Abstract
Microvascular endothelial cells in the kidney have been a neglected cell type in sepsis-induced acute kidney injury (sepsis-AKI) research; yet, they offer tremendous potential as pharmacological targets. As endothelial cells in distinct cortical microvascular segments are highly heterogeneous, this Review focuses on endothelial cells in their anatomical niche. In animal models of sepsis-AKI, reduced glomerular blood flow has been attributed to inhibition of endothelial nitric oxide synthase activation in arterioles and glomeruli, whereas decreased cortex peritubular capillary perfusion is associated with epithelial redox stress. Elevated systemic levels of vascular endothelial growth factor, reduced levels of circulating sphingosine 1-phosphate and loss of components of the glycocalyx from glomerular endothelial cells lead to increased microvascular permeability. Although coagulation disbalance occurs in all microvascular segments, the molecules involved differ between segments. Induction of the expression of adhesion molecules and leukocyte recruitment also occurs in a heterogeneous manner. Evidence of similar endothelial cell responses has been found in kidney and blood samples from patients with sepsis. Comprehensive studies are needed to investigate the relationships between segment-specific changes in the microvasculature and kidney function loss in sepsis-AKI. The application of omics technologies to kidney tissues from animals and patients will be key in identifying these relationships and in developing novel therapeutics for sepsis.
Key points
-
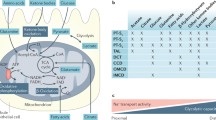
Microvascular endothelial cells in arterioles, glomeruli, peritubular capillaries and postcapillary venules in the kidney cortex have an intrinsic molecular and phenotypic heterogeneity and respond to sepsis-induced acute kidney injury (sepsis-AKI) conditions in a segment-specific manner.
-
Clinical data suggest endothelial engagement in sepsis-AKI, although the contribution of the renal microvasculature is not yet clear; changes in the levels of soluble markers in blood and urine represent endothelial responses that can originate from any organ involved in sepsis pathophysiology.
-
Not all molecules that control microvascular permeability and leukocyte recruitment in organs other than the kidney have a similar role in sepsis-AKI; this heterogeneity prevents extrapolation of mechanisms reported in other organs to the kidney.
-
As endothelial cells rapidly lose their behaviour in culture, molecular mechanisms or cell responses to sepsis-AKI conditions and pharmacological interventions that are identified in vitro must be validated in vivo.
-
Analysis of post-mortem kidney samples from patients with sepsis-AKI as well as serial plasma and urine samples and clinical data are required to effectively translate findings from animal models to the human disease.
-
Molecularly mapping endothelial responses in time in various organs will provide a rational basis for the selection of molecules that can potentially serve as (soluble) biomarkers of endothelial responses in sepsis-AKI following validation in clinical samples.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ronco, C., Bellomo, R. & Kellum, J. A. Acute kidney injury. Lancet 394, 1949–1964 (2019).
Koeze, J. et al. Incidence, timing and outcome of AKI in critically ill patients varies with the definition used and the addition of urine output criteria. BMC Nephrol. 18, 1–9 (2017).
Singer, M. et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315, 801–810 (2016).
Rudd, K. E. et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet 395, 200–211 (2020).
Singer, M., Inada-Kim, M. & Shankar-Hari, M. Sepsis hysteria: excess hype and unrealistic expectations. Lancet 394, 1513–1514 (2019).
Bellomo, R. et al. Acute kidney injury in sepsis. Intensive Care Med. 43, 816–828 (2017).
Lankadeva, Y. R., Okazaki, N., Evans, R. G., Bellomo, R. & May, C. N. Renal medullary hypoxia: a new therapeutic target for septic acute kidney injury? Semin. Nephrol. 39, 543–553 (2019).
Rubio, I. et al. Current gaps in sepsis immunology: new opportunities for translational research. Lancet Infect. Dis. 19, e422–e436 (2019).
Santacruz, C. A., Pereira, A. J., Celis, E. & Vincent, J. L. Which multicenter randomized controlled trials in critical care medicine have shown reduced mortality? A systematic review. Crit. Care Med. 47, 1680–1691 (2019).
Garofalo, A. M. et al. Histopathological changes of organ dysfunction in sepsis. Intensive Care Med. Exp. 7, 45 (2019).
Cummings, C. J. et al. Soluble E-selectin levels in sepsis and critical illness. Correlation with infection and hemodynamic dysfunction. Am. J. Respir. Crit. Care Med. 156, 431–437 (1997).
Knapp, S. et al. Prognostic value of MIP-1 alpha, TGF-beta 2, sELAM-1, and sVCAM-1 in patients with gram-positive sepsis. Clin. Immunol. Immunopathol. 87, 139–144 (1998).
Fang, Y. et al. Prognostic significance of the angiopoietin-2/angiopoietin-1 and angiopoietin-1/Tie-2 ratios for early sepsis in an emergency department. Crit. Care 19, 1–11 (2015).
Hepokoski, M. et al. Ventilator-induced lung injury increases expression of endothelial inflammatory mediators in the kidney. Am. J. Physiol. Renal Physiol. 312, F654–F660 (2017).
Cleuren, A. C. A. et al. The in vivo endothelial cell translatome is highly heterogeneous across vascular beds. Proc. Natl Acad. Sci. USA 116, 23618–23624 (2019).
Navar, L. et al. The renal microcirculation. Compr. Physiol. https://doi.org/10.1002/cphy.cp020413 (2011).
Molema, G. & Aird, W. C. Vascular heterogeneity in the kidney. Semin. Nephrol. 32, 145–155 (2012).
Bonsib, S. M. in Hepintstall’s Pathology of the Kidney Vol. I (eds Jenette, J. C., Olson, J. L., Silva, F. G. & D’Agati, V. D.) 1–66 (Wolters Kluwer, 2015).
Munoz, C. J., Lucas, A., Williams, A. T. & Cabrales, P. A review on microvascular hemodynamics: the control of blood flow distribution and tissue oxygenation. Crit. Care Clin. 36, 293–305 (2020).
Carrithers, M. et al. Enhanced susceptibility to endotoxic shock and impaired STAT3 signaling in CD31-deficient mice. Am. J. Pathol. 166, 185–196 (2005).
Lampugnani, M. G., Dejana, E. & Giampietro, C. Vascular endothelial (VE)-cadherin, endothelial adherens junctions, and vascular disease. Cold Spring Harb. Perspect. Biol. 10, a029322 (2018).
Claesson-Welsh, L., Dejana, E. & McDonald, D. M. Permeability of the endothelial barrier: identifying and reconciling controversies. Trends Mol. Med. 27, 314–331 (2021).
Lay, A. J., Donahue, D., Tsai, M. J. & Castellino, F. J. Acute inflammation is exacerbated in mice genetically predisposed to a severe protein C deficiency. Blood 109, 1984–1991 (2007).
Nozaki, Y. et al. Protective effects of recombinant human soluble thrombomodulin on lipopolysaccharide-induced acute kidney injury. Int. J. Mol. Sci. 21, 2519 (2020).
Jedlicka, J., Becker, B. F. & Chappell, D. Endothelial glycocalyx. Crit. Care Clin. 36, 217–232 (2020).
Friden, V. et al. The glomerular endothelial cell coat is essential for glomerular filtration. Kidney Int. 79, 1322–1330 (2011).
Okada, H. et al. Three-dimensional ultrastructure of capillary endothelial glycocalyx under normal and experimental endotoxemic conditions. Crit. Care 21, 261 (2017).
Lemos, D. R. et al. Maintenance of vascular integrity by pericytes is essential for normal kidney function. Am. J. Physiol. Renal Physiol. 311, F1230–F1242 (2016).
Kwei, S. et al. Early adaptive responses of the vascular wall during venous arterialization in mice. Am. J. Pathol. 164, 81–89 (2004).
Devi, S. et al. Multiphoton imaging reveals a new leukocyte recruitment paradigm in the glomerulus. Nat. Med 19, 107–112 (2013).
Minami, T. & Aird, W. C. Endothelial cell gene regulation. Trends Cardiovasc. Med. 15, 174–184 (2005).
Shirodkar, A. V. et al. A mechanistic role for DNA methylation in endothelial cell (EC)-enriched gene expression: relationship with DNA replication timing. Blood 121, 3531–3540 (2013).
Yuan, L. et al. A role of stochastic phenotype switching in generating mosaic endothelial cell heterogeneity. Nat. Commun. 7, 10160 (2016).
Sumpio, B. E. et al. MAPKs (ERK1/2, p38) and AKT can be phosphorylated by shear stress independently of platelet endothelial cell adhesion molecule-1 (CD31) in vascular endothelial cells. J. Biol. Chem. 280, 11185–11191 (2005).
Dekker, R. J. et al. Endothelial KLF2 links local arterial shear stress levels to the expression of vascular tone-regulating genes. Am. J. Pathol. 167, 609–618 (2005).
Mantilidewi, K. I. et al. Shear stress-induced redistribution of vascular endothelial-protein-tyrosine phosphatase (VE-PTP) in endothelial cells and its role in cell elongation. J. Biol. Chem. 289, 6451–6461 (2014).
Liu, M., Kluger, M. S., D’Alessio, A., Garcia-Cardena, G. & Pober, J. S. Regulation of arterial-venous differences in tumor necrosis factor responsiveness of endothelial cells by anatomic context. Am. J. Pathol. 172, 1088–1099 (2008).
Kuosmanen, S. M., Kansanen, E., Sihvola, V. & Levonen, A.-L. MicroRNA profiling reveals distinct profiles for tissue-derived and cultured endothelial cells. Sci. Rep. 7, 10943 (2017).
Yan, R. et al. Early heterogenic response of renal microvasculature to hemorrhagic shock/resuscitation and the influence of NF-κB pathway blockade. Shock 51, 200–212 (2019).
Gomez, H. & Kellum, J. A. Sepsis-induced acute kidney injury. Curr. Opin. Crit. Care 22, 546–553 (2016).
Tateda, K., Matsumoto, T., Miyazaki, S. & Yamaguchi, K. Lipopolysaccharide-induced lethality and cytokine production in aged mice. Infect. Immun. 64, 769–774 (1996).
Tidjane, N. et al. A primary role for kinin B1 receptor in inflammation, organ damage, and lethal thrombosis in a rat model of septic shock in diabetes. Eur. J. Inflamm. 13, 40–52 (2015).
Seemann, S., Zohles, F. & Lupp, A. Comprehensive comparison of three different animal models for systemic inflammation. J. Biomed. Sci. 24, 60 (2017).
Kingsley, S. M. K. & Bhat, B. V. Differential paradigms in animal models of sepsis. Curr. Infect. Dis. Rep. 18, 26 (2016).
Lewis, A. J., Seymour, C. W. & Rosengart, M. R. Current murine models of sepsis. Surg. Infect. 17, 385–393 (2016).
von Drygalski, A., Furlan-Freguia, C., Ruf, W., Griffin, J. H. & Mosnier, L. O. Organ-specific protection against lipopolysaccharide-induced vascular leak is dependent on the endothelial protein C receptor. Arterioscler. Thromb. Vasc. Biol. 33, 769–776 (2013).
Post, E. H., Kellum, J. A., Bellomo, R. & Vincent, J. L. Renal perfusion in sepsis: from macro- to microcirculation. Kidney Int. 91, 45–60 (2017).
Song, T. et al. Survival advantage depends on cecal volume rather than cecal length in a mouse model of cecal ligation and puncture. J. Surg. Res. 203, 476–482 (2016).
Lewis, A. J. et al. Use of biotelemetry to define physiology-based deterioration thresholds in a murine cecal ligation and puncture model of sepsis. Crit. Care Med. 44, e420–e431 (2016).
Matejovic, M. et al. Molecular differences in susceptibility of the kidney to sepsis-induced kidney injury. BMC Nephrol. 18, 183 (2017).
Seymour, C. W. et al. Murine sepsis phenotypes and differential treatment effects in a randomized trial of prompt antibiotics and fluids. Crit. Care 23, 384 (2019).
Scicluna, B. P. et al. Classification of patients with sepsis according to blood genomic endotype: a prospective cohort study. Lancet Respir. Med. 5, 816–826 (2017).
van Lier, D., Geven, C., Leijte, G. P. & Pickkers, P. Experimental human endotoxemia as a model of systemic inflammation. Biochimie 159, 99–106 (2019).
Fijen, J. W. et al. Inhibition of p38 mitogen-activated protein kinase: dose-dependent suppression of leukocyte and endothelial response after endotoxin challenge in humans. Crit. Care Med. 30, 841–845 (2002).
Rodriguez-Rosales, Y. A. et al. Long-term effects of experimental human endotoxemia on immune cell function: similarities and differences with sepsis. Shock 51, 678–689 (2019).
Fijen, J. W. et al. Suppression of the clinical and cytokine response to endotoxin by RWJ-67657, a p38 mitogen-activated protein-kinase inhibitor, in healthy human volunteers. Clin. Exp. Immunol. 124, 16–20 (2001).
Jilma, B. et al. The single nucleotide polymorphism Ser128Arg in the E-selectin gene is associated with enhanced coagulation during human endotoxemia. Blood 105, 2380–2383 (2005).
Zijlstra, J. G., Tulleken, J. E., Ligtenberg, J. J., de Boer, P. & van der Werf, T. S. p38-MAPK inhibition and endotoxin induced tubular dysfunction in men. J. Endotoxin Res. 10, 402–405 (2004).
Heemskerk, S. et al. Upregulation of renal inducible nitric oxide synthase during human endotoxemia and sepsis is associated with proximal tubule injury. Clin. J. Am. Soc. Nephrol. 1, 853–862 (2006).
Mera, S. et al. Multiplex cytokine profiling in patients with sepsis. APMIS 119, 155–163 (2011).
Tornblom, S. et al. Neutrophil activation in septic acute kidney injury: a post hoc analysis of the FINNAKI study. Acta Anaesthesiol. Scand. 63, 1390–1397 (2019).
Cavaillon, J.-M., Singer, M. & Skirecki, T. Sepsis therapies: learning from 30 years of failure of translational research to propose new leads. EMBO Mol. Med. 12, e10128 (2020).
Aird, W. C. Endothelium as a therapeutic target in sepsis. Curr. Drug Targets 8, 501–507 (2007).
Fels, B. & Kusche-Vihrog, K. It takes more than two to tango: mechanosignaling of the endothelial surface. Pflugers Arch. 472, 419–433 (2020).
Pober, J. S. & Sessa, W. C. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 7, 803–815 (2007).
Khakpour, S., Wilhelmsen, K. & Hellman, J. Vascular endothelial cell Toll-like receptor pathways in sepsis. Innate Immun. 21, 827–846 (2015).
Chinnaiyan, A. M. et al. Molecular signatures of sepsis: multiorgan gene expression profiles of systemic inflammation. Am. J. Pathol. 159, 1199–1209 (2001).
Kiely, J. M., Hu, Y., Garcia-Cardena, G. & Gimbrone, M. A. Jr. Lipid raft localization of cell surface E-selectin is required for ligation-induced activation of phospholipase C gamma. J. Immunol. 171, 3216–3224 (2003).
Chase, S. D., Magnani, J. L. & Simon, S. I. E-selectin ligands as mechanosensitive receptors on neutrophils in health and disease. Ann. Biomed. Eng. 40, 849–859 (2012).
Li, N. et al. Ligand-specific binding forces of LFA-1 and Mac-1 in neutrophil adhesion and crawling. Mol. Biol. Cell 29, 408–418 (2018).
Margraf, A. et al. The integrin-linked kinase is required for chemokine-triggered high-affinity conformation of the neutrophil beta2-integrin LFA-1. Blood 136, 2200–2205 (2020).
Simons, M., Gordon, E. & Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 17, 611–625 (2016).
Augustin, H. G., Koh, G. Y., Thurston, G. & Alitalo, K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat. Rev. Mol. Cell Biol. 10, 165–177 (2009).
Vestweber, D. How leukocytes cross the vascular endothelium. Nat. Rev. Immunol. 15, 692–704 (2015).
Braun, L. J. et al. Platelets docking to VWF prevent leaks during leukocyte extravasation by stimulating Tie-2. Blood 136, 627–639 (2020).
Jourde-Chiche, N. et al. Endothelium structure and function in kidney health and disease. Nat. Rev. Nephrol. 15, 87–108 (2019).
Aird, W. C. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ. Res. 100, 158–173 (2007).
Prowle, J. R. & Bellomo, R. Sepsis-associated acute kidney injury: macrohemodynamic and microhemodynamic alterations in the renal circulation. Semin. Nephrol. 35, 64–74 (2015).
Langenberg, C., Wan, L., Egi, M., May, C. N. & Bellomo, R. Renal blood flow in experimental septic acute renal failure. Kidney Int. 69, 1996–2002 (2006).
Mayeux, P. R. & MacMillan-Crow, L. A. Pharmacological targets in the renal peritubular microenvironment: implications for therapy for sepsis-induced acute kidney injury. Pharmacol. Ther. 134, 139–155 (2012).
Bellomo, R., Wan, L., Langenberg, C., Ishikawa, K. & May, C. N. Septic acute kidney injury: the glomerular arterioles. Contrib. Nephrol. 174, 98–107 (2011).
Mederle, K., Meurer, M., Castrop, H. & Hocherl, K. Inhibition of COX-1 attenuates the formation of thromboxane A2 and ameliorates the acute decrease in glomerular filtration rate in endotoxemic mice. Am. J. Physiol. Renal Physiol. 309, F332–F340 (2015).
Wu, L. et al. Peritubular capillary dysfunction and renal tubular epithelial cell stress following lipopolysaccharide administration in mice. Am. J. Physiol. Renal Physiol. 292, F261–F268 (2007).
Meurer, M. & Hocherl, K. Deregulated renal magnesium transport during lipopolysaccharide-induced acute kidney injury in mice. Pflugers Arch. 471, 619–631 (2019).
Nakano, D. et al. Reduction of tubular flow rate as a mechanism of oliguria in the early phase of endotoxemia revealed by intravital imaging. J. Am. Soc. Nephrol. 26, 3035–3044 (2015).
Nakano, D. et al. Lipopolysaccharide induces filtrate leakage from renal tubular lumina into the interstitial space via a proximal tubular Toll-like receptor 4-dependent pathway and limits sensitivity to fluid therapy in mice. Kidney Int. 97, 904–912 (2020).
Boffa, J. J. & Arendshorst, W. J. Maintenance of renal vascular reactivity contributes to acute renal failure during endotoxemic shock. J. Am. Soc. Nephrol. 16, 117–124 (2005).
Leisman, D. E. et al. Impaired angiotensin II type 1 receptor signaling contributes to sepsis-induced acute kidney injury. Kidney Int. 99, 148–160 (2021).
Calianos, T. & Muntz, K. H. Autoradiographic quantification of adrenergic receptors in rat kidney. Kidney Int. 38, 39–46 (1990).
Canessa, L. M. et al. Alpha 1B-adrenergic receptors in rat renal microvessels. Kidney Int. 48, 1412–1419 (1995).
Jesmin, S. et al. Effects of protease activated receptor (PAR)2 blocking peptide on endothelin-1 levels in kidney tissues in endotoxemic rat model. Life Sci. 102, 127–133 (2014).
Wendel, M., Knels, L., Kummer, W. & Koch, T. Distribution of endothelin receptor subtypes ETA and ETB in the rat kidney. J. Histochem. Cytochem. 54, 1193–1203 (2006).
Dhaun, N., Webb, D. J. & Kluth, D. C. Endothelin-1 and the kidney–beyond BP. Br. J. Pharmacol. 167, 720–731 (2012).
Tiwari, M. M., Brock, R. W., Megyesi, J. K., Kaushal, G. P. & Mayeux, P. R. Disruption of renal peritubular blood flow in lipopolysaccharide-induced renal failure: role of nitric oxide and caspases. Am. J. Physiol. Renal Physiol. 289, F1324–F1332 (2005).
Wu, L., Gokden, N. & Mayeux, P. R. Evidence for the role of reactive nitrogen species in polymicrobial sepsis-induced renal peritubular capillary dysfunction and tubular injury. J. Am. Soc. Nephrol. 18, 1807–1815 (2007).
Chou, D. E., Cai, H., Jayadevappa, D. & Porush, J. G. Regional expression of inducible nitric oxide synthase in the kidney stimulated by lipopolysaccharide in the rat. Exp. Physiol. 87, 153–162 (2002).
Bian, K., Davis, K., Kuret, J., Binder, L. & Murad, F. Nitrotyrosine formation with endotoxin-induced kidney injury detected by immunohistochemistry. Am. J. Physiol. 277, F33–F40 (1999).
Hua, T. et al. Micro- and macrocirculatory changes during sepsis and septic shock in a rat model. Shock 49, 591–595 (2018).
Wang, Z., Sims, C. R., Patil, N. K., Gokden, N. & Mayeux, P. R. Pharmacologic targeting of sphingosine-1-phosphate receptor 1 improves the renal microcirculation during sepsis in the mouse. J. Pharmacol. Exp. Ther. 352, 61–66 (2015).
Shieh, P., Zhou, M., Ornan, D. A., Chaudry, I. H. & Wang, P. Upregulation of inducible nitric oxide synthase and nitric oxide occurs later than the onset of the hyperdynamic response during sepsis. Shock 13, 325–329 (2000).
Bachmann, S., Bosse, H. M. & Mundel, P. Topography of nitric oxide synthesis by localizing constitutive NO synthases in mammalian kidney. Am. J. Physiol. 268, F885–F898 (1995).
Saharinen, P. et al. Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts. Nat. Cell Biol. 10, 527–537 (2008).
Di Lorenzo, A. et al. eNOS-derived nitric oxide regulates endothelial barrier function through VE-cadherin and Rho GTPases. J. Cell Sci. 126, 5541–5552 (2013).
Heemskerk, S., Masereeuw, R., Russel, F. G. & Pickkers, P. Selective iNOS inhibition for the treatment of sepsis-induced acute kidney injury. Nat. Rev. Nephrol. 5, 629–640 (2009).
Coletta, C. et al. Endothelial dysfunction is a potential contributor to multiple organ failure and mortality in aged mice subjected to septic shock: preclinical studies in a murine model of cecal ligation and puncture. Crit. Care 18, 511 (2014).
Wang, W. et al. Endothelial nitric oxide synthase-deficient mice exhibit increased susceptibility to endotoxin-induced acute renal failure. Am. J. Physiol. Renal Physiol. 287, F1044–F1048 (2004).
Schwartz, D. et al. Inhibition of constitutive nitric oxide synthase (NOS) by nitric oxide generated by inducible NOS after lipopolysaccharide administration provokes renal dysfunction in rats. J. Clin. Invest. 100, 439–448 (1997).
Condor, J. M. et al. Treatment with human Wharton’s Jelly-derived mesenchymal stem cells attenuates sepsis-induced kidney injury, liver injury, and endothelial dysfunction. Stem Cell Transl. Med. 5, 1048–1057 (2016).
Xu, C. et al. TNF-mediated damage to glomerular endothelium is an important determinant of acute kidney injury in sepsis. Kidney Int. 85, 72–81 (2014).
Yano, K. et al. Vascular endothelial growth factor is an important determinant of sepsis morbidity and mortality. J. Exp. Med. 203, 1447–1458 (2006).
Jeong, S. J., Han, S. H., Kim, C. O., Choi, J. Y. & Kim, J. M. Anti-vascular endothelial growth factor antibody attenuates inflammation and decreases mortality in an experimental model of severe sepsis. Crit. Care 17, R97 (2013).
Kumpers, P. et al. Time course of angiopoietin-2 release during experimental human endotoxemia and sepsis. Crit. Care 13, R64 (2009).
Parikh, S. M. The angiopoietin-Tie2 signaling axis in systemic inflammation. J. Am. Soc. Nephrol. 28, 1973–1982 (2017).
van Meurs, M. et al. Shock induced stress induces loss of microvascular endothelial Tie2 in the kidney which is not associated with reduced glomerular barrier function. Am. J. Physiol. Renal Physiol. 297, F272–F281 (2009).
Aslan, A. et al. Organ-specific differences in endothelial permeability-regulating molecular responses in mouse and human sepsis. Shock 48, 69–77 (2017).
Kurniati, N. F. et al. The flow dependency of Tie2 expression in endotoxemia. Intensive Care Med. 39, 1262–1271 (2013).
Bomsztyk, K. et al. Experimental acute lung injury induces multi-organ epigenetic modifications in key angiogenic genes implicated in sepsis-associated endothelial dysfunction. Crit. Care 19, 225 (2015).
Kumpers, P. et al. The synthetic Tie2 agonist peptide vasculotide protects against vascular leakage and reduces mortality in murine abdominal sepsis. Crit. Care 15, R261 (2011).
David, S. et al. Acute administration of recombinant angiopoietin-1 ameliorates multiple-organ dysfunction syndrome and improves survival in murine sepsis. Cytokine 55, 251–259 (2011).
Kim, D. H. et al. COMP-angiopoietin-1 decreases lipopolysaccharide-induced acute kidney injury. Kidney Int. 76, 1180–1191 (2009).
Kirby, R. J. et al. Dynamic regulation of sphingosine-1-phosphate homeostasis during development of mouse metanephric kidney. Am. J. Physiol. Renal Physiol. 296, F634–F641 (2009).
Sanchez, T. Sphingosine-1-phosphate signaling in endothelial disorders. Curr. Atheroscler. Rep. 18, 31 (2016).
Gaengel, K. et al. The sphingosine-1-phosphate receptor S1PR1 restricts sprouting angiogenesis by regulating the interplay between VE-cadherin and VEGFR2. Dev. Cell 23, 587–599 (2012).
Kurano, M. et al. Apolipoprotein M protects lipopolysaccharide-treated mice from death and organ injury. Thromb. Haemost. 118, 1021–1035 (2018).
Zhang, G. et al. Critical role of sphingosine-1-phosphate receptor 2 (S1PR2) in acute vascular inflammation. Blood 122, 443–455 (2013).
Kuppers, V., Vockel, M., Nottebaum, A. F. & Vestweber, D. Phosphatases and kinases as regulators of the endothelial barrier function. Cell Tissue Res. 355, 577–586 (2014).
Conway, D. E. et al. VE-cadherin phosphorylation regulates endothelial fluid shear stress responses through the polarity protein LGN. Curr. Biol. 27, 2219–2225.e5 (2017).
Xi, C. et al. Effects of alterations of glomerular fibrin deposition on renal inflammation in rats at different age stages. J. Gerontol. A Biol. Sci. Med. Sci. 60, 1099–1110 (2005).
Ruiz, S. et al. Kinin B1 receptor: a potential therapeutic target in sepsis-induced vascular hyperpermeability. J. Transl. Med. 18, 174 (2020).
Garsen, M. et al. Heparanase is essential for the development of acute experimental glomerulonephritis. Am. J. Pathol. 186, 805–815 (2016).
Adembri, C. et al. Sepsis induces albuminuria and alterations in the glomerular filtration barrier: a morphofunctional study in the rat. Crit. Care 15, R277 (2011).
Lygizos, M. I. et al. Heparanase mediates renal dysfunction during early sepsis in mice. Physiol. Rep. 1, e00153 (2013).
Margraf, A. et al. 6% Hydroxyethyl starch (HES 130/0.4) diminishes glycocalyx degradation and decreases vascular permeability during systemic and pulmonary inflammation in mice. Crit. Care 22, 111 (2018).
Madhusudhan, T., Kerlin, B. A. & Isermann, B. The emerging role of coagulation proteases in kidney disease. Nat. Rev. Nephrol. 12, 94–109 (2016).
Jesmin, S. et al. Protease-activated receptor 2 blocking peptide counteracts endotoxin-induced inflammation and coagulation and ameliorates renal fibrin deposition in a rat model of acute renal failure. Shock 32, 626–632 (2009).
Song, D., Ye, X., Xu, H. & Liu, S. F. Activation of endothelial intrinsic NF-{kappa}B pathway impairs protein C anticoagulation mechanism and promotes coagulation in endotoxemic mice. Blood 114, 2521–2529 (2009).
Pendurthi, U. R. & Rao, L. V. M. Endothelial cell protein C receptor-dependent signaling. Curr. Opin. Hematol. 25, 219–226 (2018).
Opal, S. M., Dellinger, R. P., Vincent, J. L., Masur, H. & Angus, D. C. The next generation of sepsis clinical trial designs: what is next after the demise of recombinant human activated protein C? Crit. Care Med. 42, 1714–1721 (2014).
Kang, K. et al. Heme oxygenase 1 modulates thrombomodulin and endothelial protein C receptor levels to attenuate septic kidney injury. Shock 40, 136–143 (2013).
Laszik, Z., Mitro, A., Taylor, F. B. Jr., Ferrell, G. & Esmon, C. T. Human protein C receptor is present primarily on endothelium of large blood vessels: implications for the control of the protein C pathway. Circulation 96, 3633–3640 (1997).
Terada, Y. et al. Capillary endothelial thrombomodulin expression and fibrin deposition in rats with continuous and bolus lipopolysaccharide administration. Lab. Invest. 83, 1165–1173 (2003).
Erlich, J., Fearns, C., Mathison, J., Ulevitch, R. J. & Mackman, N. Lipopolysaccharide induction of tissue factor expression in rabbits. Infect. Immun. 67, 2540–2546 (1999).
Erlich, J. H. et al. Renal expression of tissue factor pathway inhibitor and evidence for a role in crescentic glomerulonephritis in rabbits. J. Clin. Invest. 98, 325–335 (1996).
Shimokawa, T., Yamamoto, K., Kojima, T. & Saito, H. Down-regulation of murine tissue factor pathway inhibitor mRNA by endotoxin and tumor necrosis factor-alpha in vitro and in vivo. Thromb. Res. 100, 211–221 (2000).
Coughlan, A. F., Hau, H., Dunlop, L. C., Berndt, M. C. & Hancock, W. W. P-selectin and platelet-activating factor mediate initial endotoxin-induced neutropenia. J. Exp. Med. 179, 329–334 (1994).
Fries, J. W. et al. Expression of VCAM-1 and E-selectin in an in vivo model of endothelial activation. Am. J. Pathol. 143, 725–737 (1993).
Dayang, E. Z. et al. Identification of LPS-activated endothelial subpopulations with distinct inflammatory phenotypes and regulatory signaling mechanisms. Front. Immunol. 10, 1169 (2019).
Wu, X., Guo, R., Wang, Y. & Cunningham, P. N. The role of ICAM-1 in endotoxin-induced acute renal failure. Am. J. Physiol. Renal Physiol. 293, F1262–F1271 (2007).
Redl, H. et al. Expression of endothelial leukocyte adhesion molecule-1 in septic but not traumatic/hypovolemic shock in the baboon. Am. J. Pathol. 139, 461–466 (1991).
Cunningham, P. N. et al. Acute renal failure in endotoxemia is caused by TNF acting directly on TNF receptor-1 in kidney. J. Immunol. 168, 5817–5823 (2002).
Cunningham, P. N., Wang, Y., Guo, R., He, G. & Quigg, R. J. Role of Toll-like receptor 4 in endotoxin-induced acute renal failure. J. Immunol. 172, 2629–2635 (2004).
Jongman, R. M. et al. Partial deletion of Tie2 affects microvascular endothelial responses to critical illness in a vascular bed and organ-specific way. Shock 51, 757–769 (2019).
Baran, D., Vendeville, B., Ogborn, M. & Katz, N. Cell adhesion molecule expression in murine lupus-like nephritis induced by lipopolysaccharide. Nephron 84, 167–176 (2000).
Granfeldt, A., Ebdrup, L., Tonnesen, E. & Wogensen, L. Renal cytokine profile in an endotoxemic porcine model. Acta Anaesthesiol. Scand. 52, 614–620 (2008).
Herter, J. M., Rossaint, J., Spieker, T. & Zarbock, A. Adhesion molecules involved in neutrophil recruitment during sepsis-induced acute kidney injury. J. Innate Immun. 6, 597–606 (2014).
Rossaint, J. et al. Hydroxyethyl starch 130/0.4 decreases inflammation, neutrophil recruitment, and neutrophil extracellular trap formation. Br. J. Anaesth. 114, 509–519 (2015).
van Meurs, M. et al. Adiponectin diminishes organ specific microvascular endothelial cell activation associated with sepsis. Shock 37, 392–398 (2012).
Matsukawa, A. et al. Mice genetically lacking endothelial selectins are resistant to the lethality in septic peritonitis. Exp. Mol. Pathol. 72, 68–76 (2002).
Chousterman, B. G. et al. Ly6Chigh monocytes protect against kidney damage during sepsis via a CX3CR1-dependent adhesion mechanism. J. Am. Soc. Nephrol. 27, 792–803 (2016).
Zhang, J., Yang, W., Hu, B., Wu, W. & Fallon, M. B. Endothelin-1 activation of the endothelin B receptor modulates pulmonary endothelial CX3CL1 and contributes to pulmonary angiogenesis in experimental hepatopulmonary syndrome. Am. J. Pathol. 184, 1706–1714 (2014).
David, S. et al. Angiopoietin-2 may contribute to multiple organ dysfunction and death in sepsis. Crit. Care Med. 40, 3034–3041 (2012).
Ismail, H. et al. Angiopoietin-1 and vascular endothelial growth factor regulation of leukocyte adhesion to endothelial cells: role of nuclear receptor-77. Arterioscler. Thromb. Vasc. Biol. 32, 1707–1716 (2012).
Schmidt, E. P. et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat. Med. 18, 1217–1223 (2012).
Rops, A. L. et al. Modulation of heparan sulfate in the glomerular endothelial glycocalyx decreases leukocyte influx during experimental glomerulonephritis. Kidney Int. 86, 932–942 (2014).
Cox, L. A. et al. Inflammation-induced increases in plasma endocan levels are associated with endothelial dysfunction in humans in vivo. Shock 43, 322–326 (2015).
van Poelgeest, E. P. et al. Characterization of immune cell, endothelial, and renal responses upon experimental human endotoxemia. J. Pharmacol. Toxicol. Methods 89, 39–46 (2018).
Li, X. & Yan, B. Research on the effect of cytokine concentration on the immune level and survival conditions of elderly patients with sepsis. Exp. Ther. Med. 16, 842–846 (2018).
Shao, R. et al. The expression of thioredoxin-1 and inflammatory cytokines in patients with sepsis. Immunopharmacol. Immunotoxicol. 42, 280–285 (2020).
Martin, G. et al. Endothelial (NOS3 E298D) and inducible (NOS2 exon 22) nitric oxide synthase polymorphisms, as well as plasma NOx, influence sepsis development. Nitric Oxide 42, 79–86 (2014).
Hou, P. C. et al. Endothelial permeability and hemostasis in septic shock: results from the ProCESS Trial. Chest 152, 22–31 (2017).
Mikacenic, C. et al. Biomarkers of endothelial activation are associated with poor outcome in critical Illness. PLoS ONE 10, e0141251 (2015).
Fang, Y., Li, C., Shao, R., Yu, H. & Zhang, Q. The role of biomarkers of endothelial activation in predicting morbidity and mortality in patients with severe sepsis and septic shock in intensive care: a prospective observational study. Thromb. Res. 171, 149–154 (2018).
Bhatraju, P. K. et al. Identification of acute kidney injury subphenotypes with differing molecular signatures and responses to vasopressin therapy. Am. J. Respir. Crit. Care Med. 199, 863–872 (2019).
Winkler, M. S. et al. Decreased serum concentrations of sphingosine-1-phosphate in sepsis. Crit. Care 19, 372 (2015).
Skibsted, S. et al. Biomarkers of endothelial cell activation in early sepsis. Shock 39, 427–432 (2013).
Kjaergaard, A. G., Dige, A., Nielsen, J. S., Tonnesen, E. & Krog, J. The use of the soluble adhesion molecules sE-selectin, sICAM-1, sVCAM-1, sPECAM-1 and their ligands CD11a and CD49d as diagnostic and prognostic biomarkers in septic and critically ill non-septic ICU patients. APMIS 124, 846–855 (2016).
Yu, W.-K. et al. Vascular endothelial cadherin shedding is more severe in sepsis patients with severe acute kidney injury. Crit. Care 23, 18 (2019).
Schmidt, E. P. et al. Urinary glycosaminoglycans predict outcomes in septic shock and acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 194, 439–449 (2016).
Puskarich, M. A., Cornelius, D. C., Tharp, J., Nandi, U. & Jones, A. E. Plasma syndecan-1 levels identify a cohort of patients with severe sepsis at high risk for intubation after large-volume intravenous fluid resuscitation. J. Crit. Care 36, 125–129 (2016).
Uhel, F. et al. Mortality and host response aberrations associated with transient and persistent acute kidney injury in critically ill patients with sepsis: a prospective cohort study. Intensive Care Med. 46, 1576–1589 (2020).
Lerolle, N. et al. Histopathology of septic shock induced acute kidney injury: apoptosis and leukocytic infiltration. Intensive Care Med. 36, 471–478 (2010).
Aslan, A. et al. Kidney histopathology in lethal human sepsis. Crit. Care 22, 359 (2018).
Bonavia, A. & Singbartl, K. A review of the role of immune cells in acute kidney injury. Pediatr. Nephrol. 33, 1629–1639 (2018).
Molitoris, B. A. Therapeutic translation in acute kidney injury: the epithelial/endothelial axis. J. Clin. Invest. 124, 2355–2363 (2014).
Franzin, R. et al. Inflammaging and complement system: a link between acute kidney injury and chronic graft damage. Front. Immunol. 11, 734 (2020).
Kalakeche, R. et al. Endotoxin uptake by S1 proximal tubular segment causes oxidative stress in the downstream S2 segment. J. Am. Soc. Nephrol. 22, 1505–1516 (2011).
Mansson, L. E. et al. Real-time studies of the progression of bacterial infections and immediate tissue responses in live animals. Cell Microbiol. 9, 413–424 (2007).
Melican, K. et al. Bacterial infection-mediated mucosal signalling induces local renal ischaemia as a defence against sepsis. Cell. Microbiol. 10, 1987–1998 (2008).
Molema, G. Heterogeneity in endothelial responsiveness to cytokines, molecular causes and pharmacological consequences. Semin. Thromb. Hemost. 36, 246–264 (2010).
Kumar, S. & Molitoris, B. A. Renal endothelial injury and microvascular dysfunction in acute kidney injury. Semin. Nephrol. 35, 96–107 (2015).
Dauphinee, S. M. & Karsan, A. Lipopolysaccharide signaling in endothelial cells. Lab. Invest. 86, 9–22 (2006).
Abedi, F., Hayes, A. W., Reiter, R. & Karimi, G. Acute lung injury: the therapeutic role of Rho kinase inhibitors. Pharmacol. Res. 155, 104736 (2020).
Ye, X., Ding, J., Zhou, X., Chen, G. & Liu, S. F. Divergent roles of endothelial NF-kappaB in multiple organ injury and bacterial clearance in mouse models of sepsis. J. Exp. Med. 205, 1303–1315 (2008).
Meyer-Schwesinger, C. et al. Rho kinase inhibition attenuates LPS-induced renal failure in mice in part by attenuation of NF-kappaB p65 signaling. Am. J. Physiol. Renal Physiol. 296, F1088–F1099 (2009).
Mussbacher, M. et al. Cell type-specific roles of NF-kappaB linking inflammation and thrombosis. Front. Immunol. 10, 85 (2019).
Peng, Y. et al. Overexpression of toll-like receptor 2 in glomerular endothelial cells and podocytes in septic acute kidney injury mouse model. Ren. Fail. 37, 694–698 (2015).
El-Achkar, T. M. et al. Sepsis induces changes in the expression and distribution of Toll-like receptor 4 in the rat kidney. Am. J. Physiol. Renal Physiol. 290, F1034–F1043 (2006).
Jang, H. R. & Rabb, H. Immune cells in experimental acute kidney injury. Nat. Rev. Nephrol. 11, 88–101 (2015).
Gomez, H., Kellum, J. A. & Ronco, C. Metabolic reprogramming and tolerance during sepsis-induced AKI. Nat. Rev. Nephrol. 13, 143–151 (2017).
Sun, J. et al. Mitochondria in sepsis-induced AKI. J. Am. Soc. Nephrol. 30, 1151–1161 (2019).
van der Poll, T. & Parker, R. I. Platelet activation and endothelial cell dysfunction. Crit. Care Clin. 36, 233–253 (2020).
Kerchberger, V. E. & Ware, L. B. The role of circulating cell-free hemoglobin in sepsis-associated acute kidney injury. Semin. Nephrol. 40, 148–159 (2020).
Leone, M. et al. Systemic endothelial activation is greater in septic than in traumatic-hemorrhagic shock but does not correlate with endothelial activation in skin biopsies. Crit. Care Med. 30, 808–814 (2002).
Shapiro, N. I. et al. Skin biopsies demonstrate site-specific endothelial activation in mouse models of sepsis. J. Vasc. Res. 46, 495–502 (2009).
Stefanska, A. et al. Interstitial pericytes decrease in aged mouse kidneys. Aging 7, 370–382 (2015).
Wulfert, F. M. et al. Age-dependent role of microvascular endothelial and polymorphonuclear cells in lipopolysaccharide-induced acute kidney failure. Anesthesiology 117, 126–136 (2012).
Kalucka, J. et al. Single-cell transcriptome atlas of murine endothelial cells. Cell 180, 764–779.e20 (2020).
Dumas, S. J. et al. Single-cell RNA sequencing reveals renal endothelium heterogeneity and metabolic adaptation to water deprivation. J. Am. Soc. Nephrol. 31, 118–138 (2020).
Toledo, A. G. et al. Proteomic atlas of organ vasculopathies triggered by Staphylococcus aureus sepsis. Nat. Commun. 10, 4656 (2019).
de Graaf, I. A. et al. Preparation and incubation of precision-cut liver and intestinal slices for application in drug metabolism and toxicity studies. Nat. Protoc. 5, 1540–1551 (2010).
Bigaeva, E. et al. Inhibition of tyrosine kinase receptor signaling attenuates fibrogenesis in an ex vivo model of human renal fibrosis. Am. J. Physiol. Renal Physiol. 318, F117–F134 (2020).
Langenkamp, E. et al. Innovations in studying in vivo cell behavior and pharmacology in complex tissues — microvascular endothelial cells in the spotlight. Cell Tissue Res. 354, 647–669 (2013).
Skalicky, S. et al. Combining laser microdissection and microRNA expression profiling to unmask microRNA signatures in complex tissues. Biotechniques 67, 276–285 (2019).
Hasson, D., Goldstein, S. L. & Standage, S. W. The application of omic technologies to research in sepsis-associated acute kidney injury. Pediatr. Nephrol. 36, 1075–1086 (2021).
Kellum, J. A. & Prowle, J. R. Paradigms of acute kidney injury in the intensive care setting. Nat. Rev. Nephrol. 14, 217–230 (2018).
Sarma, A., Calfee, C. S. & Ware, L. B. Biomarkers and precision medicine: state of the art biomarkers precision medicine prognostic enrichment predictive enrichment. Crit. Care Clin. 36, 155–165 (2020).
Nishinakamura, R. Human kidney organoids: progress and remaining challenges. Nat. Rev. Nephrol. 15, 613–624 (2019).
Koning, M., van den Berg, C. W. & Rabelink, T. J. Stem cell-derived kidney organoids: engineering the vasculature. Cell Mol. Life Sci. 77, 2257–2273 (2020).
Zhang, J. & Hill, C. E. Differential connexin expression in preglomerular and postglomerular vasculature: accentuation during diabetes. Kidney Int. 68, 1171–1185 (2005).
Seul, K. H. & Beyer, E. C. Heterogeneous localization of connexin40 in the renal vasculature. Microvasc. Res. 59, 140–148 (2000).
Arensbak, B., Mikkelsen, H. B., Gustafsson, F., Christensen, T. & Holstein-Rathlou, N. H. Expression of connexin 37, 40, and 43 mRNA and protein in renal preglomerular arterioles. Histochem. Biol. 115, 479–487 (2001).
Hadley, T. J. et al. Postcapillary venule endothelial cells in kidney express a multispecific chemokine receptor that is structurally and functionally identical to the erythroid isoform, which is the Duffy blood group antigen. J. Clin. Invest. 94, 985–991 (1994).
Thiriot, A. et al. Differential DARC/ACKR1 expression distinguishes venular from non-venular endothelial cells in murine tissues. BMC Biol. 15, 45 (2017).
Patrakka, J. et al. Expression and subcellular distribution of novel glomerulus-associated proteins dendrin, ehd3, sh2d4a, plekhh2, and 2310066E14Rik. J. Am. Soc. Nephrol. 18, 689–697 (2007).
Samulowitz, U. et al. Human endomucin: distribution pattern, expression on high endothelial venules, and decoration with the MECA-79 epitope. Am. J. Pathol. 160, 1669–1681 (2002).
Gu, X. & Herrera, G. A. Expression of eNOS in kidneys from hypertensive patients. Int. J. Nephrol. Renovasc. Dis. 3, 11–19 (2010).
Karet, F. E. & Davenport, A. P. Localization of endothelin peptides in human kidney. Kidney Int. 49, 382–387 (1996).
Barry, D. M. et al. Molecular determinants of nephron vascular specialization in the kidney. Nat. Commun. 10, 5705 (2019).
Muczynski, K. A., Ekle, D. M., Coder, D. M. & Anderson, S. K. Normal human kidney HLA-DR-expressing renal microvascular endothelial cells: characterization, isolation, and regulation of MHC class II expression. J. Am. Soc. Nephrol. 14, 1336–1348 (2003).
Menzies, R. I. et al. Effect of P2X4 and P2X7 receptor antagonism on the pressure diuresis relationship in rats. Front. Physiol. 4, 305 (2013).
Akiyama, T., Sadahira, Y., Matsubara, K., Mori, M. & Igarashi, Y. Immunohistochemical detection of sphingosine-1-phosphate receptor 1 in vascular and lymphatic endothelial cells. J. Mol. Histol. 39, 527–533 (2008).
Naumnik, B., Borawski, J., Chyczewski, L., Pawlak, K. & Mysliwiec, M. Tissue factor and its inhibitor in human non-crescentic glomerulonephritis–immunostaining vs plasma and urinary levels. Nephrol. Dial. Transplant. 21, 3450–3457 (2006).
Al Lamki, R. S. et al. Expression of tumor necrosis factor receptors in normal kidney and rejecting renal transplants. Lab. Invest. 81, 1503–1515 (2001).
Al-Lamki, R. S. et al. TNFR1- and TNFR2-mediated signaling pathways in human kidney are cell type-specific and differentially contribute to renal injury. FASEB J. 19, 1637–1645 (2005).
Taubitz, A., Schwarz, M., Eltrich, N., Lindenmeyer, M. T. & Vielhauer, V. Distinct contributions of TNF receptor 1 and 2 to TNF-induced glomerular inflammation in mice. PLoS ONE 8, e68167 (2013).
Boffa, M. C., Burke, B. & Haudenschild, C. C. Preservation of thrombomodulin antigen on vascular and extravascular surfaces. J. Histochem. Cytochem. 35, 1267–1276 (1987).
Simon, M. et al. Expression of vascular endothelial growth factor and its receptors in human renal ontogenesis and in adult kidney. Am. J. Physiol. 268, F240–F250 (1995).
Marti, H. H. & Risau, W. Systemic hypoxia changes the organ-specific distribution of vascular endothelial growth factor and its receptors. Proc. Natl Acad. Sci. USA 95, 15809–15814 (1998).
Takahashi, K. et al. Expression of receptor-type protein tyrosine phosphatase in developing and adult renal vasculature. PLoS ONE 12, e0177192 (2017).
Pusztaszeri, M. P., Seelentag, W. & Bosman, F. T. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J. Histochem. Cytochem. 54, 385–395 (2006).
Yamamoto, K., de V., W., Fearns, C. & Loskutoff, D. J. Tissue distribution and regulation of murine von Willebrand factor gene expression in vivo. Blood 92, 2791–2801 (1998).
Pysher, T. J., Siegler, R. L., Tesh, V. L. & Taylor, F. B. Jr. von Willebrand Factor expression in a Shiga toxin-mediated primate model of hemolytic uremic syndrome. Pediatr. Dev. Pathol. 5, 472–479 (2002).
Chung, C. S. et al. Inhibition of Fas signaling prevents hepatic injury and improves organ blood flow during sepsis. Surgery 130, 339–345 (2001).
Seely, K. A. et al. Hemodynamic changes in the kidney in a pediatric rat model of sepsis-induced acute kidney injury. Am. J. Physiol. Renal Physiol. 301, F209–F217 (2011).
Holthoff, J. H., Wang, Z., Patil, N. K., Gokden, N. & Mayeux, P. R. Rolipram improves renal perfusion and function during sepsis in the mouse. J. Pharmacol. Exp. Ther. 347, 357–364 (2013).
Seymour, C. W. et al. Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA 321, 2003–2017 (2019).
Yao, Z., Petschnigg, J., Ketteler, R. & Stagljar, I. Application guide for omics approaches to cell signaling. Nat. Chem. Biol. 11, 387–397 (2015).
Langeberg, L. K. & Scott, J. D. Signalling scaffolds and local organization of cellular behaviour. Nat. Rev. Mol. Cell Biol. 16, 232–244 (2015).
Huang, J. et al. Endothelial scaffolding protein ENH (enigma homolog protein) promotes PHLPP2 (pleckstrin homology domain and leucine-rich repeat protein phosphatase 2)-mediated dephosphorylation of AKT1 and eNOS (endothelial NO synthase) promoting vascular remodeling. Arterioscler. Thromb. Vasc. Biol. 40, 1705–1721 (2020).
Ku, K. H., Subramaniam, N. & Marsden, P. A. Epigenetic determinants of flow-mediated vascular endothelial gene expression. Hypertension 74, 467–476 (2019).
Ransohoff, J. D., Wei, Y. & Khavari, P. A. The functions and unique features of long intergenic non-coding RNA. Nat. Rev. Mol. Cell Biol. 19, 143–157 (2018).
Fernandez-Hernando, C. & Suarez, Y. MicroRNAs in endothelial cell homeostasis and vascular disease. Curr. Opin. Hematol. 25, 227–236 (2018).
Bludau, I. & Aebersold, R. Proteomic and interactomic insights into the molecular basis of cell functional diversity. Nat. Rev. Mol. Cell Biol. 21, 327–340 (2020).
Round, J. L. et al. Scaffold protein Dlgh1 coordinates alternative p38 kinase activation, directing T cell receptor signals toward NFAT but not NF-kappaB transcription factors. Nat. Immunol. 8, 154–161 (2007).
Asgeirsdottir, S. A. et al. MicroRNA-126 contributes to renal microvascular heterogeneity of VCAM-1 protein expression in acute inflammation. Am. J. Physiol. Renal Physiol. 302, F1630–F1639 (2012).
Author information
Authors and Affiliations
Contributions
G.M., J.G.Z. and M.v.M. researched the data and wrote the article. G.M., J.G.Z. and J.A.A.M.K. made substantial contributions to discussion of the content. All authors reviewed or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
J.G.Z., M.v.M. and J.A.A.M.K. declare no competing interests. G.M. is co-founder and chief technology/science officer of Vivomicx, which provides tissue laser microdissection and omics analysis services.
Additional information
Peer review information
Nature Reviews Nephrology thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Immune paralysis
-
A state in which immune cells show reduced responses to stimuli. Immune paralysis can occur in response to an overload of antigen and results in susceptibility to secondary infection. The cause of immune paralysis in sepsis is not known.
- Vascular integrity
-
The homeostatic condition of blood vessels in which endothelial cells in their natural environment regulate tissue perfusion, anticoagulant–procoagulant balance, microvascular permeability and leukocyte recruitment for immune surveillance purposes.
- Perm-selective barrier
-
The barrier formed by the glomerular endothelial cells that restricts the passage of proteins but enables passage of small molecules and water.
- Dwell time
-
The time that leukocytes spend in a particular vascular segment in a tissue in vivo. Dwell time is assessed using intravital microscopy technology.
- Epigenetic mechanisms
-
Mechanisms of control of cellular phenotype that do not involve the sequence of DNA nucleotides. Epigenetic mechanisms include chemical modifications such as methylation of DNA and histones that affect the accessibility of the DNA for transcription.
- Kinases
-
Enzymes that phosphorylate target proteins in the signal transduction cascade, leading to activation of transcription factors and other molecular entities that can change the phenotype of a cell.
- Signal transduction
-
The molecular mechanisms that are used by cells to relay an external stimulus into the nucleus to enable the necessary genes to be transcribed to mount a response.
- Evans Blue Dye accumulation
-
An in vivo technique to assess organ microvascular permeability based on intravenous administration of this dye, which non-covalently associates with albumin.
- Weibel–Palade bodies
-
Storage granules for P-selectin, angiopoietin 2 and von Willebrand factor proteins that are specifically located in endothelial cells.
- Precision cut kidney slice (PCKS) technology
-
A technology that reproducibly cuts tissue slices of predetermined size from kidney biopsy samples or whole kidney tissues. This technique preserves tissue architecture and enables cellular responses to drugs and experimental conditions to be studied.
- Laser microdissection
-
A technique that combines regular microscopy with laser-based release of selected parts of a tissue section for further molecular analyses.
- Omics
-
The collective name for platform technologies that assess the molecular status of tissues and/or cells in an unbiased manner, that is, the analysis is not restricted to pre-selected molecules.
- Pharmacolomics
-
The study of drug effects on target cells and surrounding tissues using omics platform technologies.
Rights and permissions
About this article
Cite this article
Molema, G., Zijlstra, J.G., van Meurs, M. et al. Renal microvascular endothelial cell responses in sepsis-induced acute kidney injury. Nat Rev Nephrol 18, 95–112 (2022). https://doi.org/10.1038/s41581-021-00489-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-021-00489-1
This article is cited by
-
Increased Ca2 + transport across the mitochondria-associated membranes by Mfn2 inhibiting endoplasmic reticulum stress in ischemia/reperfusion kidney injury
Scientific Reports (2023)
-
GOLPH3 promotes endotoxemia-induced liver and kidney injury through Golgi stress-mediated apoptosis and inflammatory response
Cell Death & Disease (2023)
-
Understanding tumour endothelial cell heterogeneity and function from single-cell omics
Nature Reviews Cancer (2023)
-
Plasma proteomic characterization of the development of acute kidney injury in early sepsis patients
Scientific Reports (2022)
-
Predicting the risk of acute kidney injury after hematopoietic stem cell transplantation: development of a new predictive nomogram
Scientific Reports (2022)