in treatment-resistant hypertensives. J. Nephrol. 23, 563–568 (2010).
Richardson, K. L. et al. L-type calcium channel blocker use and proteinuria among children with chronic kidney diseases. Pediatr. Nephrol. 36, 2411–2419 (2021).
Gashti, C. N. & Bakris, G. L. The role of calcium antagonists in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 13, 155–161 (2004).
Pergola, P. E. et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N. Engl. J. Med. 365, 327–336 (2011).
Baigent, C. & Lennon, R. Should we increase GFR with bardoxolone in Alport syndrome? J. Am. Soc. Nephrol. 29, 357–359 (2018).
Himmelfarb, J. & Tuttle, K. R. New therapies for diabetic kidney disease. N. Engl. J. Med. 369, 2549–2550 (2013).
Silva-Islas, C. A. & Maldonado, P. D. Canonical and non-canonical mechanisms of Nrf2 activation. Pharmacol. Res. 134, 92–99 (2018).
Heerspink, H. J. L. et al. Dapagliflozin in patients with chronic kidney disease. N. Eng. J. Med. 383, 1436–1446 (2020).
Perkovic, V. et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N. Engl. J. Med. 380, 2295–2306 (2019).
Wanner, C. et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N. Engl. J. Med. 375, 323–334 (2016).
Gross, O. et al. Early angiotensin-converting enzyme inhibition in Alport syndrome delays renal failure and improves life expectancy. Kidney Int. 81, 494–501 (2012).
Kidney Disease: Improving Global Outcomes Anemia Work Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int. Suppl. 2, 279–335 (2012).
Kidney Disease: Improving Global Outcomes Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. Suppl. 2, 337–414 (2012).
Kidney Disease: Improving Global Outcomes Lipid Work Group. KDIGO clinical practice guideline for lipid management in chronic kidney disease. Kidney Int. Suppl. 3, 259–305 (2013).
Kidney Disease: Improving Global Outcomes Lipid Work Group. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney Disease. Kidney Int. 98, S1–S115 (2020).
Neal, B. et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 377, 644–657 (2017).
Neuen, B. L. et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 7, 845–854 (2019).
Wiviott, S. D. et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 380, 347–357 (2019).
Wheeler, D. C. et al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 9, 22–31 (2021).
Heerspink, H. J. L. et al. Effects of dapagliflozin on mortality in patients with chronic kidney disease: a pre-specified analysis from the DAPA-CKD randomized controlled trial. Eur. Heart J. 42, 1216–1227 (2021).
Alicic, R. Z., Cox, E. J., Neumiller, J. J. & Tuttle, K. R. Incretin drugs in diabetic kidney disease: biological mechanisms and clinical evidence. Nat. Rev. Nephrol. 17, 227–244 (2021).
Bakris, G. L. et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N. Engl. J. Med. 383, 2219–2229 (2020).
González-Blázquez, R. et al. Finerenone attenuates endothelial dysfunction and albuminuria in a chronic kidney disease model by a reduction in oxidative stress. Front. Pharmacol. 9, 1131 (2018).
Heerspink, H. J. L. et al. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet 393, 1937–1947 (2019).
Kohan, D. E. & Barton, M. Endothelin and endothelin antagonists in chronic kidney disease. Kidney Int. 86, 896–904 (2014).
Heerspink, H. J. L., Kohan, D. E. & de Zeeuw, D. New insights from SONAR indicate adding sodium glucose co-transporter 2 inhibitors to an endothelin receptor antagonist mitigates fluid retention and enhances albuminuria reduction. Kidney Int. 99, 346–349 (2021).
Rangaswami, J., Tuttle, K. & Vaduganathan, M. Cardio-renal-metabolic care models: toward achieving effective interdisciplinary care. Circ. Cardiovasc. Qual. Outcomes 13, e007264 (2020).
Liyanage, T. et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet 385, 1975–1982 (2015).
Elshahat, S. et al. The impact of chronic kidney disease on developed countries from a health economics perspective: a systematic scoping review. PLoS ONE 15, e0230512 (2020).
Gandjour, A., Armsen, W., Wehmeyer, W., Multmeier, J. & Tschulena, U. Costs of patients with chronic kidney disease in Germany. PLoS ONE 15, e0231375 (2020).
Eriksson, J. K., Neovius, M., Jacobson, S. H., Elinder, C. G. & Hylander, B. Healthcare costs in chronic kidney disease and renal replacement therapy: a population-based cohort study in Sweden. BMJ Open 6, e012062 (2016).
Subramanian, S. et al. Cost and affordability of non-communicable disease screening, diagnosis and treatment in Kenya: patient payments in the private and public sectors. PLoS ONE 13, e0190113 (2018).
Vanholder, R. et al. Reducing the costs of chronic kidney disease while delivering quality health care: a call to action. Nat. Rev. Nephrol. 13, 393–409 (2017).
Tonelli, M. & Dickinson, J. A. Early detection of CKD: implications for low-income, middle-income, and high-income countries. J. Am. Soc. Nephrol. 31, 1931–1940 (2020).
Komenda, P. et al. Cost-effectiveness of primary screening for CKD: a systematic review. Am. J. Kidney Dis. 63, 789–797 (2014).
Willis, M. et al. Cost-effectiveness of canagliflozin added to standard of care for treating diabetic kidney disease (DKD) in patients with type 2 diabetes mellitus (T2DM) in England: estimates using the CREDEM-DKD model. Diabetes Ther. 12, 313–328 (2021).
Ashuntantang, G. et al. Outcomes in adults and children with end-stage kidney disease requiring dialysis in sub-Saharan Africa: a systematic review. Lancet Glob. Health 5, e408–e417 (2017).
Chow, C. K. et al. Availability and affordability of medicines and cardiovascular outcomes in 21 high-income, middle-income and low-income countries. BMJ Glob. Health 5, e002640 (2020).
Rook, M. et al. Nephrectomy elicits impact of age and BMI on renal hemodynamics: lower postdonation reserve capacity in older or overweight kidney donors. Am. J. Transplant. 8, 2077–2085 (2008).
Pivin, E. et al. Uromodulin and nephron mass. Clin. J. Am. Soc. Nephrol. 13, 1556–1557 (2018).
Denic, A., Elsherbiny, H. & Rule, A. D. In-vivo techniques for determining nephron number. Curr. Opin. Nephrol. Hypertens. 28, 545–551 (2019).
Tofte, N. et al. Early detection of diabetic kidney disease by urinary proteomics and subsequent intervention with spironolactone to delay progression (PRIORITY): a prospective observational study and embedded randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 8, 301–312 (2020).
Pruijm, M. et al. Reduced cortical oxygenation predicts a progressive decline of renal function in patients with chronic kidney disease. Kidney Int. 93, 932–940 (2018).
Ruiz-Ortega, M., Rayego-Mateos, S., Lamas, S., Ortiz, A. & Rodrigues-Diez, R. R. Targeting the progression of chronic kidney disease. Nat. Rev. Nephrol. 16, 269–288 (2020).
Wesson, D. E. The continuum of acid stress. Clin. J. Am. Soc. Nephrol. 16, 1292–1299 (2021).
Anders, H.-J., Peired, A. J. & Romagnani, P. SGLT2 inhibition requires reconsideration of fundamental paradigms in chronic kidney disease, ‘diabetic nephropathy’, IgA nephropathy and podocytopathies with FSGS lesions. Nephrol. Dial. Transplant. https://doi.org/10.1093/ndt/gfaa329 (2020).
Acknowledgements
K.R.T. is supported by four NIDDK/NIH grants, one NCATS/NIH grant, one NIMHD/NIH grant, and a CDC contract all from the US Government, as well as research grants from Goldfinch Bio, Bayer and Travere. A.D.R. is supported by funding from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK090358). H.-J.A. is supported by the Deutsche Forschungsgemeinschaft (AN372/30-1).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, made substantial contributions to discussions of the content and wrote, reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
P.D. has received consultancy fees from Bayer and AstraZeneca. K.R.T. has received consulting fees for diabetes and CKD from Eli Lilly and Company, Boehringer Ingelheim, AstraZeneca, Gilead, Goldfinch Bio, Novo Nordisk and Bayer. A.G. has received consulting and lecture fees from Fresenius Medical Care. H.-J.A. has received consultancy fees from Bayer, Janssen, GSK, Novartis, Boehringer, AstraZeneca and PreviPharma. The other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Nephrology thanks M. Canney, A. Levin, R. Liu, P. Rossing, J. Wei and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Luyckx, V.A., Rule, A.D., Tuttle, K.R. et al. Nephron overload as a therapeutic target to maximize kidney lifespan. Nat Rev Nephrol 18, 171–183 (2022). https://doi.org/10.1038/s41581-021-00510-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-021-00510-7
This article is cited by
-
The impact of intrauterine growth restriction and prematurity on nephron endowment
Nature Reviews Nephrology (2023)
-
Ethnic disparities in pregnancy-related acute kidney injury in a United Kingdom population
Journal of Nephrology (2023)
-
Lupus Nephritis: New and Emerging Biologic and Targeted Therapies
BioDrugs (2023)
-
Glomerular hyperfiltration: part 1 — defining the threshold — is the sky the limit?
Pediatric Nephrology (2023)