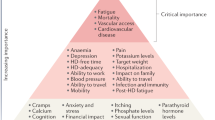
Abstract
Chronic pain is highly prevalent among adults treated with maintenance haemodialysis (HD) and has profound negative effects. Over four decades, research has demonstrated that 50–80% of adult patients treated with HD report having pain. Half of patients with HD-dependent kidney failure (HDKF) have chronic moderate-to-severe pain, which is similar to the burden of pain in patients with cancer. However, pain management in patients with HDKF is often ineffective as most patients report that their pain is inadequately treated. Opioid analgesics are prescribed more frequently for patients receiving HD than for individuals in the general population with chronic pain, and are associated with increased morbidity, mortality and health-care resource use. Furthermore, current opioid prescribing patterns are frequently inconsistent with guideline-recommended care. Evidence for the effectiveness of opioids in pain management in general, and in patients with HDKF specifically, is lacking. Nonetheless, long-term opioid therapy has a role in the treatment of some patients when used selectively, carefully and combined with an ongoing assessment of risks and benefits. Here, we provide a comprehensive overview of the use of opioid therapy in patients with HDKF and chronic pain, including a discussion of buprenorphine, which has potential as an analgesic option for patients receiving HD owing to its unique pharmacological properties.
Key points
-
Patients with kidney failure treated with haemodialysis have a pain burden that is comparable to that of patients with cancer and correlates with poor health outcomes. However, pain management is often ineffective in this population.
-
Opioid therapy can be an important pain management tool for patients treated with maintenance haemodialysis but opioids are frequently overprescribed and are associated with increased dialysis discontinuation, hospitalization and mortality.
-
Opioid use is associated with many potential risks and their risk–benefit ratio is not always clear. The use of opioids must be personalized, based on shared decision-making and implemented cautiously.
-
Kidney failure and haemodialysis can affect the pharmacokinetics and metabolism of certain opioids profoundly, which complicates their use in clinical practice; special prescribing considerations are necessary.
-
Buprenorphine has several unique pharmacological properties that make it an appealing analgesic option for patients treated with maintenance haemodialysis but effective prescribing requires a nuanced understanding of its special characteristics.
-
Monitoring for harm and the emergence of opioid use disorder in patients who are prescribed opioids is crucial. Screening for and managing opioid use disorder is an important aspect of safe opioid prescribing.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Davison, S. N. The prevalence and management of chronic pain in end-stage renal disease. J. Palliat. Med. 10, 1277–1287 (2007).
Kimmel, P. L., Emont, S. L., Newmann, J. M., Danko, H. & Moss, A. H. ESRD patient quality of life: symptoms, spiritual beliefs, psychosocial factors, and ethnicity. Am. J. Kidney Dis. 42, 713–721 (2003).
Murtagh, F. E., Addington-Hall, J. & Higginson, I. J. The prevalence of symptoms in end-stage renal disease: a systematic review. Adv. Chronic Kidney Dis. 14, 82–99 (2007).
Shayamsunder, A. K., Patel, S. S., Jain, V., Peterson, R. A. & Kimmel, P. L. Sleepiness, sleeplessness, and pain in end-stage renal disease: distressing symptoms for patients. Semin. Dial. 18, 109–118 (2005).
Weisbord, S. D. et al. Symptom burden, quality of life, advance care planning and the potential value of palliative care in severely ill haemodialysis patients. Nephrol. Dial. Transpl. 18, 1345–1352 (2003).
Weisbord, S. D. et al. Prevalence, severity, and importance of physical and emotional symptoms in chronic hemodialysis patients. J. Am. Soc. Nephrol. 16, 2487–2494 (2005).
Abdel-Kader, K., Unruh, M. L. & Weisbord, S. D. Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin. J. Am. Soc. Nephrol. 4, 1057–1064 (2009).
Brkovic, T., Burilovic, E. & Puljak, L. Prevalence and severity of pain in adult end-stage renal disease patients on chronic intermittent hemodialysis: a systematic review. Patient Preference Adherence 10, 1131–1150 (2016).
Davison, S. N. & Jhangri, G. S. Impact of pain and symptom burden on the health-related quality of life of hemodialysis patients. J. Pain. Symptom Manage. 39, 477–485 (2010).
Davison, S. N. Pain in hemodialysis patients: prevalence, cause, severity, and management. Am. J. Kidney Dis. 42, 1239–1247 (2003).
Claxton, R. N., Blackhall, L., Weisbord, S. D. & Holley, J. L. Undertreatment of symptoms in patients on maintenance hemodialysis. J. Pain. Symptom Manage. 39, 211–218 (2010).
Weisbord, S. D. et al. Renal provider recognition of symptoms in patients on maintenance hemodialysis. Clin. J. Am. Soc. Nephrol. 2, 960–967 (2007).
Jankovic, S. M. et al. Nonsteroidal antiinflammatory drugs and risk of gastrointestinal bleeding among patients on hemodialysis. J. Nephrol. 22, 502–507 (2009).
Whelton, A. Nephrotoxicity of nonsteroidal anti-inflammatory drugs: physiologic foundations and clinical implications. Am. J. Med. 106, 13S–24S (1999).
Heel, R. C., Brogden, R. N., Speight, T. M. & Avery, G. S. Buprenorphine: a review of its pharmacological properties and therapeutic efficacy. Drugs 17, 81–110 (1979).
Gudin, J. & Fudin, J. A narrative pharmacological review of buprenorphine: a unique opioid for the treatment of chronic pain. Pain. Ther. 9, 41–54 (2020).
Harris, T. J. et al. Pain, sleep disturbance and survival in hemodialysis patients. Nephrol. Dial. Transpl. 27, 758–765 (2012).
Weisbord, S. D. Patient-centered dialysis care: depression, pain, and quality of life. Semin. Dial. 29, 158–164 (2016).
Smith, D., Wilkie, R., Croft, P., Parmar, S. & McBeth, J. Pain and mortality: mechanisms for a relationship. Pain 159, 1112–1118 (2018).
Smith, D., Wilkie, R., Croft, P. & McBeth, J. Pain and mortality in older adults: the influence of pain phenotype. Arthritis Care Res. 70, 236–243 (2018).
Weisbord, S. D. et al. Associations of depressive symptoms and pain with dialysis adherence, health resource utilization, and mortality in patients receiving chronic hemodialysis. Clin. J. Am. Soc. Nephrol. 9, 1594–1602 (2014).
Davison, S. N. & Jhangri, G. S. The impact of chronic pain on depression, sleep, and the desire to withdraw from dialysis in hemodialysis patients. J. Pain. Symptom Manage. 30, 465–473 (2005).
Cohen, S. D., Patel, S. S., Khetpal, P., Peterson, R. A. & Kimmel, P. L. Pain, sleep disturbance, and quality of life in patients with chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2, 919–925 (2007).
Cheatle, M. D. Biopsychosocial approach to assessing and managing patients with chronic pain. Med. Clin. North. Am. 100, 43–53 (2016).
Wyne, A., Rai, R., Cuerden, M., Clark, W. F. & Suri, R. S. Opioid and benzodiazepine use in end-stage renal disease: a systematic review. Clin. J. Am. Soc. Nephrol. 6, 326–333 (2011).
Fillingim, R. B. et al. Sex-related psychological predictors of baseline pain perception and analgesic responses to pentazocine. Biol. Psychol. 69, 97–112 (2005).
Campbell, C. M., Edwards, R. R. & Fillingim, R. B. Ethnic differences in responses to multiple experimental pain stimuli. Pain 113, 20–26 (2005).
Fillingim, R. B. et al. Morphine responses and experimental pain: sex differences in side effects and cardiovascular responses but not analgesia. J. Pain. 6, 116–124 (2005).
Hastie, B. A., Riley, J. L. & Fillingim, R. B. Ethnic differences and responses to pain in healthy young adults. Pain. Med. 6, 61–71 (2005).
Schafer, G., Prkachin, K. M., Kaseweter, K. A. & Williams, A. C. Health care providers’ judgments in chronic pain: the influence of gender and trustworthiness. Pain 157, 1618–1625 (2016).
Hoffman, K. M., Trawalter, S., Axt, J. R. & Oliver, M. N. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc. Natl Acad. Sci. USA 113, 4296–4301 (2016).
Chen, E. H. et al. Gender disparity in analgesic treatment of emergency department patients with acute abdominal pain. Acad. Emerg. Med. 15, 414–418 (2008).
Berger, A. J. et al. Racial disparities in analgesic use amongst patients presenting to the emergency department for kidney stones in the United States. Am. J. Emerg. Med. 39, 71–74 (2021).
FitzGerald, C. & Hurst, S. Implicit bias in healthcare professionals: a systematic review. BMC Med. Ethics 18, 19 (2017).
Drwecki, B. B. Education to identify and combat racial bias in pain treatment. AMA J. Ethics 17, 221–228 (2015).
Drwecki, B. B., Moore, C. F., Ward, S. E. & Prkachin, K. M. Reducing racial disparities in pain treatment: the role of empathy and perspective-taking. Pain 152, 1001–1006 (2011).
Greenwald, A. G., McGhee, D. E. & Schwartz, J. L. Measuring individual differences in implicit cognition: the implicit association test. J. Pers. Soc. Psychol. 74, 1464–1480 (1998).
Bailie, G. R., Mason, N. A., Bragg-Gresham, J. L., Gillespie, B. W. & Young, E. W. Analgesic prescription patterns among hemodialysis patients in the DOPPS: potential for underprescription. Kidney Int. 65, 2419–2425 (2004).
Feldman, R. et al. Improving symptom management in hemodialysis patients: identifying barriers and future directions. J. Palliat. Med. 16, 1528–1533 (2013).
Green, J. A. et al. Renal provider perceptions and practice patterns regarding the management of pain, sexual dysfunction, and depression in hemodialysis patients. J. Palliat. Med. 15, 163–167 (2012).
Koncicki, H. M., Brennan, F., Vinen, K. & Davison, S. N. An approach to pain management in end stage renal disease: considerations for general management and intradialytic symptoms. Semin. Dial. 28, 384–391 (2015).
Nagar, V. R., Birthi, P., Salles, S. & Sloan, P. A. Opioid use in chronic pain patients with chronic kidney disease: a systematic review. Pain Med. 18, 1416–1449 (2017).
Ishida, J. H., McCulloch, C. E., Steinman, M. A., Grimes, B. A. & Johansen, K. L. Opioid analgesics and adverse outcomes among hemodialysis patients. Clin. J. Am. Soc. Nephrol. 13, 746–753 (2018).
Butler, A. M., Kshirsagar, A. V. & Brookhart, M. A. Opioid use in the US hemodialysis population. Am. J. Kidney Dis. 63, 171–173 (2014).
Daubresse, M., Alexander, G. C., Crews, D. C., Segev, D. L. & McAdams-DeMarco, M. A. Trends in opioid prescribing among hemodialysis patients, 2007-2014. Am. J. Nephrol. 49, 20–31 (2019).
Kimmel, P. L. et al. Opioid prescription, morbidity, and mortality in United States dialysis patients. J. Am. Soc. Nephrol. 28, 3658–3670 (2017).
Manley, H. J. et al. Factors associated with medication-related problems in ambulatory hemodialysis patients. Am. J. Kidney Dis. 41, 386–393 (2003).
Institute of Medicine (US) Committee on Advancing Pain Research, Care and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research (The National Academies Press, 2011).
Interagency Pain Research Coordinating Committee. National Pain Strategy: a comprehensive population health-level strategy for pain (IPRCC, 2016).
Busse, J. W. et al. Opioids for chronic noncancer pain: a systematic review and meta-analysis. JAMA 320, 2448–2460 (2018).
Chou, R. et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann. Intern. Med. 162, 276–286 (2015).
Roy, P. J. et al. Pain management in patients with chronic kidney disease and end-stage kidney disease. Curr. Opin. Nephrol. Hypertens. 29, 671–680 (2020).
Clive, D. M. & Clive, P. H. in Chronic Renal Disease 2nd edn Ch 65 (eds Kimmel, P. L. & Rosenberg, M. E.) 1071–1092 (Academic Press, 2019).
Kienzler, J. L., Gold, M. & Nollevaux, F. Systemic bioavailability of topical diclofenac sodium gel 1% versus oral diclofenac sodium in healthy volunteers. J. Clin. Pharmacol. 50, 50–61 (2010).
Federation of State Medical Boards. Guidelines for the chronic use of opioid analgesics (FSMB, 2017).
Dowell, D., Haegerich, T. M. & Chou, R. CDC guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm. Rep. 65, 1–49 (2016).
US Department of Veterans Affairs. VA/DoD clinical practice guideline for opioid therapy for chronic pain (USDVA, 2017).
Wachterman, M. W. et al. One-year mortality after dialysis initiation among older adults. JAMA Intern. Med. 179, 987–990 (2019).
Clarke, H., Soneji, N., Ko, D. T., Yun, L. & Wijeysundera, D. N. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ 348, g1251 (2014).
Deyo, R. A. et al. Association between initial opioid prescribing patterns and subsequent long-term use among opioid-naive patients: a statewide retrospective cohort study. J. Gen. Intern. Med. 32, 21–27 (2017).
Meske, D. S. et al. Efficacy of opioids versus placebo in chronic pain: a systematic review and meta-analysis of enriched enrollment randomized withdrawal trials. J. Pain Res. 11, 923–934 (2018).
Tobin, D. G., Keough Forte, K. & Johnson McGee, S. Breaking the pain contract: a better controlled-substance agreement for patients on chronic opioid therapy. Cleve Clin. J. Med. 83, 827–835 (2016).
Bialas, P., Maier, C., Klose, P. & Hauser, W. Efficacy and harms of long-term opioid therapy in chronic non-cancer pain: systematic review and meta-analysis of open-label extension trials with a study duration ≥26 weeks. Eur. J. Pain 24, 265–278 (2020).
Krebs, E. E. et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the SPACE randomized clinical trial. JAMA 319, 872–882 (2018).
Vowles, K. E. et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain 156, 569–576 (2015).
Klimas, J. et al. Strategies to identify patient risks of prescription opioid addiction when initiating opioids for pain: a systematic review. JAMA Netw. Open 2, e193365 (2019).
Bronstein, K., Passik, S., Munitz, L. & Leider, H. Can clinicians accurately predict which patients are misusing their medications? J. Pain 12, P3 (2011).
Moore, T. M., Jones, T., Browder, J. H., Daffron, S. & Passik, S. D. A comparison of common screening methods for predicting aberrant drug-related behavior among patients receiving opioids for chronic pain management. Pain Med. 10, 1426–1433 (2009).
Cheatle, M. D., Compton, P. A., Dhingra, L., Wasser, T. E. & O’Brien, C. P. Development of the revised opioid risk tool to predict opioid use disorder in patients with chronic nonmalignant pain. J. Pain 20, 842–851 (2019).
Paulozzi, L., Dellinger, A. & Degutis, L. Lessons from the past. Inj. Prev. 18, 70 (2012).
Benyamin, R. et al. Opioid complications and side effects. Pain Physician 11, S105–S120 (2008).
Wiese, A. D., Griffin, M. R., Schaffner, W., Stein, C. M. & Grijalva, C. G. Opioid analgesic use and risk for invasive pneumococcal diseases. Ann. Intern. Med. 169, 355 (2018).
Edelman, E. J. et al. Association of prescribed opioids with increased risk of community-acquired pneumonia among patients with and without HIV. JAMA Intern. Med. 179, 297–304 (2019).
Saunders, K. W. et al. Relationship of opioid use and dosage levels to fractures in older chronic pain patients. J. Gen. Intern. Med. 25, 310–315 (2010).
Fountas, A., Van Uum, S. & Karavitaki, N. Opioid-induced endocrinopathies. Lancet Diabetes Endocrinol. 8, 68–80 (2020).
Yi, P. & Pryzbylkowski, P. Opioid induced hyperalgesia. Pain. Med. 16 (Suppl. 1), 32–36 (2015).
Ren, Z. Y. et al. The impact of genetic variation on sensitivity to opioid analgesics in patients with postoperative pain: a systematic review and meta-analysis. Pain Physician 18, 131–152 (2015).
Gourlay, D. L., Heit, H. A. & Almahrezi, A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med. 6, 107–112 (2005).
Manchikanti, L. et al. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: Part 2–guidance. Pain Physician 15, S67–S116 (2012).
Chou, R. et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J. Pain 10, 113–130 (2009).
Centers for Disease Control and Prevention. Quality improvement and care coordination: implementing the CDC guideline for prescribing opioids for chronic pain. https://www.cdc.gov/drugoverdose/pdf/prescribing/CDC-DUIP-QualityImprovementAndCareCoordination-508.pdf (2018).
McGee, S. & Silverman, R. D. Treatment agreements, informed consent, and the role of state medical boards in opioid prescribing. Pain Med. 16, 25–29 (2015).
Buchman, D. Z. & Ho, A. What’s trust got to do with it? Revisiting opioid contracts. J. Med. Ethics 40, 673–677 (2014).
Dunn, K. M. et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann. Intern. Med. 152, 85–92 (2010).
Braden, J. B. et al. Emergency department visits among recipients of chronic opioid therapy. Arch. Intern. Med. 170, 1425–1432 (2010).
Bohnert, A. S. et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA 305, 1315–1321 (2011).
Paulozzi, L. J. et al. A history of being prescribed controlled substances and risk of drug overdose death. Pain Med. 13, 87–95 (2012).
Volkow, N. D. & McLellan, A. T. Opioid abuse in chronic pain — misconceptions and mitigation strategies. N. Engl. J. Med. 374, 1253–1263 (2016).
Cassidy, T. A., DasMahapatra, P., Black, R. A., Wieman, M. S. & Butler, S. F. Changes in prevalence of prescription opioid abuse after introduction of an abuse-deterrent opioid formulation. Pain Med. 15, 440–451 (2014).
Dasgupta, N. et al. Cohort study of the impact of high-dose opioid analgesics on overdose mortality. Pain Med. 17, 85–98 (2016).
Hirschtritt, M. E., Olfson, M. & Kroenke, K. Balancing the risks and benefits of benzodiazepines. JAMA 325, 347–348 (2021).
Gomes, T. et al. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med. 14, e1002396 (2017).
Gomes, T. et al. Pregabalin and the risk for opioid-related death: a nested case-control study. Ann. Intern. Med. 169, 732–734 (2018).
Ruchi, R. et al. Opioid safety and concomitant benzodiazepine use in end-stage renal disease patients. Pain Res. Manag. 2019, 3865924 (2019).
Waddy, S. P. et al. Concomitant use of gabapentinoids with opioids is associated with increased mortality and morbidity among dialysis patients. Am. J. Nephrol. 51, 424–432 (2020).
Inciardi, J. A., Surratt, H. L., Lugo, Y. & Cicero, T. J. The diversion of prescription opioid analgesics. Law Enforc. Exec. Forum 7, 127–141 (2007).
Singh, N., Fishman, S., Rich, B. & Orlowski, A. Prescription opioid forgery: reporting to law enforcement and protection of medical information. Pain Med. 14, 792–798 (2013).
van Eeghen, C., Edwards, M., Libman, B. S., MacLean, C. D. & Kennedy, A. G. Order from chaos: an initiative to improve opioid prescribing in rheumatology using lean A3. ACR Open Rheumatol. 1, 546–551 (2019).
Longo, L. P., Parran, T., Johnson, B. & Kinsey, W. Addiction: part II. Identification and management of the drug-seeking patient. Am. Fam. Physician 61, 2401–2408 (2000).
US Food and Drug Administration. Where and how to dispose of unused medicines. https://www.fda.gov/consumers/consumer-updates/where-and-how-dispose-unused-medicines (2021).
Lea-Henry, T. N., Carland, J. E., Stocker, S. L., Sevastos, J. & Roberts, D. M. Clinical pharmacokinetics in kidney disease. Clin. J. Am. Soc. Nephrol. 13, 1085–1095 (2018).
Déri, M. T. et al. End-stage renal disease reduces the expression of drug-metabolizing cytochrome P450s. Pharmacol. Rep. 72, 1695–1705 (2020).
Dreisbach, A. W. & Lertora, J. J. L. The effect of chronic renal failure on drug metabolism and transport. Expert. Opin. Drug Metab. Toxicol. 4, 1065–1074 (2008).
Davison, S. N. Clinical pharmacology considerations in pain management in patients with advanced kidney failure. Clin. J. Am. Soc. Nephrol. 14, 917–931 (2019).
King, S., Forbes, K., Hanks, G. W., Ferro, C. J. & Chambers, E. J. A systematic review of the use of opioid medication for those with moderate to severe cancer pain and renal impairment: a European Palliative Care Research Collaborative opioid guidelines project. Palliat. Med. 25, 525–552 (2011).
Paglialunga, S., Offman, E., Ichhpurani, N., Marbury, T. C. & Morimoto, B. H. Update and trends on pharmacokinetic studies in patients with impaired renal function: practical insight into application of the FDA and EMA guidelines. Expert. Rev. Clin. Pharmacol. 10, 273–283 (2017).
Goedel, W. C. et al. Association of racial/ethnic segregation with treatment capacity for opioid use disorder in counties in the United States. JAMA Netw. Open 3, e203711 (2020).
Hansen, H., Siegel, C., Wanderling, J. & DiRocco, D. Buprenorphine and methadone treatment for opioid dependence by income, ethnicity and race of neighborhoods in New York City. Drug Alcohol. Depend. 164, 14–21 (2016).
Schuckit, M. A. Treatment of opioid-use disorders. N. Engl. J. Med. 375, 357–368 (2016).
Davis, M. P., Pasternak, G. & Behm, B. Treating chronic pain: an overview of clinical studies centered on the buprenorphine option. Drugs 78, 1211–1228 (2018).
Daitch, J. et al. Conversion of chronic pain patients from full-opioid agonists to sublingual buprenorphine. Pain Physician 15, ES59–ES66 (2012).
Daitch, D. et al. Conversion from high-dose full-opioid agonists to sublingual buprenorphine reduces pain scores and improves quality of life for chronic pain patients. Pain Med. 15, 2087–2094 (2014).
Malinoff, H. L., Barkin, R. L. & Wilson, G. Sublingual buprenorphine is effective in the treatment of chronic pain syndrome. Am. J. Ther. 12, 379–384 (2005).
Sorge, J. & Sittl, R. Transdermal buprenorphine in the treatment of chronic pain: results of a phase III, multicenter, randomized, double-blind, placebo-controlled study. Clin. Ther. 26, 1808–1820 (2004).
Center for Substance Abuse Treatment. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction (Substance Abuse and Mental Health Services Administration, 2004).
Harris, S. C., Morganroth, J., Ripa, S. R., Thorn, M. D. & Colucci, S. Effects of buprenorphine on QT intervals in healthy subjects: results of 2 randomized positive- and placebo-controlled trials. Postgrad. Med. 129, 69–80 (2017).
Kao, D. P., Haigney, M. C., Mehler, P. S. & Krantz, M. J. Arrhythmia associated with buprenorphine and methadone reported to the Food and Drug Administration. Addiction 110, 1468–1475 (2015).
Volpe, D. A. et al. Uniform assessment and ranking of opioid μ receptor binding constants for selected opioid drugs. Regul. Toxicol. Pharmacol. 59, 385–390 (2011).
Becker, W. C., Frank, J. W. & Edens, E. L. Switching from high-dose, long-term opioids to buprenorphine: a case series. Ann. Intern. Med. 173, 70–71 (2020).
Ghosh, S. M., Klaire, S., Tanguay, R., Manek, M. & Azar, P. A review of novel methods to support the transition from methadone and other full agonist opioids to buprenorphine/naloxone sublingual in both community and acute care settings. Can. J. Addict. 10, 41–50 (2019).
Filitz, J. et al. Effects of intermittent hemodialysis on buprenorphine and norbuprenorphine plasma concentrations in chronic pain patients treated with transdermal buprenorphine. Eur. J. Pain 10, 743–748 (2006).
Elkader, A. & Sproule, B. Buprenorphine: clinical pharmacokinetics in the treatment of opioid dependence. Clin. Pharmacokinet. 44, 661–680 (2005).
Salili, A. R., Muller, D., Skendaj, R., Jehle, A. W. & Taegtmeyer, A. B. Breakthrough pain associated with a reduction in serum buprenorphine concentration during dialysis. Clin. Ther. 38, 212–215 (2016).
Substance Abuse and Mental Health Services Administration. Medications for opioid use disorder. Treatment Improvement Protocol: TIP 63 (SAMHSA, 2021).
Becker, W. C. et al. Evaluation of an integrated, multidisciplinary program to address unsafe use of opioids prescribed for pain. Pain Med. 19, 1419–1424 (2018).
Jannetto, P. J. & Langman, L. J. Using clinical laboratory tests to monitor drug therapy in pain management patients. J. Appl. Lab. Med. 2, 471–472 (2019).
Argoff, C. E. et al. Rational urine drug monitoring in patients receiving opioids for chronic pain: consensus recommendations. Pain Med. 19, 97–117 (2018).
Tobin, D. G. A rational approach to opioid use disorder in primary care. Cleve Clin. J. Med. 84, 385–387 (2017).
Substance Abuse and Mental Health Services Administration. Federal guidelines for opioid treatment programs (SAMHSA, 2015).
Ward, M. B., Hackenmueller, S. A. & Strathmann, F. G. for the Education Committee of the Academy of Clinical Laboratory Physicians and Scientists. Pathology consultation on urine compliance testing and drug abuse screening. Am. J. Clin. Pathol. 142, 586–593 (2014).
Kluge, J., Rentzsch, L., Remane, D., Peters, F. T. & Wissenbach, D. K. Systematic investigations of novel validity parameters in urine drug testing and prevalence of urine adulteration in a two-year cohort. Drug Test. Anal. 10, 1536–1542 (2018).
Allen, K. R. Screening for drugs of abuse: which matrix, oral fluid or urine? Ann. Clin. Biochem. 48, 531–541 (2011).
Honarmand, M., Farhad-Mollashahi, L., Nakhaee, A. & Sargolzaie, F. Oral manifestation and salivary changes in renal patients undergoing hemodialysis. J. Clin. Exp. Dent. 9, e207–e210 (2017).
Chiu, Y. W. et al. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin. J. Am. Soc. Nephrol. 4, 1089–1096 (2009).
Park, H. et al. Adherence and persistence to prescribed medication therapy among Medicare part D beneficiaries on dialysis: comparisons of benefit type and benefit phase. J. Manag. Care Spec. Pharm. 20, 862–876 (2014).
St. Peter, W. L. Management of polypharmacy in dialysis patients. Semin. Dialysis 28, 427–432 (2015).
Pai, A. B. et al. Medication reconciliation and therapy management in dialysis-dependent patients: need for a systematic approach. Clin. J. Am. Soc. Nephrol. 8, 1988–1999 (2013).
Kaplan, B., Mason, N. A., Shimp, L. A. & Ascione, F. J. Chronic hemodialysis patients. Part I: characterization and drug-related problems. Ann. Pharmacother. 28, 316–319 (1994).
Nakhaee, S. et al. Tramadol and the occurrence of seizures: a systematic review and meta-analysis. Crit. Rev. Toxicol. 49, 710–723 (2019).
Hanes, S. D., Franklin, M., Kuhl, D. A. & Headley, A. S. Prolonged opioid antagonism with naloxone in chronic renal failure. Pharmacotherapy 19, 897–901 (1999).
Sohn, M., Talbert, J. C., Huang, Z., Lofwall, M. R. & Freeman, P. R. Association of naloxone coprescription laws with naloxone prescription dispensing in the United States. JAMA Netw. Open 2, e196215 (2019).
Guy, G. P. Jr et al. Vital signs: pharmacy-based naloxone dispensing–United States, 2012–2018. MMWR Morb. Mortal. Wkly. Rep. 68, 679–686 (2019).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5™ 5th ed (American Psychiatric Publishing, 2013).
Banta-Green, C. J. et al. The Prescribed Opioids Difficulties Scale: a patient-centered assessment of problems and concerns. Clin. J. Pain. 26, 489–497 (2010).
Tobin, D. G., Andrews, R. & Becker, W. C. Prescribing opioids in primary care: safely starting, monitoring, and stopping. Cleve Clin. J. Med. 83, 207–215 (2016).
Bruneau, J. et al. Management of opioid use disorders: a national clinical practice guideline. CMAJ 190, E247–E257 (2018).
Kampman, K. & Jarvis, M. American Society of Addiction Medicine (ASAM) National Practice Guideline for the use of medications in the treatment of addiction involving opioid use. J. Addict. Med. 9, 358–367 (2015).
European Monitoring Centre for Drugs and Drug Addiction. Health and social responses to drug problems: a European guide (Publications Office of the European Union, 2017).
Zhan, M. et al. Association of opioids and nonsteroidal anti-inflammatory drugs with outcomes in CKD: findings from the CRIC (Chronic Renal Insufficiency Cohort) study. Am. J. Kidney Dis. 76, 184–193 (2020).
Jaffe, J. A. & Kimmel, P. L. Chronic nephropathies of cocaine and heroin abuse: a critical review. Clin. J. Am. Soc. Nephrol. 1, 655–667 (2006).
Konstantinidis, I. et al. Representation of patients with kidney disease in trials of cardiovascular interventions: an updated systematic review. JAMA Intern. Med. 176, 121–124 (2016).
Kitchlu, A. et al. Representation of patients with chronic kidney disease in trials of cancer therapy. JAMA 319, 2437–2439 (2018).
US Food and Drug Administration. Enhancing the diversity of clinical trial populations — eligibility criteria, enrollment practices, and trial designs guidance for industry. https://www.fda.gov/media/127712/download (2020).
US Food and Drug Administration. Guidance for industry: pharmacokinetics in patients with impaired renal function–study design, data analysis, and impact on dosing. https://www.fda.gov/media/78573/download (2020).
Agarwal, D., Udoji, M. A. & Trescot, A. Genetic testing for opioid pain management: a primer. Pain. Ther. 6, 93–105 (2017).
Ettienne, E. B. et al. Pharmacogenomics-guided policy in opioid use disorder (OUD) management: an ethnically-diverse case-based approach. Addict. Behav. Rep. 6, 8–14 (2017).
Kapur, B. M., Lala, P. K. & Shaw, J. L. Pharmacogenetics of chronic pain management. Clin. Biochem. 47, 1169–1187 (2014).
Raouf, M., Bettinger, J., Wegrzyn, E. W., Mathew, R. O. & Fudin, J. J. Pharmacotherapeutic management of neuropathic pain in end-stage renal disease. Kidney Dis. 6, 157–167 (2020).
He, X., Fan, L., Wu, Z., He, J. & Cheng, B. Gene expression profiles reveal key pathways and genes associated with neuropathic pain in patients with spinal cord injury. Mol. Med. Rep. 15, 2120–2128 (2017).
Descalzi, G. et al. Neuropathic pain promotes adaptive changes in gene expression in brain networks involved in stress and depression. Sci. Signal. 10, eaaj1549 (2017).
Minerbi, A. et al. Altered microbiome composition in individuals with fibromyalgia. Pain 160, 2589–2602 (2019).
Martin, C. R., Osadchiy, V., Kalani, A. & Mayer, E. A. The brain-gut-microbiome axis. Cell Mol. Gastroenterol. Hepatol. 6, 133–148 (2018).
Dworsky-Fried, Z., Kerr, B. J. & Taylor, A. M. W. Microbes, microglia, and pain. Neurobiol. Pain 7, 100045–100045 (2020).
Kaye, A. D. et al. Update on the pharmacogenomics of pain management. Pharmgenomics Pers. Med. 12, 125–143 (2019).
Ruano, G. & Kost, J. A. Fundamental considerations for genetically-guided pain management with opioids based on CYP2D6 and OPRM1 polymorphisms. Pain Physician 21, E611–E621 (2018).
Molanaei, H. et al. Influence of the CYP2D6 polymorphism and hemodialysis on codeine disposition in patients with end-stage renal disease. Eur. J. Clin. Pharmacol. 66, 269–273 (2010).
Guay, D. R. et al. Pharmacokinetics and pharmacodynamics of codeine in end-stage renal disease. Clin. Pharmacol. Ther. 43, 63–71 (1988).
Koehntop, D. E. & Rodman, J. H. Fentanyl pharmacokinetics in patients undergoing renal transplantation. Pharmacotherapy 17, 746–752 (1997).
Joh, J., Sila, M. K. & Bastani, B. Nondialyzability of fentanyl with high-efficiency and high-flux membranes. Anesth. Analg. 86, 447 (1998).
Bastani, B. & Jamal, J. A. Removal of morphine but not fentanyl during haemodialysis. Nephrol. Dial. Transpl. 12, 2802–2804 (1997).
Darwish, M., Yang, R., Tracewell, W., Robertson, P. Jr & Bond, M. Effects of renal impairment and hepatic impairment on the pharmacokinetics of hydrocodone after administration of a hydrocodone extended-release tablet formulated with abuse-deterrence technology. Clin. Pharmacol. Drug Dev. 5, 141–149 (2016).
Perlman, R. et al. Intradialytic clearance of opioids: methadone versus hydromorphone. Pain 154, 2794–2800 (2013).
Lee, M. A., Leng, M. E. & Tiernan, E. J. Retrospective study of the use of hydromorphone in palliative care patients with normal and abnormal urea and creatinine. Palliat. Med. 15, 26–34 (2001).
Davison, S. N. & Mayo, P. R. Pain management in chronic kidney disease: the pharmacokinetics and pharmacodynamics of hydromorphone and hydromorphone-3-glucuronide in hemodialysis patients. J. Opioid Manag. 4, 335–344 (2008).
Hsu, C. H., Lin, T. C., Lu, C. C., Lin, S. H. & Ho, S. T. Clearance of meperidine and its metabolite normeperidine in hemodialysis patients with chronic noncancer pain. J. Pain Symptom Manage 47, 801–805 (2014).
Hassan, H., Bastani, B. & Gellens, M. Successful treatment of normeperidine neurotoxicity by hemodialysis. Am. J. Kidney Dis. 35, 146–149 (2000).
Opdal, M. S. et al. Effects of hemodialysis on methadone pharmacokinetics and QTc. Clin. Ther. 37, 1594–1599 (2015).
Kreek, M. J., Schecter, A. J., Gutjahr, C. L. & Hecht, M. Methadone use in patients with chronic renal disease. Drug Alcohol. Depend. 5, 197–205 (1980).
Furlan, V. et al. Methadone is poorly removed by haemodialysis. Nephrol. Dial. Transpl. 14, 254–255 (1999).
Dean, M. Opioids in renal failure and dialysis patients. J. Pain. Symptom Manage 28, 497–504 (2004).
Osborne, R., Joel, S., Grebenik, K., Trew, D. & Slevin, M. The pharmacokinetics of morphine and morphine glucuronides in kidney failure. Clin. Pharmacol. Ther. 54, 158–167 (1993).
O’Connor, N. R. & Corcoran, A. M. End-stage renal disease: symptom management and advance care planning. Am. Fam. Physician 85, 705–710 (2012).
Smith, H. S. Opioid metabolism. Mayo Clin. Proc. 84, 613–624 (2009).
Leuppi-Taegtmeyer, A. et al. Pharmacokinetics of oxycodone/naloxone and its metabolites in patients with end-stage renal disease during and between haemodialysis sessions. Nephrol. Dial. Transpl. 34, 692–702 (2019).
Samolsky Dekel, B. G. et al. Dialyzability of oxycodone and its metabolites in chronic noncancer pain patients with end-stage renal disease. Pain. Pract. 17, 604–615 (2017).
Kirvela, M., Lindgren, L., Seppala, T. & Olkkola, K. T. The pharmacokinetics of oxycodone in uremic patients undergoing renal transplantation. J. Clin. Anesth. 8, 13–18 (1996).
Pham, P. C. et al. 2017 update on pain management in patients with chronic kidney disease. Clin. Kidney J. 10, 688–697 (2017).
Izzedine, H. et al. Pharmacokinetics of tramadol in a hemodialysis patient. Nephron 92, 755–756 (2002).
Vardanyan, R. S. & Hruby, V. J. Fentanyl-related compounds and derivatives: current status and future prospects for pharmaceutical applications. Future Med. Chem. 6, 385–412 (2014).
Paramanandam, G., Prommer, E. & Schwenke, D. C. Adverse effects in hospice patients with chronic kidney disease receiving hydromorphone. J. Palliat. Med. 14, 1029–1033 (2011).
Aiyer, R., Mehta, N., Gungor, S. & Gulati, A. A systematic review of NMDA receptor antagonists for treatment of neuropathic pain in clinical practice. Clin. J. Pain 34, 450–467 (2018).
Chou, R. et al. Methadone safety: a clinical practice guideline from the American Pain Society and College on Problems of Drug Dependence, in collaboration with the Heart Rhythm Society. J. Pain 15, 321–337 (2014).
Grond, S. & Sablotzki, A. Clinical pharmacology of tramadol. Clin. Pharmacokinet. 43, 879–923 (2004).
[No authors listed] Transdermal buprenorphine (Butrans) for chronic pain. Med. Lett. Drugs Ther. 53, 31–32 (2011).
US Food and Drug Administration. Highlights of prescribing information: Butrans. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021306s015s019lbl.pdf (2014).
[No authors listed] Buprenorphine buccal film (Belbuca) for chronic pain. Med. Lett. Drugs Ther. 58, 47–48 (2016).
Chou, R., Ballantyne, J. & Lembke, A. Rethinking opioid dose tapering, prescription opioid dependence, and indications for buprenorphine. Ann. Intern. Med. 171, 427–429 (2019).
Heit, H. A., Covington, E. & Good, P. M. Dear DEA. Pain. Med. 5, 303–308 (2004).
Acknowledgements
M.B.L., W.C.B. and M.J.F. were supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the NIH under Award Number U01DK123787. M.J. was supported by the NIDDK of the NIH under Award Number U01DK123812A. P.L.K. is a Senior Advisor at NIDDK. M.B.L. was supported by the National Institute of Nursing Research of the NIH under Award Number K23NR018482. L.M.D. was supported by the NIDDK of the NIH under Award Number U01DK123813. The content is solely the responsibility of the authors. The views expressed in this paper do not necessarily represent the views of the NIDDK, the NIH, the Department of Health and Human Services, the Department of Veterans Affairs, or the government of the United States. The authors acknowledge A. Abelleira from the VA Connecticut Healthcare System in West Haven, CT, for assistance in drafting and reviewing pharmacological content presented in the tables before submission.
Author information
Authors and Affiliations
Consortia
Contributions
All authors researched data for the article, made substantial contributions to discussions of the content and wrote, reviewed or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
D.G.T. works as an expert witness for cases involving opioids. P.L.K. is a Co-Editor of Chronic Renal Disease (Academic Press) and Psychosocial Aspects of Chronic Kidney Disease, and receives royalties from Elsevier. L.M.D. receives compensation from the National Kidney Foundation for her role as a Deputy Editor of the American Journal of Kidney Diseases, and consulting fees from Merck, Cara Therapeutics and Astra Zeneca. N.D.E. receives fees from work as a scientific advisor for Somatus. T.D.N. receives consulting fees from MediBeacon and CytoSorbents, and royalties from McGraw-Hill Education. The other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Nephrology thanks M. Bair, I. Istampoulouoglou, A. Leuppi-Taegtmeyer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
American Medical association opioid CME course: https://edhub.ama-assn.org/pages/opioid-cme-course
Hemodialysis Opioid Prescribing Effort (HOPE) Consortium: https://www.niddk.nih.gov/research-funding/researchprograms/hemodialysis-opioid-prescription-effort-consortium
Opioid use disorder symptoms and severity: https://pcssnow.org/resource/opioid-use-disorder-opioid-addiction/
PDMP: https://www.pdmpassist.org/
Risk Evaluation and Mitigation Strategies courses: https://search.opioidanalgesicrems.com/RPC-RMS-PROD/Guest/GuestPageExternal.aspx
Supplementary information
Glossary
- Nociceptive pain
-
A type of pain that occurs due to the activation of nociceptors in the peripheral nervous system by noxious stimuli, including tissue injury, inflammation or disease.
- Neuropathic pain
-
A form of pain caused by a lesion or disease of the somatosensory nervous system.
- Morphine milligram equivalents
-
(MMEs). A dose that is calculated to be equivalent to a morphine dose based on the equi-analgesic potency of the opioid relative to morphine.
- Abuse-deterrent opioid formulations
-
Opioid formulations designed to resist or discourage physical or chemical adulteration of the drug (for example, by crushing, smoking, extracting or injecting the drug) while remaining safe and effective when used as intended.
- Prescription drug monitoring programs
-
(PDMPs). An electronic database that tracks and records controlled substance prescribing and dispensing data for patients.
- Partial opioid agonist
-
A drug that binds to and activates opioid receptors, but causes less receptor conformational change and activation than a full opioid agonist.
- Opioid antagonist
-
A drug that competitively binds to an opioid receptor without activating it, thus preventing receptor conformational change, and/or displacing and reversing the effects of a drug that previously activated the receptor.
- Corrected QT interval
-
(QTc). The time from the Q wave to the T wave (Q-T interval) on an electrocardiogram, divided by the square root of the time between successive R waves (R-R interval).
- Torsades de pointes
-
A form of polymorphic ventricular tachycardia that is characterized by a gradual change in the amplitude and twisting of the QRS complexes around the isoelectric line in an electrocardiogram.
- Opioid-tolerant individual
-
A patient with previous opioid exposure who can safely receive an opioid dose that would be otherwise dangerous without earlier repeated opioid exposure.
- Volume of distribution
-
The volume necessary to contain the total amount of an administered drug at the same concentration measured in plasma.
Rights and permissions
About this article
Cite this article
Tobin, D.G., Lockwood, M.B., Kimmel, P.L. et al. Opioids for chronic pain management in patients with dialysis-dependent kidney failure. Nat Rev Nephrol 18, 113–128 (2022). https://doi.org/10.1038/s41581-021-00484-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-021-00484-6
This article is cited by
-
Epidemiology of haemodialysis outcomes
Nature Reviews Nephrology (2022)