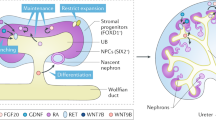
Abstract
The lineage relationships of cells provide information about the origins of component cell types during development and repair as well as the source of aberrant cells during disease. Genetic approaches to lineage tracing applied in the mouse have revealed much about how the mammalian kidney forms, including the identification of key progenitors for the nephrons and stromal compartments. Inducible Cre systems have also facilitated lineage tracing studies in the postnatal animal that illustrate the changes in cellular fate that can occur during kidney injury. With the advent of single-cell transcriptional profiling and trajectory analyses, predictions of cellular relationships across development are now being made in model systems, such as the mouse, as well as in human fetal kidney. Importantly, these approaches provide predictions of lineage relationships rather than definitive evidence. Although genetic approaches to the study of lineage have not previously been possible in a human setting, the application of CRISPR–Cas9 gene editing of pluripotent stem cells is beginning to teach us about human lineage relationships.
Key points
-
Genetic lineage tracing in mouse has informed our understanding of mammalian kidney development; this approach has been particularly valuable in identifying the key progenitor cell types that give rise to the final organ.
-
Inducible lineage tracing has also led to improved understanding of responses to postnatal kidney injury; such studies can identify the normal and maladaptive responses of the tubular epithelium, potentially providing approaches for improving repair.
-
Pseudotime analyses of single-cell transcriptional data can predict likely lineage relationships but do not provide proof of lineage.
-
CRISPR–Cas9-based gene editing enables lineage tracing within pluripotent stem cell-derived organoid models of the human kidney; this approach provides a unique opportunity to investigate lineage during human development.
-
Advances in genetic scarring approaches combined with single-cell RNA sequencing will facilitate lineage tracing at the single-cell level, thereby providing a much higher resolution to analyses of lineage relationships across time.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kobayashi, A. et al. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3, 169–181 (2008). This manuscript applied genetic lineage tracing in mice to identify a self-renewing progenitor population within the cap mesenchyme that gives rise to all segments of the epithelial nephron.
Taguchi, A. et al. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 14, 53–67 (2014). This study used lineage tracing to define the embryological orgins of the metanephric nephron progenitors, paving the way for future studies using human pluripotent stem cells to recreate these populations.
Barker, N. et al. Lgr5+ve stem/progenitor cells contribute to nephron formation during kidney development. Cell Rep. 2, 540–552 (2012). Lineage tracing for Lgr5-derived cells showed the presence of a clonal cellular expansion giving rise to the medial segment of the forming nephron in mouse.
Rinkevich, Y. et al. In vivo clonal analysis reveals lineage-restricted progenitor characteristics in mammalian kidney development, maintenance, and regeneration. Cell Rep. 7, 1270–1283 (2014). Using an adaptation of the Brainbow approach to unique fluorescent protein lineage tracing, this study investigated the origin of cells along the length of the developing, postnatal and repairing nephrons in mouse, concluding that there were tubular progenitors within individual nephron segments that contribute specifically to that segment during development and repair.
Lazzeri, E. et al. Endocycle-related tubular cell hypertrophy and progenitor proliferation recover renal function after acute kidney injury. Nat. Commun. 9, 1344 (2018). Using a combination of Confetti and FUCCI2 lineage tracing mouse models, this study discounted the proliferation of surviving tubular epithelial cells as the predominant reparative mechanism during acute kidney injury, instead demonstrating their hypertrophic response. Tubular repair was found to occur via a distinct subpopulation of Pax2 -expressing epithelial progenitors more resistant to apoptosis and possessing clonogenic capacity.
Appel, D. et al. Recruitment of podocytes from glomerular parietal epithelial cells. J. Am. Soc. Nephrol. 20, 333–343 (2009). After identifying transitionary cells at the glomerular vascular stalk possessing features of both PECs and podoctyes, this study utilized metabolic and genetic lineage tracing from the podocalyxin promoter to reveal that podocytes can be recruited from PECs under physiological conditions.
Pippin, J. W. et al. Cells of renin lineage take on a podocyte phenotype in aging nephropathy. Am. J. Physiol. Renal Physiol. 306, F1198–F1209 (2014).
Eng, D. G. et al. Detection of renin lineage cell transdifferentiation to podocytes in the kidney glomerulus with dual lineage tracing. Kidney Int. 93, 1240–1246 (2018). Using a novel FLP–FRT recombination lineage tracing technique to simultaneously track two kidney cell types, this study provided evidence of renin lineage cell transdifferentiation into podocytes following focal segmental glomerulosclerosis-induced podocyte depletion.
McKenna, A. et al. Whole-organism lineage tracing by combinatorial and cumulative genome editing. Science 353, aaf7907 (2016). One of the first proof-of-principle studies that couples CRISPR–Cas9 gene editing and next-generation sequencing technologies to interrogate lineage relationships during zebrafish development. Using this approach, the authors show that most cells in each organ are derived from a small number of progenitor cells.
Spanjaard, B. et al. Simultaneous lineage tracing and cell-type identification using CRISPR-Cas9-induced genetic scars. Nat. Biotechnol. 36, 469–473 (2018). This study reconstructs lineage trees in developing zebrafish using a strategy that combines CRISPR–Cas9-based scarring and transcriptional profiling to simultaneously track both cell lineage and cell identity.
Cotterell, J., Vila-Cejudo, M., Batlle-Morera, L. & Sharpe, J. Endogenous CRISPR/Cas9 arrays for scalable whole-organism lineage tracing. Development 147, dev184481 (2020). This study identifies endogenous genomic loci in both zebrafish and mouse that are suitable for CRISPR–Cas9-based scarring lineage tracing approaches and therefore avoids the need to introduce synthetic genetic arrays into the host genome.
Kalhor, R. et al. Developmental barcoding of whole mouse via homing CRISPR. Science 361, eaat9804 (2018).
Chan, M. M. et al. Molecular recording of mammalian embryogenesis. Nature 570, 77–82 (2019).
Saxen, L. Organogenesis of the Kidney (Cambridge Univ. Press, 1987).
Self, M. et al. Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. EMBO J. 25, 5214–5228 (2006).
Hatini, V., Huh, S. O., Herzlinger, D., Soares, V. C. & Lai, E. Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of Winged Helix transcription factor BF-2. Genes Dev. 10, 1467–1478 (1996).
Kobayashi, A. et al. Identification of a multipotent self-renewing stromal progenitor population during mammalian kidney organogenesis. Stem Cell Rep. 3, 650–662 (2014). Based upon lineage tracing from the Foxd1 promoter, this study provided evidence that the cortical stroma of the developing mouse kidney also represents a self-renewing progenitor able to give rise to multiple resulting stromal compartments in the final organ but unable to contribute to the nephrons.
Hu, Y., Li, M., Gothert, J. R., Gomez, R. A. & Sequeira-Lopez, M. L. Hemovascular progenitors in the kidney require sphingosine-1-phosphate receptor 1 for vascular development. J. Am. Soc. Nephrol. 27, 1984–1995 (2016).
Mohamed, T. & Sequeira-Lopez, M. L. S. Development of the renal vasculature. Semin. Cell Dev. Biol. 91, 132–146 (2019).
Seibler, J. et al. Rapid generation of inducible mouse mutants. Nucleic Acids Res. 31, e12 (2003).
Mugford, J. W., Sipila, P., McMahon, J. A. & McMahon, A. P. Osr1 expression demarcates a multi-potent population of intermediate mesoderm that undergoes progressive restriction to an Osr1-dependent nephron progenitor compartment within the mammalian kidney. Dev. Biol. 324, 88–98 (2008).
Takasato, M. et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526, 564–568 (2015).
Taguchi, A. & Nishinakamura, R. Higher-order kidney organogenesis from pluripotent stem cells. Cell Stem Cell 21, 730–746.e6 (2017).
Boyle, S. et al. Fate mapping using Cited1-CreERT2 mice demonstrates that the cap mesenchyme contains self-renewing progenitor cells and gives rise exclusively to nephronic epithelia. Dev. Biol. 313, 234–245 (2008). Published in the same year as Kobayashi et al. (2008), this also confirmed the origin of the nephron epithelia from the cap mesenchyme, in this instance using lineage tracing from the Cited1 promoter.
Xu, J. et al. Eya1 interacts with Six2 and Myc to regulate expansion of the nephron progenitor pool during nephrogenesis. Dev. Cell 31, 434–447 (2014).
Brown, A. C., Muthukrishnan, S. D. & Oxburgh, L. A synthetic niche for nephron progenitor cells. Dev. Cell 34, 229–241 (2015).
Tanigawa, S., Sharma, N., Hall, M. D., Nishinakamura, R. & Perantoni, A. O. Preferential propagation of competent SIX2+ nephronic progenitors by LIF/ROCKi treatment of the metanephric mesenchyme. Stem Cell Rep. 5, 435–447 (2015).
Carroll, T. J., Park, J. S., Hayashi, S., Majumdar, A. & McMahon, A. P. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev. Cell 9, 283–292 (2005).
Karner, C. M. et al. Canonical Wnt9b signaling balances progenitor cell expansion and differentiation during kidney development. Development 138, 1247–1257 (2011).
Ramalingam, H. et al. Disparate levels of beta-catenin activity determine nephron progenitor cell fate. Dev. Biol. 440, 13–21 (2018).
Georgas, K. et al. Analysis of early nephron patterning reveals a role for distal RV proliferation in fusion to the ureteric tip via a cap mesenchyme-derived connecting segment. Dev. Biol. 332, 273–286 (2009). Using Six2 lineage tracing, this study showed that the connecting segment between the forming nephrons and the ureteric tip in the developing mouse kidney is derived from the cap mesenchyme and not from the ureteric epithelium.
Rinkevich, Y., Lindau, P., Ueno, H., Longaker, M. T. & Weissman, I. L. Germ-layer and lineage-restricted stem/progenitors regenerate the mouse digit tip. Nature 476, 409–413 (2011).
Costantini, F. & Kopan, R. Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev. Cell 18, 698–712 (2010).
Thiagarajan, R. D. et al. Identification of anchor genes during kidney development defines ontological relationships, molecular subcompartments and regulatory pathways. PLoS One 6, e17286 (2011).
Yu, J., Carroll, T. J. & McMahon, A. P. Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development 129, 5301–5312 (2002).
Chi, X. et al. Ret-dependent cell rearrangements in the Wolffian duct epithelium initiate ureteric bud morphogenesis. Dev. Cell 17, 199–209 (2009).
Riccio, P., Cebrian, C., Zong, H., Hippenmeyer, S. & Costantini, F. Ret and Etv4 promote directed movements of progenitor cells during renal branching morphogenesis. PLoS Biol. 14, e1002382 (2016).
Zong, H., Espinosa, J. S., Su, H. H., Muzumdar, M. D. & Luo, L. Mosaic analysis with double markers in mice. Cell 121, 479–492 (2005). This novel approach to lineage marking, referred to as MADM, was designed to fluorescently label somatic mutant clones generated by interchromosomal recombination events.
Yu, J. et al. Identification of molecular compartments and genetic circuitry in the developing mammalian kidney. Development 139, 1863–1873 (2012).
Gandhi, D. et al. Retinoid signaling in progenitors controls specification and regeneration of the urothelium. Dev. Cell 26, 469–482 (2013).
Yu, J. et al. A Wnt7b-dependent pathway regulates the orientation of epithelial cell division and establishes the cortico-medullary axis of the mammalian kidney. Development 136, 161–171 (2009).
Das, A. et al. Stromal-epithelial crosstalk regulates kidney progenitor cell differentiation. Nat. Cell Biol. 15, 1035–1044 (2013).
Brunskill, E. W., Georgas, K., Rumballe, B., Little, M. H. & Potter, S. S. Defining the molecular character of the developing and adult kidney podocyte. PLoS ONE 6, e24640 (2011).
Wang, Y. et al. Cre/lox recombination in the lower urinary tract. Genesis 47, 409–413 (2009).
Airik, R., Bussen, M., Singh, M. K., Petry, M. & Kispert, A. Tbx18 regulates the development of the ureteral mesenchyme. J. Clin. Invest. 116, 663–674 (2006).
Bohnenpoll, T. et al. Tbx18 expression demarcates multipotent precursor populations in the developing urogenital system but is exclusively required within the ureteric mesenchymal lineage to suppress a renal stromal fate. Dev. Biol. 380, 25–36 (2013). Genetic lineage tracing in mice from the Tbx18 promoter revealed a contribution to the medullary stroma from the Tbx18-expressing stroma along the invading ureteric bud.
Bohnenpoll, T. et al. Diversification of cell lineages in ureter development. J. Am. Soc. Nephrol. 28, 1792–1801 (2017).
England, A. R. et al. Identification and characterization of cellular heterogeneity within the developing renal interstitium. Development 147, dev190108 (2020).
Combes, A. N. et al. Single cell analysis of the developing mouse kidney provides deeper insight into marker gene expression and ligand-receptor crosstalk. Development 146, dev178673 (2019).
Schmidt-Ott, K. M. et al. c-kit delineates a distinct domain of progenitors in the developing kidney. Dev. Biol. 299, 238–249 (2006).
Munro, D. A. D., Hohenstein, P. & Davies, J. A. Cycles of vascular plexus formation within the nephrogenic zone of the developing mouse kidney. Sci. Rep. 7, 3273 (2017).
Sequeira-Lopez, M. L. et al. The earliest metanephric arteriolar progenitors and their role in kidney vascular development. Am. J. Physiol. Regul. Integr. Comp. Physiol. 308, R138–R149 (2015).
Halt, K. J. et al. CD146+ cells are essential for kidney vasculature development. Kidney Int. 90, 311–324 (2016).
Grassmeyer, J. et al. Elf5 is a principal cell lineage specific transcription factor in the kidney that contributes to Aqp2 and Avpr2 gene expression. Dev. Biol. 424, 77–89 (2017).
Howden, S. E., Vanslambrouck, J. M., Wilson, S. B., Tan, K. S. & Little, M. H. Reporter-based fate mapping in human kidney organoids confirms nephron lineage relationships and reveals synchronous nephron formation. EMBO Rep. 20, e47483 (2019). Using CRISPR–Cas9-engineered human iPSCs, this study demonstrated that nephrons arising within a kidney organoid arise from a SIX2-expressing mesenchymal population in response to a canonical WNT signal, validating conservation of the anticipated murine nephrogenic programme in human organoids. This represents the only application of lineage tracing in kidney organoids.
Rae, F. et al. Characterisation and trophic functions of murine embryonic macrophages based upon the use of a Csf1r-EGFP transgene reporter. Dev. Biol. 308, 232–246 (2007).
Muthukrishnan, S. D., Ryzhov, S., Karolak, M. & Oxburgh, L. Nephron progenitor cell death elicits a limited compensatory response associated with interstitial expansion in the neonatal kidney. Dis. Model. Mech. 11, dmm030544 (2018).
Salei, N. et al. The kidney contains ontogenetically distinct dendritic cell and macrophage subtypes throughout development that differ in their inflammatory properties. J. Am. Soc. Nephrol. 31, 257–278 (2020).
Park, J. et al. Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science 360, 758–763 (2018).
Combes, A. N., Lefevre, J. G., Wilson, S., Hamilton, N. A. & Little, M. H. Cap mesenchyme cell swarming during kidney development is influenced by attraction, repulsion, and adhesion to the ureteric tip. Dev. Biol. 418, 297–306 (2016).
Lawlor, K. T. et al. Nephron progenitor commitment is a stochastic process influenced by cell migration. eLife 8, e41156 (2019).
Magella, B. et al. Cross-platform single cell analysis of kidney development shows stromal cells express Gdnf. Dev. Biol. 434, 36–47 (2018).
Takebayashi, H., Usui, N., Ono, K. & Ikenaka, K. Tamoxifen modulates apoptosis in multiple modes of action in CreER mice. Genesis 46, 775–781 (2008).
Ved, N., Curran, A., Ashcroft, F. M. & Sparrow, D. B. Tamoxifen administration in pregnant mice can be deleterious to both mother and embryo. Lab. Anim. 53, 630–633 (2019).
Wagner, D. E. & Klein, A. M. Lineage tracing meets single-cell omics: opportunities and challenges. Nat. Rev. Genet. 21, 410–427 (2020).
VanHorn, S. & Morris, S. A. Next-generation lineage tracing and fate mapping to interrogate development. Dev. Cell 56, 7–21 (2020).
McDole, K. et al. In toto imaging and reconstruction of post-implantation mouse development at the single-cell level. Cell 175, 859–876.e33 (2018).
Diep, C. Q. et al. Identification of adult nephron progenitors capable of kidney regeneration in zebrafish. Nature 470, 95–100 (2011).
Abuelo, J. G. Normotensive ischemic acute renal failure. N. Engl. J. Med. 357, 797–805 (2007).
Basile, D. P., Anderson, M. D. & Sutton, T. A. Pathophysiology of acute kidney injury. Compr. Physiol. 2, 1303–1353 (2012).
Chawla, L. S., Eggers, P. W., Star, R. A. & Kimmel, P. L. Acute kidney injury and chronic kidney disease as interconnected syndromes. N. Engl. J. Med. 371, 58–66 (2014).
Kaufman, J. M., Hardy, R. & Hayslett, J. P. Age-dependent characteristics of compensatory renal growth. Kidney Int. 8, 21–26 (1975).
Prescott, L. F. The normal urinary excretion rates of renal tubular cells, leucocytes and red blood cells. Clin. Sci. 31, 425–435 (1966).
Bonventre, J. V. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J. Am. Soc. Nephrol. 14, S55–S61 (2003).
Messier, B. & Leblond, C. P. Cell proliferation and migration as revealed by radioautography after injection of thymidine-H3 into male rats and mice. Am. J. Anat. 106, 247–285 (1960).
Venkatachalam, M. A., Bernard, D. B., Donohoe, J. F. & Levinsky, N. G. Ischemic damage and repair in the rat proximal tubule: differences among the S1, S2, and S3 segments. Kidney Int. 14, 31–49 (1978).
Witzgall, R., Brown, D., Schwarz, C. & Bonventre, J. V. Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J. Clin. Invest. 93, 2175–2188 (1994). Exploring the mechanism of recovery post-AKI, this study identifies the S3 segment of the proximal tubule as a source of mitotically active epithelial cells that undergo dedifferentiation post-ischemia, supporting the suggestions that adult tubular repair occurs via a population of mature S3 renal epithelial progenitors.
Guo, J. K., Schedl, A. & Krause, D. S. Bone marrow transplantation can attenuate the progression of mesangial sclerosis. Stem Cell 24, 406–415 (2006).
Kale, S. et al. Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J. Clin. Invest. 112, 42–49 (2003).
Lin, F. et al. Hematopoietic stem cells contribute to the regeneration of renal tubules after renal ischemia-reperfusion injury in mice. J. Am. Soc. Nephrol. 14, 1188–1199 (2003).
Prodromidi, E. I. et al. Bone marrow-derived cells contribute to podocyte regeneration and amelioration of renal disease in a mouse model of Alport syndrome. Stem Cell 24, 2448–2455 (2006).
Broekema, M. et al. Bone marrow-derived myofibroblasts contribute to the renal interstitial myofibroblast population and produce procollagen I after ischemia/reperfusion in rats. J. Am. Soc. Nephrol. 18, 165–175 (2007).
Li, J., Deane, J. A., Campanale, N. V., Bertram, J. F. & Ricardo, S. D. The contribution of bone marrow-derived cells to the development of renal interstitial fibrosis. Stem Cell 25, 697–706 (2007).
LeBleu, V. S. et al. Origin and function of myofibroblasts in kidney fibrosis. Nat. Med. 19, 1047–1053 (2013). Exploring the source of myofibroblasts in the injured kidney, this study employed multiple genetic lineage tracing mice to reveal that myofibroblasts predominantly arrise from proliferating resident fibroblasts and non-proliferating extrinsic bone marrow-derived cells. In addition, a role for pericyte-derived myofibroblasts in fibrosis was discounted.
Humphreys, B. D. et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am. J. Pathol. 176, 85–97 (2010).
Kramann, R. et al. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 16, 51–66 (2015).
Zeisberg, E. M., Potenta, S. E., Sugimoto, H., Zeisberg, M. & Kalluri, R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J. Am. Soc. Nephrol. 19, 2282–2287 (2008).
Bussolati, B. et al. Hypoxia modulates the undifferentiated phenotype of human renal inner medullary CD133+ progenitors through Oct4/miR-145 balance. Am. J. Physiol. Renal Physiol. 302, F116–F128 (2012).
Dekel, B. et al. Isolation and characterization of nontubular Sca-1+Lin− multipotent stem/progenitor cells from adult mouse kidney. J. Am. Soc. Nephrol. 17, 3300–3314 (2006).
Oliver, J. A. et al. Proliferation and migration of label-retaining cells of the kidney papilla. J. Am. Soc. Nephrol. 20, 2315–2327 (2009).
Oliver, J. A., Maarouf, O., Cheema, F. H., Martens, T. P. & Al-Awqati, Q. The renal papilla is a niche for adult kidney stem cells. J. Clin. Invest. 114, 795–804 (2004).
Challen, G. A., Bertoncello, I., Deane, J. A., Ricardo, S. D. & Little, M. H. Kidney side population reveals multilineage potential and renal functional capacity but also cellular heterogeneity. J. Am. Soc. Nephrol. 17, 1896–1912 (2006).
Gupta, S. et al. Isolation and characterization of kidney-derived stem cells. J. Am. Soc. Nephrol. 17, 3028–3040 (2006).
Kitamura, S. et al. Establishment and characterization of renal progenitor like cells from S3 segment of nephron in rat adult kidney. FASEB J. 19, 1789–1797 (2005).
Langworthy, M., Zhou, B., de Caestecker, M., Moeckel, G. & Baldwin, H. S. NFATc1 identifies a population of proximal tubule cell progenitors. J. Am. Soc. Nephrol. 20, 311–321 (2009).
Maeshima, A., Sakurai, H. & Nigam, S. K. Adult kidney tubular cell population showing phenotypic plasticity, tubulogenic capacity, and integration capability into developing kidney. J. Am. Soc. Nephrol. 17, 188–198 (2006).
Ronconi, E. et al. Regeneration of glomerular podocytes by human renal progenitors. J. Am. Soc. Nephrol. 20, 322–332 (2009).
Sagrinati, C. et al. Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J. Am. Soc. Nephrol. 17, 2443–2456 (2006).
Humphreys, B. D. et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2, 284–291 (2008).
Humphreys, B. D. et al. Repair of injured proximal tubule does not involve specialized progenitors. Proc. Natl Acad. Sci. USA 108, 9226–9231 (2011). Employing an unbiased DNA-labeling lineage-tracing approach, this study contradicts reports of a papillary stem cell niche with LRCs. Despite confirming a reduction of LRCs in the renal papillla post-AKI, this study revealed that LRCs do not proliferate or migrate to contribute to the regeneration of injured tubular epithelium.
Song, J. et al. Characterization and fate of telomerase-expressing epithelia during kidney repair. J. Am. Soc. Nephrol. 22, 2256–2265 (2011).
Adams, D. C. & Oxburgh, L. The long-term label retaining population of the renal papilla arises through divergent regional growth of the kidney. Am. J. Physiol. Renal Physiol. 297, F809–F815 (2009).
He, W. et al. Sirt1 activation protects the mouse renal medulla from oxidative injury. J. Clin. Invest. 120, 1056–1068 (2010).
Angelotti, M. L. et al. Characterization of renal progenitors committed toward tubular lineage and their regenerative potential in renal tubular injury. Stem Cell 30, 1714–1725 (2012).
Lindgren, D. et al. Isolation and characterization of progenitor-like cells from human renal proximal tubules. Am. J. Pathol. 178, 828–837 (2011).
Schutgens, F. et al. Troy/TNFRSF19 marks epithelial progenitor cells during mouse kidney development that continue to contribute to turnover in adult kidney. Proc. Natl Acad. Sci. USA 114, E11190–E11198 (2017).
Chang-Panesso, M. et al. FOXM1 drives proximal tubule proliferation during repair from acute ischemic kidney injury. J. Clin. Invest. 129, 5501–5517 (2019).
Berger, K. et al. The regenerative potential of parietal epithelial cells in adult mice. J. Am. Soc. Nephrol. 25, 693–705 (2014).
Schulte, K. et al. Origin of parietal podocytes in atubular glomeruli mapped by lineage tracing. J. Am. Soc. Nephrol. 25, 129–141 (2014).
Wanner, N. et al. Unraveling the role of podocyte turnover in glomerular aging and injury. J. Am. Soc. Nephrol. 25, 707–716 (2014).
Kaverina, N. V. et al. Dual lineage tracing shows that glomerular parietal epithelial cells can transdifferentiate toward the adult podocyte fate. Kidney Int. 96, 597–611 (2019).
Villanueva, S., Céspedes, C. & Vio, C. P. Ischemic acute renal failure induces the expression of a wide range of nephrogenic proteins. Am. J. Physiol. 290, R861–R870 (2006).
Imgrund, M. et al. Re-expression of the developmental gene Pax-2 during experimental acute tubular necrosis in mice 1. Kidney Int. 56, 1423–1431 (1999).
Houghton, D. C., Hartnett, M., Campbell-Boswell, M., Porter, G. & Bennett, W. A light and electron microscopic analysis of gentamicin nephrotoxicity in rats. Am. J. Pathol. 82, 589–612 (1976).
Kusaba, T., Lalli, M., Kramann, R., Kobayashi, A. & Humphreys, B. D. Differentiated kidney epithelial cells repair injured proximal tubule. Proc. Natl Acad. Sci. USA 111, 1527–1532 (2014). Utilizing tamoxifen-inducible genetic lineage-tracing of Slc34a1-expressing proximal tubule cells, this study lends evidence to the hypopthesis that terminally differentiated proximal tubule cells repair tubular damage via a process of dedifferentiation and proliferation.
Liu, J. et al. Cell-specific translational profiling in acute kidney injury. J. Clin. Invest. 124, 1242–1254 (2014).
Kumar, S., Liu, J. & McMahon, A. P. Defining the acute kidney injury and repair transcriptome. Semin. Nephrol. 34, 404–417 (2014).
Kumar, S. et al. Sox9 activation highlights a cellular pathway of renal repair in the acutely injured mammalian kidney. Cell Rep. 12, 1325–1338 (2015). Following their identification of Sox9 as an early epithelial injury response marker, the current study performed genetic lineage-tracing of Sox9-expressing cells following AKI, showing evidence of their proliferation and contribution to exensive proximal tubule repair. These findings serve to highlight Sox9 activity in the repair process and the parallels between repair and mammalian nephrogenesis.
Kamei, C. N., Gallegos, T. F., Liu, Y., Hukriede, N. & Drummond, I. A. Wnt signaling mediates new nephron formation during zebrafish kidney regeneration. Development 29, 146 (2019).
Kato, H. et al. Wnt/β-catenin pathway in podocytes integrates cell adhesion, differentiation, and survival. J. Biol. Chem. 286, 26003–26015 (2011).
Kato, H. & Susztak, K. Repair problems in podocytes: Wnt, Notch, and glomerulosclerosis. Semin. Nephrol. 32, 350–356 (2012).
Nawaz, M. et al. Extracellular vesicles: evolving factors in stem cell biology. Stem Cell Int. 2016, 1073140 (2016).
Tögel, F. et al. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am. J. Physiol. Renal Physiol. 289, F31–F42 (2005).
Tögel, F. et al. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am. J. Physiol. Renal Physiol. 292, F1626–F1635 (2007).
Zhao, L., Hu, C., Zhang, P., Jiang, H. & Chen, J. Genetic communication by extracellular vesicles is an important mechanism underlying stem cell-based therapy-mediated protection against acute kidney injury. Stem Cell Res. Ther. 10, 119 (2019).
Ryan, D. et al. Development of the human fetal kidney from mid to late gestation in male and female infants. EBioMedicine 27, 275–283 (2018).
Little, M. H. Improving our resolution of kidney morphogenesis across time and space. Curr. Opin. Genet. Dev. 32, 135–143 (2015).
Lindström, N. O. et al. Conserved and divergent features of mesenchymal progenitor cell types within the cortical nephrogenic niche of the human and mouse kidney. J. Am. Soc. Nephrol. 29, 806–824 (2018).
Lindström, N. O. et al. Conserved and divergent features of human and mouse kidney organogenesis. J. Am. Soc. Nephrol. 29, 785–805 (2018).
O’Brien, L. L. et al. Differential regulation of mouse and human nephron progenitors by the Six family of transcriptional regulators. Development 143, 595–608 (2016).
Kester, L. & van Oudenaarden, A. Single-cell transcriptomics meets lineage tracing. Cell Stem Cell 23, 166–179 (2018).
Trapnell, C. et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat. Biotech. 32, 381–386 (2014).
Qiu, X. et al. Reversed graph embedding resolves complex single-cell trajectories. Nat. Methods 14, 979–982 (2017).
Lindström, N. O. et al. Progressive recruitment of mesenchymal progenitors reveals a time-dependent process of cell fate acquisition in mouse and human nephrogenesis. Dev. Cell 45, 651–660.e4 (2018).
Tran, T. et al. In vivo developmental trajectories of human podocyte inform in vitro differentiation of pluripotent stem cell-derived podocytes. Dev. Cell 50, 102–116.e6 (2019).
Ader, M. & Tanaka, E. M. Modeling human development in 3D culture. Curr. Opin. Cell Biol. 31, 23–28 (2014).
Freedman, B. S. et al. Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat. Commun. 6, 8715 (2015).
Low, J. H. et al. Generation of human PSC-derived kidney organoids with patterned nephron segments and a de novo vascular network. Cell Stem Cell 25, 373–387.e9 (2019).
Morizane, R. & Bonventre, J. V. Generation of nephron progenitor cells and kidney organoids from human pluripotent stem cells. Nat. Protoc. 12, 195–207 (2017).
Phipson, B. et al. Evaluation of variability in human kidney organoids. Nat. Methods 16, 79–87 (2019).
Forbes, T. A. et al. Patient-iPSC-derived kidney organoids show functional validation of a ciliopathic renal phenotype and reveal underlying pathogenetic mechanisms. Am. J. Hum. Genet. 102, 816–831 (2018).
Cruz, N. M. et al. Organoid cystogenesis reveals a critical role of microenvironment in human polycystic kidney disease. Nat. Mater. 16, 1112–1119 (2017).
Howden, S. E. et al. Plasticity of distal nephron epithelia from human kidney organoids enables the induction of ureteric tip and stalk. Cell Stem Cell 28, 671–684.e6 (2021).
Boreström, C. et al. A CRISP(e)R view on kidney organoids allows generation of an induced pluripotent stem cell-derived kidney model for drug discovery. Kidney Int. 94, 1099–1110 (2018).
Vanslambrouck, J. M. et al. A toolbox to characterize human induced pluripotent stem cell-derived kidney cell types and organoids. J. Am. Soc. Nephrol. 30, 1811–1823 (2019).
Bao, X. et al. Gene editing to generate versatile human pluripotent stem cell reporter lines for analysis of differentiation and lineage tracing. Stem Cell 37, 1556–1566 (2019).
Kao, T. et al. GAPTrap: a simple expression system for pluripotent stem cells and their derivatives. Stem Cell Rep. 7, 518–526 (2016).
Little, M. H. & Combes, A. N. Kidney organoids: accurate models or fortunate accidents. Genes Dev. 33, 1319–1345 (2019).
Nishinakamura, R. Human kidney organoids: progress and remaining challenges. Nat. Rev. Nephrol. 15, 613–624 (2019).
Mae, S. I. et al. Expansion of Human iPSC-derived ureteric bud organoids with repeated branching potential. Cell Rep. 32, 107963 (2020).
Uchimura, K., Wu, H., Yoshimura, Y. & Humphreys, B. D. Human pluripotent stem cell-derived kidney organoids with improved collecting duct maturation and injury modeling. Cell Rep. 33, 108514 (2020).
Combes, A. N., Zappia, L., Er, P. X., Oshlack, A. & Little, M. H. Single-cell analysis reveals congruence between kidney organoids and human fetal kidney. Genome Med. 11, 3 (2019).
Wu, H. et al. Comparative analysis and refinement of human PSC-derived kidney organoid differentiation with single-cell transcriptomics. Cell Stem Cell 23, 869–881.e8 (2018).
Pei, W. et al. Polylox barcoding reveals haematopoietic stem cell fates realized in vivo. Nature 548, 456–460 (2017).
Perli, S. D., Cui, C. H. & Lu, T. K. Continuous genetic recording with self-targeting CRISPR-Cas in human cells. Science 353, aag0511 (2016).
Schmidt, S. T., Zimmerman, S. M., Wang, J., Kim, S. K. & Quake, S. R. Quantitative analysis of synthetic cell lineage tracing using nuclease barcoding. ACS Synth. Biol. 6, 936–942 (2017).
Alemany, A., Florescu, M., Baron, C. S., Peterson-Maduro, J. & van Oudenaarden, A. Whole-organism clone tracing using single-cell sequencing. Nature 556, 108–112 (2018).
Yao, Z. et al. A single-cell roadmap of lineage bifurcation in human ESC models of embryonic brain development. Cell Stem Cell 20, 120–134 (2017).
Biddy, B. A. et al. Single-cell mapping of lineage and identity in direct reprogramming. Nature 564, 219–224 (2018).
He, Z. et al. Lineage recording reveals dynamics of cerebral organoid regionalization. Preprint at bioRxiv https://doi.org/10.1101/2020.06.19.162032 (2020).
Acknowledgements
M.H.L. is a senior principal research fellow of the National Health and Medical Research Council, Australia (APP1136085). The authors were supported by the National Institutes of Health (UH3DK107344), Australian Research Council (DP190101705), NHMRC (GNT1156440), Medical Research Future Fund and the Stafford Fox Medical Research Foundation.
Author information
Authors and Affiliations
Contributions
All authors researched the data for the article, discussed the content, wrote the text and reviewed or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Nephrology thanks Benjamin Humphreys and Nicholas Barker for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Metanephric kidney
-
The postnatal kidney. The metanephric kidneys arise from the metanephric mesenchyme during embryogenesis.
- Transgenic mice
-
Mice into which foreign DNA has been delivered, usually via pronuclear injection. This term is also used more broadly to describe mice in which genetically modified pluripotent cells are introduced at the morula or blastocyst stage to generate chimeric animals.
- Lineage relationships
-
The relationships between founder and progeny. In the context of tissues and organisms, the lineage of a cell describes which cell it has arisen from.
- Morphogenesis
-
The development of morphological characteristics. In developmental biology, this term refers to the generation of form during organogenesis.
- Nephron progenitor
-
A stem cell able to give rise to nephrons via a mesenchyme to epithelial transition.
- Intermediate mesoderm
-
The region of the axial mesoderm between the paraxial and lateral plate mesoderm of the developing embryo. The intermediate mesoderm gives rise to the kidney and gonads.
- Pronephric mesenchyme
-
The region of the intermediate mesoderm that gives rise to the pronephros.
- Mesonephric mesenchyme
-
The nephrogenic region of the intermediate mesoderm that gives rise to the mesonephros.
- Metanephric mesenchyme
-
The nephrogenic region of the intermediate mesoderm that gives rise to the metanephros, including the nephron progenitors, stroma and angioblasts.
- Barcoding
-
A process of tagging with a molecular sequence for the purpose of identifying the origin of that sequence. Molecular barcodes can be introduced into individual cells via gene editing to facilitate lineage tracing or into transcripts during mRNA amplification or cDNA synthesis to facilitate multiplexing of samples in next-generation sequencing.
- Human pluripotent stem cell
-
(hPSC). Cells that are able to give rise to all germ layers. HPSCs include human embryonic stem cells, which were initially isolated from the inner cell mass of a human blastocyst, and induced pluripotent stem cells, which are generated by reprogramming of any somatic cell to an equivalent pluripotent stem cell state.
- Organoids
-
A group of cells that resemble a tissue or organ. This term has been applied to in vitro structures generated from postnatal epithelial progenitors and tissue organoids generated via the directed differentiation of pluripotent stem cells.
- CRISPR–Cas9 gene editing
-
A gene editing system in which clustered regularly interspaced short palindromic repeats (CRISPR) sequences together with a CRIPSR-associated protein 9 (Cas9) facilitates the cutting of DNA in a targeted location. CRISPR–Cas9 gene editing is now readily used for targeted gene disruption or high-fidelity gene editing in transgenic animals and in cell lines, including pluripotent stem cell lines.
- Transcriptional profiling
-
Evaluation of the global gene expression of a sample.
- Nephric duct
-
Also referred to as the Wolffian duct or the mesonephric duct, this paired structure extends along the body axis within the intermediate mesoderm into which the pronephros and mesonephros drain and from which the ureteric bud arises.
- Self-renewal
-
The process by which a dividing cell gives rise to more cells with identical cell identities. Stem cells are regarded as having a capacity to self-renew.
- Mesenchymal-to-epithelial transition
-
The process whereby a mesenchymal cell type transitions to a polarized epithelial state.
- Cre recombinase
-
A tyrosine recombinase enzyme derived from P1 bacteriophages that is able to carry out site-specific recombination events between two regions of DNA based upon recognition of a specific DNA sequence, the loxP site.
- loxP sites
-
A DNA recognition sequence consisting of two 13-bp palindromic sequences with an asymmetric core spacer measuring 8 bp in length that gives the site directionality. loxP sites are recognized by Cre recombinase as a site for cutting and recombination.
- Cap mesenchyme
-
A nephron progenitor population within the metanephros that is associated with the tips of the branching ureteric tree. Lineage tracing has identified this mesenchymal population as the progenitor for all regions of the nephron epithelium.
- Renal vesicle
-
The earliest nephron structure that arises as the immediate result of nephron progenitor mesenchyme to epithelial transition.
- Comma-shaped body
-
An early nephron stage between the renal vesicle and S-shaped body.
- S-shaped body
-
A stage II nephron comprising proximal, distal and medial segments together with a distal connecting tubule marking the point at which the nephron joins the adjacent ureteric epithelium.
- Rainbow reporter
-
A reporter system that applies the random combinatorial expression of multiple fluorescent reporters to enable colour-based lineage tracing. The reporters are flanked by Cre–loxP sites to enable stochastic labelling of each cell with the expression of three or more fluorophores in different ratios, which results in distinctive colours.
- Ureteric epithelium
-
Epithelium that gives rise to the ureteric tree and ureter of the developing metanephric kidney. Cells of this lineage ultimately contribute to the collecting ducts and ureter of the final kidney.
- Mosaic analysis with double markers
-
A genetic labelling method that enables simultaneous labelling and gene knockout within a subset of somatic cells. This method can be used to generate a small number of clonal cells that are homozygous for a deleterious gene mutation in the context of a functional heterozygous tissue.
- Angiogenesis
-
The formation of new blood vessels via sprouting from existing endothelial vessels.
- Vasculogenesis
-
A process of de novo blood vessel formation in which endothelial networks arise from endothelial progenitors known as angioblasts via coalescence
- Plasticity
-
The ability of a cell type to change identity in response to changes in culture environment.
- Transdifferentiation
-
The process by which cells of one identity adopt a distinct identity. Transdifferentiation is sometimes referred to as lineage reprogramming.
- Label-retaining cells
-
(LRCs). Cells that retain a label, usually a uridine-based tag, inserted into the DNA. As such labels reduce with cell division, slow-dividing cells, such as quiescent stem cells, retain these labels in the long term.
- Confetti lineage tracing
-
A lineage tracing approach in which a Brainbow construct is inserted into the Rosa26 locus and driven by a strong, ubiquitous promoter. This cassette yields the R26R-confetti allele, a general multicolour Cre recombinase-driven reporter.
- Fluorescent ubiquitin-based cell cycle indicator
-
(FUCCI2). A derivative of fluorescent ubiquitin-based cell cycle indicator (FUCCI) that uses dual-colour imaging to identify live cells in G(1) and S/G(2)/M cellcycle phases. FUCCI2 provides improved colour contrast via mCherry and mVenus fluorescence.
- FLP–FRT recombination
-
Flippase (FLP) is a sequence-targeted recombinase enzyme adapted from Saccharomyces cerevisiae that is able to cut DNA at flippase recognition target (FRT) sequences. FLP–FRT recombination can be combined with Cre recombinase to enable separable control of gene editing.
- Renin lineage cells
-
Juxtaglomerular perivascular cells derived from renin-expressing progenitors.
- Single-cell mRNA sequencing
-
(scRNA-seq). A method of transcriptional profiling that provides a global view of the mRNA present within each individual cell of a sample. This approach became available with the advent of next-generation sequencing technologies.
- Pseudotime analyses
-
A mathematical evaluation of distance along a trajectory of cells within a population that is generally based upon relative similarity of transcriptional profiles. Pseudotime analyses can predict potential cell identity relationships, but they do not formally prove lineage.
- Safe harbour locus
-
A site where genes or other genetic elements can be safely inserted and expressed with minimal negative impact on the host genome.
Rights and permissions
About this article
Cite this article
Little, M.H., Howden, S.E., Lawlor, K.T. et al. Determining lineage relationships in kidney development and disease. Nat Rev Nephrol 18, 8–21 (2022). https://doi.org/10.1038/s41581-021-00485-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-021-00485-5
This article is cited by
-
Preeclampsia impedes foetal kidney development by delivering placenta-derived exosomes to glomerular endothelial cells
Cell Communication and Signaling (2023)
-
An integrated organoid omics map extends modeling potential of kidney disease
Nature Communications (2023)
-
Regulation of nephron progenitor cell lifespan and nephron endowment
Nature Reviews Nephrology (2022)
-
Creating a kidney organoid-vasculature interaction model using a novel organ-on-chip system
Scientific Reports (2022)
-
Organs-on-chip technology: a tool to tackle genetic kidney diseases
Pediatric Nephrology (2022)