Abstract
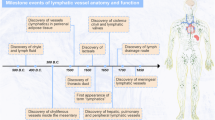
The mammalian vascular system consists of two networks: the blood vascular system and the lymphatic vascular system. Throughout the body, the lymphatic system contributes to homeostatic mechanisms by draining extravasated interstitial fluid and facilitating the trafficking and activation of immune cells. In the kidney, lymphatic vessels exist mainly in the kidney cortex. In the medulla, the ascending vasa recta represent a hybrid lymphatic-like vessel that performs lymphatic-like roles in interstitial fluid reabsorption. Although the lymphatic network is mainly derived from the venous system, evidence supports the existence of lymphatic beds that are of non-venous origin. Following their development and maturation, lymphatic vessel density remains relatively stable; however, these vessels undergo dynamic functional changes to meet tissue demands. Additionally, new lymphatic growth, or lymphangiogenesis, can be induced by pathological conditions such as tissue injury, interstitial fluid overload, hyperglycaemia and inflammation. Lymphangiogenesis is also associated with conditions such as polycystic kidney disease, hypertension, ultrafiltration failure and transplant rejection. Although lymphangiogenesis has protective functions in clearing accumulated fluid and immune cells, the kidney lymphatics may also propagate an inflammatory feedback loop, exacerbating inflammation and fibrosis. Greater understanding of lymphatic biology, including the developmental origin and function of the lymphatics and their response to pathogenic stimuli, may aid the development of new therapeutic agents that target the lymphatic system.
Key points
-
Advances in imaging and genetics technologies have furthered our understanding of the role of lymphatic vascular systems in both homeostasis and disease.
-
Key advances include the discovery of hybrid lymphatic-like vessels in multiple tissues including in the kidney, where the ascending vasa recta express a combination of both blood and lymphatic endothelial markers and perform a lymphatic-like role in reabsorbing interstitial fluid in the medulla.
-
Kidney lymphangiogenesis is strongly associated with injury, inflammation and the progression of fibrosis.
-
Lymphangiogenesis can perform a protective role in clearing the accumulated fluid and immune cells associated with inflammation from the interstitial space; however, the kidney lymphatics also function to propagate an inflammatory feedback loop in coordination with the draining lymph nodes, which may exacerbate inflammation and fibrosis. In addition, chronic inflammation can result in the disorganized growth of leaky, poorly functioning lymphatic vessels, further contributing to tissue injury.
-
Targeting the lymphatic system is a potential future direction for new therapeutics for kidney disease, and several therapies are undergoing investigation in preclinical models.
-
Better understanding of the context-dependent consequences of kidney lymphangiogenesis, as well as the mechanisms of action and potential off-target consequences of targeting the proposed molecular pathways are needed prior to their clinical use.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Escobedo, N. & Oliver, G. Lymphangiogenesis: origin, specification, and cell fate determination. Annu. Rev. Cell Dev. Biol. 32, 677–691 (2016).
Cifarelli, V. & Eichmann, A. The intestinal lymphatic system: functions and metabolic implications. Cell Mol. Gastroenterol. Hepatol. 7, 503–513 (2019).
Bernier-Latmani, J. & Petrova, T. V. Intestinal lymphatic vasculature: structure, mechanisms and functions. Nat. Rev. Gastroenterol. Hepatol. 14, 510–526 (2017).
Martinez-Corral, I. et al. Nonvenous origin of dermal lymphatic vasculature. Circ. Res. 116, 1649–1654 (2015).
Brakenhielm, E. & Alitalo, K. Cardiac lymphatics in health and disease. Nat. Rev. Cardiol. 16, 56–68 (2019).
Louveau, A. et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat. Neurosci. 21, 1380–1391 (2018).
Aspelund, A. et al. The Schlemm’s canal is a VEGF-C/VEGFR-3-responsive lymphatic-like vessel. J. Clin. Invest. 124, 3975–3986 (2014).
Pawlak, J. B. et al. Lymphatic mimicry in maternal endothelial cells promotes placental spiral artery remodeling. J. Clin. Invest. 129, 4912–4921 (2019).
Kenig-Kozlovsky, Y. et al. Ascending vasa recta are angiopoietin/Tie2-dependent lymphatic-like vessels. J. Am. Soc. Nephrol. 29, 1097–1107 (2018).
Baluk, P. et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 204, 2349–2362 (2007).
Zhang, F., Zarkada, G., Yi, S. & Eichmann, A. Lymphatic endothelial cell junctions: molecular regulation in physiology and diseases. Front. Physiol. 11, 509 (2020).
Moore, J. E. Jr & Bertram, C. D. Lymphatic system flows. Annu. Rev. Fluid Mech. 50, 459–482 (2018).
Reed, H. O. et al. Lymphatic impairment leads to pulmonary tertiary lymphoid organ formation and alveolar damage. J. Clin. Invest. 129, 2514–2526 (2019).
Drake, R. E., Weiss, D. & Gabel, J. C. Active lymphatic pumping and sheep lung lymph flow. J. Appl. Physiol. 71, 99–103 (1991).
Rouhani, S. J. et al. Roles of lymphatic endothelial cells expressing peripheral tissue antigens in CD4 T-cell tolerance induction. Nat. Commun. 6, 6771 (2015).
Rantakari, P. et al. The endothelial protein PLVAP in lymphatics controls the entry of lymphocytes and antigens into lymph nodes. Nat. Immunol. 16, 386–396 (2015).
Hons, M. & Sixt, M. The lymph node filter revealed. Nat. Immunol. 16, 338–340 (2015).
Telinius, N. et al. Human thoracic duct in vitro: diameter-tension properties, spontaneous and evoked contractile activity. Am. J. Physiol. Heart Circ. Physiol. 299, H811–H818 (2010).
Wigle, J. T. & Oliver, G. Prox1 function is required for the development of the murine lymphatic system. Cell 98, 769–778 (1999).
Johnson, N. C. et al. Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes Dev. 22, 3282–3291 (2008).
Lee, H. W. et al. Expression of lymphatic endothelium-specific hyaluronan receptor LYVE-1 in the developing mouse kidney. Cell Tissue Res. 343, 429–444 (2011).
Tanabe, M. et al. Development of lymphatic vasculature and morphological characterization in rat kidney. Clin. Exp. Nephrol. 16, 833–842 (2012).
Sosa-Pineda, B., Wigle, J. T. & Oliver, G. Hepatocyte migration during liver development requires Prox1. Nat. Genet. 25, 254–255 (2000).
Risebro, C. A. et al. Prox1 maintains muscle structure and growth in the developing heart. Development 136, 495–505 (2009).
Norden, P. R. et al. Shear stimulation of FOXC1 and FOXC2 differentially regulates cytoskeletal activity during lymphatic valve maturation. eLife 9, e53814 (2020).
Petrova, T. V. et al. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat. Med. 10, 974–981 (2004).
Fatima, A. et al. Foxc1 and Foxc2 deletion causes abnormal lymphangiogenesis and correlates with ERK hyperactivation. J. Clin. Invest. 126, 2437–2451 (2016).
Dagenais, S. L. et al. Foxc2 is expressed in developing lymphatic vessels and other tissues associated with lymphedema-distichiasis syndrome. Gene Expr. Patterns. 4, 611–619 (2004).
Irrthum, A. et al. Mutations in the transcription factor gene SOX18 underlie recessive and dominant forms of hypotrichosis-lymphedema-telangiectasia. Am. J. Hum. Genet. 72, 1470–1478 (2003).
Francois, M., Harvey, N. L. & Hogan, B. M. The transcriptional control of lymphatic vascular development. Physiology 26, 146–155 (2011).
You, L. R. et al. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature 435, 98–104 (2005).
Lee, S. et al. Prox1 physically and functionally interacts with COUP-TFII to specify lymphatic endothelial cell fate. Blood 113, 1856–1859 (2009).
Yamazaki, T., Yoshimatsu, Y., Morishita, Y., Miyazono, K. & Watabe, T. COUP-TFII regulates the functions of Prox1 in lymphatic endothelial cells through direct interaction. Genes Cells 14, 425–434 (2009).
Lin, F. J. et al. Direct transcriptional regulation of neuropilin-2 by COUP-TFII modulates multiple steps in murine lymphatic vessel development. J. Clin. Invest. 120, 1694–1707 (2010).
Karkkainen, M. J. et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 5, 74–80 (2004).
Mishima, K. et al. Prox1 induces lymphatic endothelial differentiation via integrin alpha9 and other signaling cascades. Mol. Biol. Cell 18, 1421–1429 (2007).
Srinivasan, R. S. et al. The Prox1-Vegfr3 feedback loop maintains the identity and the number of lymphatic endothelial cell progenitors. Genes Dev. 28, 2175–2187 (2014).
Kaipainen, A. et al. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc. Natl Acad. Sci. USA 92, 3566–3570 (1995).
Daniel, E. et al. Spatiotemporal heterogeneity and patterning of developing renal blood vessels. Angiogenesis 21, 617–634 (2018).
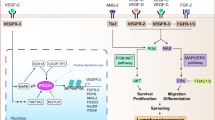
Foster, R. R. et al. Vascular endothelial growth factor-C, a potential paracrine regulator of glomerular permeability, increases glomerular endothelial cell monolayer integrity and intracellular calcium. Am. J. Pathol. 173, 938–948 (2008).
Kukk, E. et al. VEGF-C receptor binding and pattern of expression with VEGFR-3 suggests a role in lymphatic vascular development. Development 122, 3829–3837 (1996).
Haiko, P. et al. Deletion of vascular endothelial growth factor C (VEGF-C) and VEGF-D is not equivalent to VEGF receptor 3 deletion in mouse embryos. Mol. Cell Biol. 28, 4843–4850 (2008).
Baeyens, N. et al. Vascular remodeling is governed by a VEGFR3-dependent fluid shear stress set point. eLife 4, e04645 (2015).
Heinolainen, K. et al. VEGFR3 modulates vascular permeability by controlling VEGF/VEGFR2 signaling. Circ. Res. 120, 1414–1425 (2017).
Lee, A. S. et al. Vascular endothelial growth factor-C and -D are involved in lymphangiogenesis in mouse unilateral ureteral obstruction. Kidney Int. 83, 50–62 (2013).
Foster, R. R. et al. VEGF-C promotes survival in podocytes. Am. J. Physiol. Renal Physiol. 291, F196–F207 (2006).
Onions, K. L. et al. VEGFC reduces glomerular albumin permeability and protects against alterations in VEGF receptor expression in diabetic nephropathy. Diabetes 68, 172–187 (2019).
Dellinger, M. T. & Brekken, R. A. Phosphorylation of Akt and ERK1/2 is required for VEGF-A/VEGFR2-induced proliferation and migration of lymphatic endothelium. PLoS ONE 6, e28947 (2011).
Dellinger, M. T., Meadows, S. M., Wynne, K., Cleaver, O. & Brekken, R. A. Vascular endothelial growth factor receptor-2 promotes the development of the lymphatic vasculature. PLoS ONE 8, e74686 (2013).
Zheng, W. et al. Angiopoietin 2 regulates the transformation and integrity of lymphatic endothelial cell junctions. Genes Dev. 28, 1592–1603 (2014).
Gale, N. W. et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev. Cell 3, 411–423 (2002).
Kim, J. et al. Impaired angiopoietin/Tie2 signaling compromises Schlemm’s canal integrity and induces glaucoma. J. Clin. Invest. 127, 3877–3896 (2017).
Leppänen, V.-M., Saharinen, P. & Alitalo, K. Structural basis of Tie2 activation and Tie2/Tie1 heterodimerization. Proc. Natl Acad. Sci. USA 114, 4376–4381 (2017).
Qu, X., Zhou, B. & Scott Baldwin, H. Tie1 is required for lymphatic valve and collecting vessel development. Dev. Biol. 399, 117–128 (2015).
Banerji, S. et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J. Cell Biol. 144, 789–801 (1999).
Gordon, E. J., Gale, N. W. & Harvey, N. L. Expression of the hyaluronan receptor LYVE-1 is not restricted to the lymphatic vasculature; LYVE-1 is also expressed on embryonic blood vessels. Dev. Dyn. 237, 1901–1909 (2008).
Carreira, C. M. et al. LYVE-1 is not restricted to the lymph vessels: expression in normal liver blood sinusoids and down-regulation in human liver cancer and cirrhosis. Cancer Res. 61, 8079–8084 (2001).
Gale, N. W. et al. Normal lymphatic development and function in mice deficient for the lymphatic hyaluronan receptor LYVE-1. Mol. Cell. Biol. 27, 595–604 (2007).
Johnson, L. A. et al. Dendritic cells enter lymph vessels by hyaluronan-mediated docking to the endothelial receptor LYVE-1. Nat. Immunol. 18, 762–770 (2017).
Schacht, V. et al. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 22, 3546–3556 (2003).
Pan, Y., Wang, W. D. & Yago, T. Transcriptional regulation of podoplanin expression by Prox1 in lymphatic endothelial cells. Microvascular Res. 94, 96–102 (2014).
Pan, Y. & Xia, L. Emerging roles of podoplanin in vascular development and homeostasis. Front. Med. 9, 421–430 (2015).
Xu, Y. et al. Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together with VEGFR3. J. Cell Biol. 188, 115–130 (2010).
Yuan, L. et al. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development 129, 4797–4806 (2002).
Karpanen, T. et al. Functional interaction of VEGF-C and VEGF-D with neuropilin receptors. FASEB J. 20, 1462–1472 (2006).
Karpanen, T. et al. An evolutionarily conserved role for polydom/Svep1 during lymphatic vessel formation. Circ. Res. 120, 1263–1275 (2017).
Sato-Nishiuchi, R. et al. Polydom/SVEP1 is a ligand for integrin. α9β1. J. Biol. Chem. 287, 25615–25630 (2012).
Souma, T. et al. Context-dependent functions of angiopoietin 2 are determined by the endothelial phosphatase VEPTP. Proc. Natl Acad. Sci. USA 115, 1298–1303 (2018).
Bazigou, E. et al. Integrin-alpha9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev. Cell 17, 175–186 (2009).
Huntington, G. S. & McClure, C. F. W. The anatomy and development of the jugular lymph sacs in the domestic cat (Felis domestica). Am. J. Anat. 10, 177–312 (1910).
Sabin, F. R. On the origin of the lymphatic system from the veins and the development of the lymph hearts and thoracic duct in the pig. Am. J. Anat. 1, 367–389 (1902).
Yang, Y. & Oliver, G. Development of the mammalian lymphatic vasculature. J. Clin. Invest. 124, 888–897 (2014).
Stanczuk, L. et al. cKit lineage hemogenic endothelium-derived cells contribute to mesenteric lymphatic vessels. Cell Rep. 10, 1708–1721 (2015).
Mahadevan, A. et al. The left-right Pitx2 pathway drives organ-specific arterial and lymphatic development in the intestine. Dev. Cell 31, 690–706 (2014).
Yang, Y. et al. Lymphatic endothelial progenitors bud from the cardinal vein and intersomitic vessels in mammalian embryos. Blood 120, 2340–2348 (2012).
Planas-Paz, L. et al. Mechanoinduction of lymph vessel expansion. EMBO J. 31, 788–804 (2012).
Zheng, W. et al. Notch restricts lymphatic vessel sprouting induced by vascular endothelial growth factor. Blood 118, 1154–1162 (2011).
Zhang, Y. et al. Heterogeneity in VEGFR3 levels drives lymphatic vessel hyperplasia through cell-autonomous and non-cell-autonomous mechanisms. Nat. Commun. 9, 1296 (2018).
Stone, O. A. & Stainier, D. Y. R. Paraxial mesoderm is the major source of lymphatic endothelium. Dev. Cell 50, 247–255 e243 (2019).
Peirce, E. C. N. Renal lymphatics. Anat. Rec. 131, 315–335 (1944).
Bell, R. D., Keyl, M. J., Shrader, F. R., Jones, E. W. & Henry, L. P. Renal lymphatics: the internal distribution. Nephron 5, 454–463 (1968).
Cuttino, J. T. Jr, Jennette, J. C., Clark, R. L. & Kwock, L. Renal medullary lymphatics: microradiographic, light, and electron microscopic studies in pigs. Lymphology 18, 24–30 (1985).
Nordquist, R. E., Bell, R. D., Sinclair, R. J. & Keyl, M. J. The distribution and ultrastructural morphology of lymphatic vessels in the canine renal cortex. Lymphology 6, 13–19 (1973).
Eliska, O. Topography of intrarenal lymphatics. Lymphology 17, 135–141 (1984).
Tenstad, O., Heyeraas, K. J., Wiig, H. & Aukland, K. Drainage of plasma proteins from the renal medullary interstitium in rats. J. Physiol. 536, 533–539 (2001).
Kriz, W. & Kaissling, B. in Seldin and Giebisch’s the Kidney (eds Alpern, R. J., Moe, O. W., & Caplan, M. J.) Ch. 20, 595–691 (Elsevier, 2013).
Holmes, M. J., O’Morchoe, P. J. & O’Morchoe, C. C. Morphology of the intrarenal lymphatic system. Capsular and hilar communications. Am. J. Anat. 149, 333–351 (1977).
Niiro, G. K., Jarosz, H. M., O’Morchoe, P. J. & O’Morchoe, C. C. The renal cortical lymphatic system in the rat, hamster, and rabbit. Am. J. Anat. 177, 21–34 (1986).
O’Morchoe, C. C. & O’Morchoe, P. J. The renal lymphatic system: a brief review. Contrib. Nephrol. 68, 230–237 (1988).
McIntosh, G. H. & Morris, B. The lymphatics of the kidney and the formation of renal lymph. J. Physiol. 214, 365–376 (1971).
Jafree, D. J. et al. Spatiotemporal dynamics and heterogeneity of renal lymphatics in mammalian development and cystic kidney disease. eLife 8, e48183 (2019).
Albertine, K. H. & O’Morchoe, C. C. Distribution and density of the canine renal cortical lymphatic system. Kidney Int. 16, 470–480 (1979).
Cuttino, J. T. Jr, Clark, R. L. & Jennette, J. C. Microradiographic demonstration of human intrarenal microlymphatic pathways. Urol. Radiol. 11, 83–87 (1989).
Ishikawa, Y. et al. The human renal lymphatics under normal and pathological conditions. Histopathology 49, 265–273 (2006).
Munro, D. A. D., Hohenstein, P., Coate, T. M. & Davies, J. A. Refuting the hypothesis that semaphorin-3f/neuropilin-2 exclude blood vessels from the cap mesenchyme in the developing kidney. Dev. Dyn. 246, 1047–1056 (2017).
Gancz, D. et al. Distinct origins and molecular mechanisms contribute to lymphatic formation during cardiac growth and regeneration. eLife 8, e44153 (2019).
Pallone, T. L., Turner, M. R., Edwards, A. & Jamison, R. L. Countercurrent exchange in the renal medulla. Am. J. Physiol. Regul. Integr. Comp. Physiol. 284, R1153–R1175 (2003).
Kim, Y. M. et al. Role of Prox1 in the transforming ascending thin limb of Henle’s loop during mouse kidney development. PLoS ONE 10, e0127429 (2015).
Matsui, K. et al. Lymphatic microvessels in the rat remnant kidney model of renal fibrosis: aminopeptidase p and podoplanin are discriminatory markers for endothelial cells of blood and lymphatic vessels. J. Am. Soc. Nephrol. 14, 1981–1989 (2003).
Motojima, M., Kume, T. & Matsusaka, T. Foxc1 and Foxc2 are necessary to maintain glomerular podocytes. Exp. Cell Res. 352, 265–272 (2017).
Levick, J. R. & Michel, C. C. Microvascular fluid exchange and the revised Starling principle. Cardiovasc. Res. 87, 198–210 (2010).
Olszewski, W. L. The lymphatic system in body homeostasis: physiological conditions. Lymphat. Res. Biol. 1, 11–21 discussion 21–24 (2003).
Schulte-Merker, S., Sabine, A. & Petrova, T. V. Lymphatic vascular morphogenesis in development, physiology, and disease. J. Cell Biol. 193, 607–618 (2011).
Leak, L. V. & Burke, J. F. Ultrastructural studies on the lymphatic anchoring filaments. J. Cell Biol. 36, 129–149 (1968).
Lemley, K. V. & Kriz, W. Anatomy of the renal interstitium. Kidney Int. 39, 370–381 (1991).
Shelton, E. L., Yang, H. C., Zhong, J., Salzman, M. M. & Kon, V. Renal lymphatic vessel dynamics. Am. J. Physiol. Renal Physiol. 319, F1027–F1036 (2020).
Russell, P. S., Hong, J., Windsor, J. A., Itkin, M. & Phillips, A. R. J. Renal lymphatics: anatomy, physiology, and clinical implications. Front. Physiol. 10, 251 (2019).
Ranghino, A., Segoloni, G. P., Lasaponara, F. & Biancone, L. Lymphatic disorders after renal transplantation: new insights for an old complication. Clin. Kidney J. 8, 615–622 (2015).
Pedersen, M. S. et al. Lymphangiogenesis in a mouse model of renal transplant rejection extends life span of the recipients. Kidney Int. 97, 89–94 (2019).
Schineis, P., Runge, P. & Halin, C. Cellular traffic through afferent lymphatic vessels. Vasc. Pharmacol. 112, 31–41 (2019).
Card, C. M., Yu, S. S. & Swartz, M. A. Emerging roles of lymphatic endothelium in regulating adaptive immunity. J. Clin. Invest. 124, 943–952 (2014).
Lane, R. S. et al. IFNgamma-activated dermal lymphatic vessels inhibit cytotoxic T cells in melanoma and inflamed skin. J. Exp. Med. 215, 3057–3074 (2018).
Kedl, R. M. et al. Migratory dendritic cells acquire and present lymphatic endothelial cell-archived antigens during lymph node contraction. Nat. Commun. 8, 2034 (2017).
Brown, F. D. & Turley, S. J. Fibroblastic reticular cells: organization and regulation of the T lymphocyte life cycle. J. Immunol. 194, 1389–1394 (2015).
Thomas, S. N. et al. Impaired humoral immunity and tolerance in K14-VEGFR-3-Ig mice that lack dermal lymphatic drainage. J. Immunol. 189, 2181–2190 (2012).
Lukacs-Kornek, V. et al. The kidney-renal lymph node-system contributes to cross-tolerance against innocuous circulating antigen. J. Immunol. 180, 706–715 (2008).
Vieira, J. M. et al. The cardiac lymphatic system stimulates resolution of inflammation following myocardial infarction. J. Clin. Invest. 128, 3402–3412 (2018).
Machnik, A. et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat. Med. 15, 545–552 (2009).
Choi, S. Y. et al. Tonicity-responsive enhancer-binding protein mediates hyperglycemia-induced inflammation and vascular and renal injury. J. Am. Soc. Nephrol. 29, 492–504 (2018).
Sakamoto, I. et al. Lymphatic vessels develop during tubulointerstitial fibrosis. Kidney Int. 75, 828–838 (2009).
Hasegawa, S. et al. Vascular endothelial growth factor-C ameliorates renal interstitial fibrosis through lymphangiogenesis in mouse unilateral ureteral obstruction. Lab. Invest. 97, 1439–1452 (2017).
Hwang, S. D. et al. Inhibition of lymphatic proliferation by the selective VEGFR-3 inhibitor SAR131675 ameliorates diabetic nephropathy in db/db mice. Cell Death Dis. 10, 219 (2019).
Huang, J. L. et al. Vascular endothelial growth factor C for polycystic kidney diseases. J. Am. Soc. Nephrol. 27, 69–77 (2016).
Zarjou, A. et al. Dynamic signature of lymphangiogenesis during acute kidney injury and chronic kidney disease. Lab. Invest. 99, 1376–1388 (2019).
Abouelkheir, G. R., Upchurch, B. D. & Rutkowski, J. M. Lymphangiogenesis: fuel, smoke, or extinguisher of inflammation’s fire? Exp. Biol. Med. 242, 884–895 (2017).
Rabb, H. et al. Inflammation in AKI: current understanding, key questions, and knowledge gaps. J. Am. Soc. Nephrol. 27, 371–379 (2016).
Zhang, Y. et al. Activation of vascular endothelial growth factor receptor-3 in macrophages restrains TLR4-NF-kappaB signaling and protects against endotoxin shock. Immunity 40, 501–514 (2014).
Guo, Y. C. et al. Macrophages regulate unilateral ureteral obstruction-induced renal lymphangiogenesis through C-C motif chemokine receptor 2-dependent phosphatidylinositol 3-kinase-AKT-mechanistic target of rapamycin signaling and hypoxia-inducible factor-1alpha/vascular endothelial growth factor-C expression. Am. J. Pathol. 187, 1736–1749 (2017).
Pei, G. et al. Lymphangiogenesis in kidney and lymph node mediates renal inflammation and fibrosis. Sci. Adv. 5, eaaw5075 (2019).
Kajiya, K. & Detmar, M. An important role of lymphatic vessels in the control of UVB-induced edema formation and inflammation. J. Invest. Dermatol. 126, 919–921 (2006).
Guo, R. et al. Inhibition of lymphangiogenesis and lymphatic drainage via vascular endothelial growth factor receptor 3 blockade increases the severity of inflammation in a mouse model of chronic inflammatory arthritis. Arthritis Rheum. 60, 2666–2676 (2009).
Zhang, Y. et al. Lymphangiogenesis in renal fibrosis arises from macrophages via VEGF-C/VEGFR3-dependent autophagy and polarization. Cell Death Dis. 12, 109 (2021).
Kerjaschki, D. et al. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat. Med. 12, 230–234 (2006).
Mumprecht, V., Roudnicky, F. & Detmar, M. Inflammation-induced lymph node lymphangiogenesis is reversible. Am. J. Pathol. 180, 874–879 (2012).
Snelgrove, S. L. et al. Activated renal dendritic cells cross present intrarenal antigens after ischemia-reperfusion injury. Transplantation 101, 1013–1024 (2017).
Herzog, B. H. et al. Podoplanin maintains high endothelial venule integrity by interacting with platelet CLEC-2. Nature 502, 105–109 (2013).
Kasinath, V. et al. Activation of fibroblastic reticular cells in kidney lymph node during crescentic glomerulonephritis. Kidney Int. 95, 310–320 (2019).
Kataru, R. P. et al. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood 113, 5650–5659 (2009).
Wang, N. et al. Fluid balance and mortality in critically ill patients with acute kidney injury: a multicenter prospective epidemiological study. Crit. Care 19, 371 (2015).
Firth, J. D., Raine, A. E. & Ledingham, J. G. Raised venous pressure: a direct cause of renal sodium retention in oedema? Lancet 1, 1033–1035 (1988).
Prowle, J. R., Echeverri, J. E., Ligabo, E. V., Ronco, C. & Bellomo, R. Fluid balance and acute kidney injury. Nat. Rev. Nephrol. 6, 107–115 (2010).
Boor, P., Ostendorf, T. & Floege, J. Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nat. Rev. Nephrol. 6, 643–656 (2010).
Kinashi, H., Ito, Y., Sun, T., Katsuno, T. & Takei, Y. Roles of the TGF-beta(-)VEGF-C pathway in fibrosis-related lymphangiogenesis. Int. J. Mol. Sci. 19, 2487 (2018).
Cheng, J. et al. Renal lymphatic ligation aggravates renal dysfunction through induction of tubular epithelial cell apoptosis in mononephrectomized rats. Clin. Nephrol. 79, 124–131 (2013).
Zhang, T. et al. Disturbance of lymph circulation develops renal fibrosis in rats with or without contralateral nephrectomy. Nephrology 13, 128–138 (2008).
Liu, X. et al. Lymphoangiocrine signals promote cardiac growth and repair. Nature 588, 705–711 (2020).
Yazdani, S. et al. Proteinuria triggers renal lymphangiogenesis prior to the development of interstitial fibrosis. PLoS ONE 7, e50209 (2012).
Kim, Y. et al. Attenuated lymphatic proliferation ameliorates diabetic nephropathy and high-fat diet-induced renal lipotoxicity. Sci. Rep. 9, 1994 (2019).
Uchiyama, T., Takata, S., Ishikawa, H. & Sawa, Y. Altered dynamics in the renal lymphatic circulation of type 1 and type 2 diabetic mice. Acta Histochem. Cytochem. 46, 97–104 (2013).
Maruyama, K. et al. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am. J. Pathol. 170, 1178–1191 (2007).
Kajiya, K., Hirakawa, S. & Detmar, M. Vascular endothelial growth factor-A mediates ultraviolet B-induced impairment of lymphatic vessel function. Am. J. Pathol. 169, 1496–1503 (2006).
Bergmann, C. et al. Polycystic kidney disease. Nat. Rev. Dis. Prim. 4, 50 (2018).
Outeda, P. et al. Polycystin signaling is required for directed endothelial cell migration and lymphatic development. Cell Rep. 7, 634–644 (2014).
Coxam, B. et al. Pkd1 regulates lymphatic vascular morphogenesis during development. Cell Rep. 7, 623–633 (2014).
Abu-Hijleh, M. F., Habbal, O. A. & Moqattash, S. T. The role of the diaphragm in lymphatic absorption from the peritoneal cavity. J. Anat. 186, 453–467 (1995).
Mactier, R. A., Khanna, R., Twardowski, Z., Moore, H. & Nolph, K. D. Contribution of lymphatic absorption to loss of ultrafiltration and solute clearances in continuous ambulatory peritoneal dialysis. J. Clin. Invest. 80, 1311–1316 (1987).
Churchill, D. N. et al. Increased peritoneal membrane transport is associated with decreased patient and technique survival for continuous peritoneal dialysis patients. J. Am. Soc. Nephrol. 9, 1285–1292 (1998).
Brimble, K. S., Walker, M., Margetts, P. J., Kundhal, K. K. & Rabbat, C. G. Meta-analysis: peritoneal membrane transport, mortality, and technique failure in peritoneal dialysis. J. Am. Soc. Nephrol. 17, 2591–2598 (2006).
Williams, J. D. et al. Morphologic changes in the peritoneal membrane of patients with renal disease. J. Am. Soc. Nephrol. 13, 470–479 (2002).
Parikova, A., Smit, W., Struijk, D. G. & Krediet, R. T. Analysis of fluid transport pathways and their determinants in peritoneal dialysis patients with ultrafiltration failure. Kidney Int. 70, 1988–1994 (2006).
Kinashi, H. et al. TGF-beta1 promotes lymphangiogenesis during peritoneal fibrosis. J. Am. Soc. Nephrol. 24, 1627–1642 (2013).
Coester, A. M., Smit, W., Struijk, D. G., Parikova, A. & Krediet, R. T. Longitudinal analysis of peritoneal fluid transport and its determinants in a cohort of incident peritoneal dialysis patients. Perit. Dial. Int. 34, 195–203 (2014).
Yang, W. S. et al. Intraperitoneal vascular endothelial growth factor C level is related to peritoneal dialysis ultrafiltration. Blood Purif. 28, 69–74 (2009).
Terabayashi, T. et al. Vascular endothelial growth factor receptor-3 is a novel target to improve net ultrafiltration in methylglyoxal-induced peritoneal injury. Lab. Invest. 95, 1029–1043 (2015).
Kinashi, H. et al. Connective tissue growth factor is correlated with peritoneal lymphangiogenesis. Sci. Rep. 9, 12175 (2019).
Mizutani, M. et al. Connective tissue growth factor (CTGF/CCN2) is increased in peritoneal dialysis patients with high peritoneal solute transport rate. Am. J. Physiol. Renal Physiol 298, F721–F733 (2010).
Zarrinkalam, K. H., Stanley, J. M., Gray, J., Oliver, N. & Faull, R. J. Connective tissue growth factor and its regulation in the peritoneal cavity of peritoneal dialysis patients. Kidney Int. 64, 331–338 (2003).
Toda, N. et al. Deletion of connective tissue growth factor ameliorates peritoneal fibrosis by inhibiting angiogenesis and inflammation. Nephrol. Dial. Transpl. 33, 943–953 (2018).
Kinashi, H. et al. Connective tissue growth factor regulates fibrosis-associated renal lymphangiogenesis. Kidney Int. 92, 850–863 (2017).
Sakai, N. et al. Inhibition of CTGF ameliorates peritoneal fibrosis through suppression of fibroblast and myofibroblast accumulation and angiogenesis. Sci. Rep. 7, 5392 (2017).
Pi, L. et al. CCN2/CTGF regulates neovessel formation via targeting structurally conserved cystine knot motifs in multiple angiogenic regulators. FASEB J. 26, 3365–3379 (2012).
Mekarski, J. E. Essential hypertension is lymphatic: a working hypothesis. Med. Hypotheses 51, 101–103 (1998).
Lopez Gelston, C. A. & Mitchell, B. M. Recent advances in immunity and hypertension. Am. J. Hypertens. 30, 643–652 (2017).
Lopez Gelston, C. A. et al. Enhancing renal lymphatic expansion prevents hypertension in mice. Circ. Res. 122, 1094–1101 (2018).
Adrogué, H. J. & Madias, N. E. Sodium and potassium in the pathogenesis of hypertension. N. Engl. J. Med. 356, 1966–1978 (2007).
Ziomber, A. et al. Sodium-, potassium-, chloride-, and bicarbonate-related effects on blood pressure and electrolyte homeostasis in deoxycorticosterone acetate-treated rats. Am. J. Physiol. Renal Physiol. 295, F1752–F1763 (2008).
Wiig, H. et al. Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J. Clin. Invest. 123, 2803–2815 (2013).
Machnik, A. et al. Mononuclear phagocyte system depletion blocks interstitial tonicity-responsive enhancer binding protein/vascular endothelial growth factor C expression and induces salt-sensitive hypertension in rats. Hypertension 55, 755–761 (2010).
Song, L. et al. Lymphangiogenic therapy prevents cardiac dysfunction by ameliorating inflammation and hypertension. eLife 9, e58376 (2020).
Yang, G. H. et al. VEGF-C-mediated cardiac lymphangiogenesis in high salt intake accelerated progression of left ventricular remodeling in spontaneously hypertensive rats. Clin. Exp. Hypertens. 39, 740–747 (2017).
Beaini, S. et al. VEGF-C attenuates renal damage in salt-sensitive hypertension. J. Cell Physiol. 234, 9616–9630 (2019).
Balasubbramanian, D. et al. Kidney-specific lymphangiogenesis increases sodium excretion and lowers blood pressure in mice. J. Hypertens. 38, 874–885 (2020).
Kneedler, S. C. et al. Renal inflammation and injury are associated with lymphangiogenesis in hypertension. Am. J. Physiol. Renal Physiol. 312, F861–F869 (2017).
Balasubbramanian, D. et al. Augmenting renal lymphatic density prevents angiotensin II-induced hypertension in male and female mice. Am. J. Hypertens. 33, 61–69 (2019).
Franco, M. et al. Impaired pressure natriuresis resulting in salt-sensitive hypertension is caused by tubulointerstitial immune cell infiltration in the kidney. Am. J. Physiol. Renal Physiol. 304, F982–F990 (2013).
De Miguel, C., Lund, H. & Mattson, D. L. High dietary protein exacerbates hypertension and renal damage in Dahl SS rats by increasing infiltrating immune cells in the kidney. Hypertension 57, 269–274 (2011).
Balasubbramanian, D., Lopez Gelston, C. A., Rutkowski, J. M. & Mitchell, B. M. Immune cell trafficking, lymphatics and hypertension. Br. J. Pharmacol. 176, 1978–1988 (2019).
Espinosa, J. R., Samy, K. P. & Kirk, A. D. Memory T cells in organ transplantation: progress and challenges. Nat. Rev. Nephrol. 12, 339–347 (2016).
Stuht, S. et al. Lymphatic neoangiogenesis in human renal allografts: results from sequential protocol biopsies. Am. J. Transpl. 7, 377–384 (2007).
Kerjaschki, D. et al. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J. Am. Soc. Nephrol. 15, 603–612 (2004).
Phillips, S. et al. Endothelial activation, lymphangiogenesis, and humoral rejection of kidney transplants. Hum. Pathol. 51, 86–95 (2016).
Talsma, D. T. et al. Increased migration of antigen presenting cells to newly-formed lymphatic vessels in transplanted kidneys by glycol-split heparin. PLoS ONE 12, e0180206 (2017).
Palin, N. K., Savikko, J. & Koskinen, P. K. Sirolimus inhibits lymphangiogenesis in rat renal allografts, a novel mechanism to prevent chronic kidney allograft injury. Transpl. Int. 26, 195–205 (2013).
Tsuchimoto, A. et al. The potential role of perivascular lymphatic vessels in preservation of kidney allograft function. Clin. Exp. Nephrol. 21, 721–731 (2017).
Cui, Y., Liu, K., Lamattina, A. M., Visner, G. & El-Chemaly, S. Lymphatic vessels: the next frontier in lung transplant. Ann. Am. Thorac. Soc. 14, S226–S232 (2017).
Norrmen, C., Tammela, T., Petrova, T. V. & Alitalo, K. Biological basis of therapeutic lymphangiogenesis. Circulation 123, 1335–1351 (2011).
Yazdani, S. et al. Targeting tubulointerstitial remodeling in proteinuric nephropathy in rats. Dis. Model Mech. 8, 919–930 (2015).
Visuri, M. T. et al. VEGF-C and VEGF-C156S in the pro-lymphangiogenic growth factor therapy of lymphedema: a large animal study. Angiogenesis 18, 313–326 (2015).
Huggenberger, R. et al. Stimulation of lymphangiogenesis via VEGFR-3 inhibits chronic skin inflammation. J. Exp. Med. 207, 2255–2269 (2010).
Raghu, G. et al. FG-3019 anti-connective tissue growth factor monoclonal antibody: results of an open-label clinical trial in idiopathic pulmonary fibrosis. Eur. Respir. J. 47, 1481–1491 (2016).
Payne, H., Ponomaryov, T., Watson, S. P. & Brill, A. Mice with a deficiency in CLEC-2 are protected against deep vein thrombosis. Blood 129, 2013–2020 (2017).
Krishnan, H. et al. Podoplanin: an emerging cancer biomarker and therapeutic target. Cancer Sci. 109, 1292–1299 (2018).
Matsui, K., Breiteneder-Geleff, S. & Kerjaschki, D. Epitope-specific antibodies to the 43-kD glomerular membrane protein podoplanin cause proteinuria and rapid flattening of podocytes. J. Am. Soc. Nephrol. 9, 2013 (1998).
Wigle, J. T. et al. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 21, 1505–1513 (2002).
Leppanen, V. M. et al. Characterization of ANGPT2 mutations associated with primary lymphedema. Sci. Transl. Med. 12, eaax8013 (2020).
Willimann, K. et al. The chemokine SLC is expressed in T cell areas of lymph nodes and mucosal lymphoid tissues and attracts activated T cells via CCR7. Eur. J. Immunol. 28, 2025–2034 (1998).
McKimmie, C. S. et al. An analysis of the function and expression of D6 on lymphatic endothelial cells. Blood 121, 3768–3777 (2013).
Young, T. L. et al. SVEP1 as a genetic modifier of TEK-related primary congenital glaucoma. Invest. Ophthalmol. Vis. Sci. 61, 6 (2020).
Hoye, A. M., Couchman, J. R., Wewer, U. M., Fukami, K. & Yoneda, A. The newcomer in the integrin family: integrin alpha9 in biology and cancer. Adv. Biol. Regul. 52, 326–339 (2012).
Sarfarazi, A. et al. Therapeutic delivery to the peritoneal lymphatics: Current understanding, potential treatment benefits and future prospects. Int. J. Pharm. 567, 118456 (2019).
Dong, J. et al. Unipedal diagnostic lymphangiography followed by sequential CT examinations in patients with idiopathic chyluria: a retrospective study. AJR Am. J. Roentgenol. 210, 792–798 (2018).
Arrive, L., Monnier-Cholley, L. & El Mouhadi, S. Use of unenhanced MR lymphography to characterize idiopathic chyluria. AJR Am. J. Roentgenol, 211, W200 (2018).
Yildirim, I. O. et al. A novel technique in the treatment of lymphoceles after renal transplantation: C-arm cone beam CT-guided percutaneous embolization of lymphatic leakage after lymphangiography. Transplantation 102, 1955–1960 (2018).
Iwai, T. et al. Experience of lymphangiography as a therapeutic tool for lymphatic leakage after kidney transplantation. Transplant. Proc. 50, 2526–2530 (2018).
Kuusk, T. et al. Lymphatic drainage from renal tumors in vivo: a prospective sentinel node study using SPECT/CT imaging. J. Urol. 199, 1426–1432 (2018).
Pawlak, J. B. & Caron, K. M. Lymphatic programing and specialization in hybrid vessels. Front. Physiol. 11, 114 (2020).
Thomson, B. R. et al. A lymphatic defect causes ocular hypertension and glaucoma in mice. J. Clin. Invest. 124, 4320–4324 (2014).
Keyl, M. J. et al. Composition of canine renal hilar lymph. Am. J. Physiol. 209, 1031–1033 (1965).
Cockett, A. T., Roberts, A. P. & Moore, R. S. Renal lymphatic transport of fluid and solutes. Investig. Urol. 7, 10–14 (1969).
Cook, V. L., Reese, A. H., Wilson, P. D. & Pinter, G. G. Access of reabsorbed glucose to renal lymph. Experientia 38, 108–109 (1982).
Bell, R. D. Renal lymph flow and composition during acetazolamide and furosemide diuresis. Lymphology 17, 10–14 (1984).
Wilcox, C. S. & Peart, W. S. Release of renin and angiotensin II into plasma and lymph during hyperchloremia. Am. J. Physiol. 253, F734–F741 (1987).
Bivol, L. M. et al. Unilateral renal ischaemia in rats induces a rapid secretion of inflammatory markers to renal lymph and increased capillary permeability. J. Physiol. 594, 1709–1726 (2016).
Acknowledgements
M.D.D. was supported by the National Institutes of Health (T32DK108738) and is currently supported by the American Society of Nephrology Ben J. Lipps Research Fellowship. S.E.Q is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (P30DK114857) and National Eye Institute (R01EY025799).
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to the drafting, researching and writing of this article. All authors reviewed and/or edited this manuscript prior to submission.
Corresponding author
Ethics declarations
Competing interests
S.E.Q. has applied for patents related to therapeutic targeting of the ANGPT–TEK pathway in ocular hypertension, glaucoma and kidney disease, receives research support, owns stocks in and is a director of Mannin Research, is an external advisory board member of AstraZeneca and receives consulting and advisory board fees from Roche, Janssen, Genentech and AstraZeneca. The other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Nephrology thanks A. Phillips, who co-reviewed with P. Russell; J. Rutkowski; and J. Titze for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Hypotrichosis–lymphoedema–telangiectasia
-
(HLT). A rare genetic syndrome characterized by lymphoedema in the lower limbs and eyelids, cutaneous telangiectasia and dilatations of superficial vasculature, and defects in hair follicle development.
- Chylous ascites
-
The accumulation of lipid-rich lymph in the peritoneal cavity as a result of lymphatic vessel dysfunction.
- Lymphocele
-
A post-surgical complication in which lymphatic fluid collects in the body.
Rights and permissions
About this article
Cite this article
Donnan, M.D., Kenig-Kozlovsky, Y. & Quaggin, S.E. The lymphatics in kidney health and disease. Nat Rev Nephrol 17, 655–675 (2021). https://doi.org/10.1038/s41581-021-00438-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-021-00438-y
This article is cited by
-
Role of the Renal Lymphatic System in Heart Failure
Current Heart Failure Reports (2023)
-
Serum soluble LYVE1 is a promising non-invasive biomarker of renal fibrosis: a population-based retrospective cross-sectional study
Immunologic Research (2023)
-
The role of lymphangiogenesis in cardiovascular diseases and heart transplantation
Heart Failure Reviews (2022)