Abstract
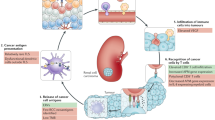
Renal cell carcinoma (RCC) is the most common type of kidney cancer and comprises several subtypes with unique characteristics. The most common subtype (~70% of cases) is clear-cell RCC. RCC is considered to be an immunogenic tumour but is known to mediate immune dysfunction in large part by eliciting the infiltration of immune-inhibitory cells, such as regulatory T cells and myeloid-derived suppressor cells, into the tumour microenvironment. Several possible mechanisms have been proposed to explain how these multiple tumour-infiltrating cell types block the development of an effective anti-tumour immune response, including inhibition of the activity of effector T cells and of antigen presenting cells via upregulation of suppressive factors such as checkpoint molecules. Targeting immune suppression using checkpoint inhibition has resulted in clinical responses in some patients with RCC and combinatorial approaches involving checkpoint blockade are now standard of care in patients with advanced RCC. However, a substantial proportion of patients do not benefit from checkpoint blockade. The identification of reliable biomarkers of response to checkpoint blockade is crucial to facilitate improvements in the clinical efficacy of these therapies. In addition, there is a need for the development of other immune-based strategies that address the shortcomings of checkpoint blockade, such as adoptive cell therapies.
Key points
-
Renal cell carcinoma (RCC) tumours are heavily infiltrated by T cells and myeloid cells; however, the tumour-infiltrating T cells do not mount effective anti-tumour responses, probably owing to the suppressive activity of regulatory T cells and myeloid cells.
-
The immunosuppressed state of RCC tumours provides an opportunity to restore anti-tumour immune responses by targeting negative regulators such as immune checkpoint molecules
-
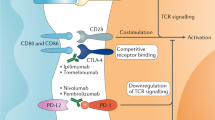
Single and double agent immune checkpoint inhibition (ICI) and combinations of immune checkpoint inhibitors (ICIs) with vascular endothelial growth factor tyrosine kinase inhibitors are now the standard of care in patients with advanced RCC.
-
Despite these important developments, only a minority of patients with RCC will obtain durable benefit from ICI therapies, underscoring the need for reliable biomarkers of response to these therapies
-
Elucidation of the mechanisms that underlie responses or resistance to ICIs will enable the rational development of combinatorial strategies aimed at improving the efficacy of these therapies
-
A better understanding of the functions of immune mediators within the tumour microenvironment in RCC could lead to the development of novel therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Wagner, R. P. Anecdotal, historical and critical commentaries on genetics. Rudolph Virchow and the genetic basis of somatic ecology. Genetics 151, 917–920 (1999).
Balkwill, F. & Mantovani, A. Inflammation and cancer: back to Virchow? Lancet 357, 539–545 (2001).
Schreiber, R. D., Old, L. J. & Smyth, M. J. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331, 1565–1570 (2011).
O’Donnell, J. S., Teng, M. W. L. & Smyth, M. J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 16, 151–167 (2019).
Lee, S. & Margolin, K. Cytokines in cancer immunotherapy. Cancers 3, 3856–3893 (2011).
Cheever, M. A. & Higano, C. S. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin. Cancer Res. 17, 3520–3526 (2011).
Hargadon, K. M., Johnson, C. E. & Williams, C. J. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int. Immunopharmacol. 62, 29–39 (2018).
Conry, R. M., Westbrook, B., McKee, S. & Norwood, T. G. Talimogene laherparepvec: first in class oncolytic virotherapy. Hum. Vaccin. Immunother. 14, 839–846 (2018).
Leach, D. R., Krummel, M. F. & Allison, J. P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 271, 1734–1736 (1996).
Keir, M. E. et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J. Exp. Med. 203, 883–895 (2006).
Raedler, L. A. Keytruda (pembrolizumab): first PD-1 inhibitor approved for previously treated unresectable or metastatic melanoma. Am. Health Drug. Benefits 8, 96–100 (2015).
Gong, J., Chehrazi-Raffle, A., Reddi, S. & Salgia, R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J. Immunother. Cancer 6, 8 (2018).
Amin, A. & Hammers, H. The evolving landscape of immunotherapy-based combinations for frontline treatment of advanced renal cell carcinoma. Front. Immunol. 9, 3120 (2018).
Escudier, B. Combination therapy as first-line treatment in metastatic renal-cell carcinoma. N. Engl. J. Med. 380, 1176–1178 (2019).
Cohen, H. T. & McGovern, F. J. Renal-cell carcinoma. N. Engl. J. Med. 353, 2477–2490 (2005).
Choueiri, T. K. Renal cell carcinoma. Hematol. Oncol. Clin. North. Am. 25, xiii–xiv (2011).
Linehan, W. M., Srinivasan, R. & Garcia, J. A. Non-clear cell renal cancer: disease-based management and opportunities for targeted therapeutic approaches. Semin. Oncol. 40, 511–520 (2013).
Gerlinger, M. et al. Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing. Nat. Genet. 46, 225–233 (2014).
Latif, F. et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science 260, 1317–1320 (1993).
Cancer Genome Atlas Research Network. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 499, 43–49 (2013).
Kaelin, W. G. Jr. The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat. Rev. Cancer 8, 865–873 (2008).
Turajlic, S. et al. Deterministic evolutionary trajectories influence primary tumor growth: TRACERx Renal. Cell 173, 595–610.e11 (2018).
Mitchell, T. J. et al. Timing the landmark events in the evolution of clear cell renal cell cancer: TRACERx Renal. Cell 173, 611–623.e17 (2018).
Brannon, A. R. et al. Molecular stratification of clear cell renal cell carcinoma by consensus clustering reveals distinct subtypes and survival patterns. Genes Cancer 1, 152–163 (2010).
Beuselinck, B. et al. Molecular subtypes of clear cell renal cell carcinoma are associated with sunitinib response in the metastatic setting. Clin. Cancer Res. 21, 1329–1339 (2015).
Beuselinck, B. et al. Pro-angiogenic gene expression is associated with better outcome on sunitinib in metastatic clear-cell renal cell carcinoma. Acta Oncol. 57, 498–508 (2018).
Verbiest, A. et al. Molecular subtypes of clear cell renal cell carcinoma are associated with outcome during pazopanib therapy in the metastatic setting. Clin. Genitourin. Cancer 16, e605–e612 (2018).
Cowey, C. L. & Rathmell, W. K. VHL gene mutations in renal cell carcinoma: role as a biomarker of disease outcome and drug efficacy. Curr. Oncol. Rep. 11, 94–101 (2009).
D’Avella, C., Abbosh, P., Pal, S. K. & Geynisman, D. M. Mutations in renal cell carcinoma. Urol. Oncol. https://doi.org/10.1016/j.urolonc.2018.10.027 (2018).
Hsieh, J. J. et al. Renal cell carcinoma. Nat. Rev. Dis. Prim. 3, 17009 (2017).
Chen, F. et al. Multilevel genomics-based taxonomy of renal cell carcinoma. Cell Rep. 14, 2476–2489 (2016).
Durinck, S. et al. Spectrum of diverse genomic alterations define non-clear cell renal carcinoma subtypes. Nat. Genet. 47, 13–21 (2015).
Cancer Genome Atlas Research Network et al. Comprehensive molecular characterization of papillary renal-cell carcinoma. N. Engl. J. Med. 374, 135–145 (2016).
Ji, S., Xiong, Y., Zhao, X., Liu, Y. & Yu, L. Q. Effect of the Nrf2-ARE signaling pathway on biological characteristics and sensitivity to sunitinib in renal cell carcinoma. Oncol. Lett. 17, 5175–5186 (2019).
Malouf, G. G., Joseph, R. W., Shah, A. Y. & Tannir, N. M. Non-clear cell renal cell carcinomas: biological insights and therapeutic challenges and opportunities. Clin. Adv. Hematol. Oncol. 15, 409–418 (2017).
Blankenstein, T., Coulie, P. G., Gilboa, E. & Jaffee, E. M. The determinants of tumour immunogenicity. Nat. Rev. Cancer 12, 307–313 (2012).
Kirkwood, J. M. & Ernstoff, M. S. Interferons in the treatment of human cancer. J. Clin. Oncol. 2, 336–352 (1984).
McDermott, D. F. Immunotherapy of metastatic renal cell carcinoma. Cancer 115, 2298–2305 (2009).
Kopecky, O. et al. Phenotype analysis of tumour-infiltrating lymphocytes and lymphocytes in peripheral blood in patients with renal carcinoma. Acta Medica 50, 207–212 (2007).
Komohara, Y. et al. Macrophage infiltration and its prognostic relevance in clear cell renal cell carcinoma. Cancer Sci. 102, 1424–1431 (2011).
Nakano, O. et al. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res. 61, 5132–5136 (2001).
Seliger, B. et al. Characterization of human lymphocyte antigen class I antigen-processing machinery defects in renal cell carcinoma lesions with special emphasis on transporter-associated with antigen-processing down-regulation. Clin. Cancer Res. 9, 1721–1727 (2003).
Atkins, D., Ferrone, S., Schmahl, G. E., Storkel, S. & Seliger, B. Down-regulation of HLA class I antigen processing molecules: an immune escape mechanism of renal cell carcinoma? J. Urol. 171, 885–889 (2004).
Dunker, K. et al. Expression and regulation of non-classical HLA-G in renal cell carcinoma. Tissue Antigens 72, 137–148 (2008).
Alegre, E. et al. Some basic aspects of HLA-G biology. J. Immunol. Res. 2014, 657625 (2014).
Kochan, G., Escors, D., Breckpot, K. & Guerrero-Setas, D. Role of non-classical MHC class I molecules in cancer immunosuppression. Oncoimmunology 2, e26491 (2013).
Kren, L. et al. HLA-G and HLA-E specific mRNAs connote opposite prognostic significance in renal cell carcinoma. Diagn. Pathol. 7, 58 (2012).
Seliger, B. et al. HLA-E expression and its clinical relevance in human renal cell carcinoma. Oncotarget 7, 67360–67372 (2016).
Zhang, S. et al. Immune infiltration in renal cell carcinoma. Cancer Sci. 110, 1564–1572 (2019).
Choueiri, T. K. et al. PD-L1 expression in nonclear-cell renal cell carcinoma. Ann. Oncol. 25, 2178–2184 (2014).
Chevrier, S. et al. An immune atlas of clear cell renal cell carcinoma. Cell 169, 736–749.e18 (2017).
Baine, M. K. et al. Characterization of tumor infiltrating lymphocytes in paired primary and metastatic renal cell carcinoma specimens. Oncotarget 6, 24990–25002 (2015).
Zhang, X. et al. Differential expression of TIM-3 between primary and metastatic sites in renal cell carcinoma. BMC Cancer 19, 49 (2019).
Porta, C. et al. Renal cell carcinoma-induced immunosuppression: an immunophenotypic study of lymphocyte subpopulations and circulating dendritic cells. Anticancer. Res. 27, 165–173 (2007).
Remark, R. et al. Characteristics and clinical impacts of the immune environments in colorectal and renal cell carcinoma lung metastases: influence of tumor origin. Clin. Cancer Res. 19, 4079–4091 (2013).
Giraldo, N. A. et al. Orchestration and prognostic significance of immune checkpoints in the microenvironment of primary and metastatic renal cell cancer. Clin. Cancer Res. 21, 3031–3040 (2015).
Finke, J. H. et al. Characterization of tumor-infiltrating lymphocyte subsets from human renal cell carcinoma: specific reactivity defined by cytotoxicity, interferon-gamma secretion, and proliferation. J. Immunother. Emphas. Tumor Immunol. 15, 91–104 (1994).
Finke, J. H. et al. Characterization of the cytolytic activity of CD4+ and CD8+ tumor-infiltrating lymphocytes in human renal cell carcinoma. Cancer Res. 50, 2363–2370 (1990).
Schoof, D. D. et al. CD4+ T cell clones isolated from human renal cell carcinoma possess the functional characteristics of Th2 helper cells. Cell Immunol. 150, 114–123 (1993).
Angevin, E., Kremer, F., Gaudin, C., Hercend, T. & Triebel, F. Analysis of T-cell immune response in renal cell carcinoma: polarization to type 1-like differentiation pattern, clonal T-cell expansion and tumor-specific cytotoxicity. Int. J. Cancer 72, 431–440 (1997).
Giraldo, N. A. et al. Tumor-infiltrating and peripheral blood T-cell immunophenotypes predict early relapse in localized clear cell renal cell carcinoma. Clin. Cancer Res. 23, 4416–4428 (2017).
Sharma, P., Hu-Lieskovan, S., Wargo, J. A. & Ribas, A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168, 707–723 (2017).
Sharma, P. & Allison, J. P. The future of immune checkpoint therapy. Science 348, 56–61 (2015).
Topalian, S. L., Drake, C. G. & Pardoll, D. M. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27, 450–461 (2015).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Wei, S. C., Duffy, C. R. & Allison, J. P. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 8, 1069–1086 (2018).
Khattri, R., Auger, J. A., Griffin, M. D., Sharpe, A. H. & Bluestone, J. A. Lymphoproliferative disorder in CTLA-4 knockout mice is characterized by CD28-regulated activation of Th2 responses. J. Immunol. 162, 5784–5791 (1999).
Paterson, A. M. et al. Deletion of CTLA-4 on regulatory T cells during adulthood leads to resistance to autoimmunity. J. Exp. Med. 212, 1603–1621 (2015).
Ishida, Y., Agata, Y., Shibahara, K. & Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 11 3887–3895 (1992).
Sharpe, A. H. & Pauken, K. E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 18, 153–167 (2018).
Chen, S. et al. Mechanisms regulating PD-L1 expression on tumor and immune cells. J. Immunother. Cancer 7, 305 (2019).
Noman, M. Z. et al. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J. Exp. Med. 211, 781–790 (2014).
Barsoum, I. B., Smallwood, C. A., Siemens, D. R. & Graham, C. H. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 74, 665–674 (2014).
Palsson-McDermott, E. M. et al. Pyruvate kinase M2 is required for the expression of the immune checkpoint PD-L1 in immune cells and tumors. Front. Immunol. 8, 1300 (2017).
Zhang, C. et al. TFEB mediates immune evasion and resistance to mTOR inhibition of renal cell carcinoma via induction of PD-L1. Clin. Cancer Res. 25, 6827–6838 (2019).
Lu, D. et al. Beyond T cells: understanding the role of PD-1/PD-L1 in tumor-associated macrophages. J. Immunol. Res. 2019, 1919082 (2019).
Tatsumi, T. et al. Disease-associated bias in T helper type 1 (Th1)/Th2 CD4(+) T cell responses against MAGE-6 in HLA-DRB10401(+) patients with renal cell carcinoma or melanoma. J. Exp. Med. 196, 619–628 (2002).
Tatsumi, T. et al. MAGE-6 encodes HLA-DRbeta1*0401-presented epitopes recognized by CD4+ T cells from patients with melanoma or renal cell carcinoma. Clin. Cancer Res. 9, 947–954 (2003).
Li, L. et al. Skewed T-helper (Th)1/2- and Th17/T regulatory cell balances in patients with renal cell carcinoma. Mol. Med. Rep. 11, 947–953 (2015).
DeNardo, D. G. et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 16, 91–102 (2009).
Abel, A. M., Yang, C., Thakar, M. S. & Malarkannan, S. Natural killer cells: development, maturation, and clinical utilization. Front. Immunol. 9, 1869 (2018).
Cozar, J. M. et al. Analysis of NK cells and chemokine receptors in tumor infiltrating CD4 T lymphocytes in human renal carcinomas. Cancer Immunol. Immunother. 54, 858–866 (2005).
Schleypen, J. S. et al. Cytotoxic markers and frequency predict functional capacity of natural killer cells infiltrating renal cell carcinoma. Clin. Cancer Res. 12, 718–725 (2006).
Eckl, J. et al. Transcript signature predicts tissue NK cell content and defines renal cell carcinoma subgroups independent of TNM staging. J. Mol. Med. 90, 55–66 (2012).
Donskov, F. et al. Intratumoural and peripheral blood lymphocyte subsets in patients with metastatic renal cell carcinoma undergoing interleukin-2 based immunotherapy: association to objective response and survival. Br. J. Cancer 87, 194–201 (2002).
Toliou, T., Stravoravdi, P., Polyzonis, M. & Vakalikos, J. Natural killer cell activation after interferon administration in patients with metastatic renal cell carcinoma: an ultrastructural and immunohistochemical study. Eur. Urol. 29, 252–256 (1996).
Trotta, A. M. et al. Mutated von Hippel-Lindau-renal cell carcinoma (RCC) promotes patients specific natural killer (NK) cytotoxicity. J. Exp. Clin. Cancer Res. 37, 297 (2018).
Engblom, C., Pfirschke, C. & Pittet, M. J. The role of myeloid cells in cancer therapies. Nat. Rev. Cancer 16, 447–462 (2016).
Soos, T. J. et al. CX3CR1+ interstitial dendritic cells form a contiguous network throughout the entire kidney. Kidney Int. 70, 591–596 (2006).
Toma, M. et al. Accumulation of tolerogenic human 6-sulfo LacNAc dendritic cells in renal cell carcinoma is associated with poor prognosis. Oncoimmunology 4, e1008342 (2015).
Hamada, I. et al. Clinical effects of tumor-associated macrophages and dendritic cells on renal cell carcinoma. Anticancer. Res. 22, 4281–4284 (2002).
Toge, H., Inagaki, T., Kojimoto, Y., Shinka, T. & Hara, I. Angiogenesis in renal cell carcinoma: the role of tumor-associated macrophages. Int. J. Urol. 16, 801–807 (2009).
Kovaleva, O. V., Samoilova, D. V., Shitova, M. S. & Gratchev, A. Tumor associated macrophages in kidney cancer. Anal. Cell Pathol. 2016, 9307549 (2016).
Roumenina, L. T. et al. Tumor cells hijack macrophage-produced complement C1q to promote tumor growth. Cancer Immunol. Res. 7, 1091–1105 (2019).
Ko, J. S. et al. Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer Res. 70, 3526–3536 (2010).
Ko, J. S. et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin. Cancer Res. 15, 2148–2157 (2009).
Najjar, Y. G. et al. Myeloid-derived suppressor cell subset accumulation in renal cell carcinoma parenchyma is associated with intratumoral expression of IL1β, IL8, CXCL5, and Mip-1α. Clin. Cancer Res. 23, 2346–2355 (2017).
Diaz-Montero, C. M. et al. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol. Immunother. 58, 49–59 (2009).
Peranzoni, E. et al. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr. Opin. Immunol. 22, 238–244 (2010).
Tannenbaum, C. S. et al. Mediators of inflammation-driven expansion, trafficking, and function of tumor-infiltrating MDSCs. Cancer Immunol. Res. 7, 1687–1699 (2019).
Feng, S. et al. Myeloid-derived suppressor cells inhibit T cell activation through nitrating LCK in mouse cancers. Proc. Natl Acad. Sci. USA 115, 10094–10099 (2018).
Najjar, Y. G. & Finke, J. H. Clinical perspectives on targeting of myeloid derived suppressor cells in the treatment of cancer. Front. Oncol. 3, 49 (2013).
Finke, J. et al. MDSC as a mechanism of tumor escape from sunitinib mediated anti-angiogenic therapy. Int. Immunopharmacol. 11, 856–861 (2011).
Corzo, C. A. et al. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J. Immunol. 182, 5693–5701 (2009).
Serafini, P., Mgebroff, S., Noonan, K. & Borrello, I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 68, 5439–5449 (2008).
Rodriguez, P. C., Quiceno, D. G. & Ochoa, A. C. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood 109, 1568–1573 (2007).
Rodriguez, P. C. et al. Regulation of T cell receptor CD3ζ chain expression by L-arginine. J. Biol. Chem. 277, 21123–21129 (2002).
Rodriguez, P. C. & Ochoa, A. C. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol. Rev. 222, 180–191 (2008).
Ostrand-Rosenberg, S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol. Immunother. 59, 1593–1600 (2010).
Gabrilovich, D. I. & Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174 (2009).
Condamine, T., Ramachandran, I., Youn, J. I. & Gabrilovich, D. I. Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu. Rev. Med. 66, 97–110 (2015).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Shalapour, S. & Karin, M. Immunity, inflammation, and cancer: an eternal fight between good and evil. J. Clin. Invest. 125, 3347–3355 (2015).
Petrella, B. L. & Vincenti, M. P. Interleukin-1β mediates metalloproteinase-dependent renal cell carcinoma tumor cell invasion through the activation of CCAAT enhancer binding protein β. Cancer Med. 1, 17–27 (2012).
Chittezhath, M. et al. Molecular profiling reveals a tumor-promoting phenotype of monocytes and macrophages in human cancer progression. Immunity 41, 815–829 (2014).
Kaminska, K., Czarnecka, A. M., Escudier, B., Lian, F. & Szczylik, C. Interleukin-6 as an emerging regulator of renal cell cancer. Urol. Oncol. 33, 476–485 (2015).
Fu, Q. et al. Prognostic value of interleukin-6 and interleukin-6 receptor in organ-confined clear-cell renal cell carcinoma: a 5-year conditional cancer-specific survival analysis. Br. J. Cancer 113, 1581–1589 (2015).
Harrison, M. L. et al. Tumor necrosis factor α as a new target for renal cell carcinoma: two sequential phase II trials of infliximab at standard and high dose. J. Clin. Oncol. 25, 4542–4549 (2007).
Ho, M. Y. et al. TNF-α induces epithelial-mesenchymal transition of renal cell carcinoma cells via a GSK3β-dependent mechanism. Mol. Cancer Res. 10, 1109–1119 (2012).
Sun, K. H. et al. TNF-α augments CXCR2 and CXCR3 to promote progression of renal cell carcinoma. J. Cell Mol. Med. 20, 2020–2028 (2016).
Mantovani, A., Allavena, P., Sica, A. & Balkwill, F. Cancer-related inflammation. Nature 454, 436–444 (2008).
Elinav, E. et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 13, 759–771 (2013).
Triner, D. & Shah, Y. M. Hypoxia-inducible factors: a central link between inflammation and cancer. J. Clin. Invest. 126, 3689–3698 (2016).
Reuter, S., Gupta, S. C., Chaturvedi, M. M. & Aggarwal, B. B. Oxidative stress, inflammation, and cancer: how are they linked? Free. Radic. Biol. Med. 49, 1603–1616 (2010).
Li, Y., Patel, S. P., Roszik, J. & Qin, Y. Hypoxia-driven immunosuppressive metabolites in the tumor microenvironment: new approaches for combinational immunotherapy. Front. Immunol. 9, 1591 (2018).
Fahey, E. & Doyle, S. L. IL-1 family cytokine regulation of vascular permeability and angiogenesis. Front. Immunol. 10, 1426 (2019).
Johnson, D. E., O’Keefe, R. A. & Grandis, J. R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 15, 234–248 (2018).
Ward, P. S. & Thompson, C. B. Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell 21, 297–308 (2012).
Kim, J. Regulation of immune cell functions by metabolic reprogramming. J. Immunol. Res. 2018, 8605471 (2018).
Li, H. et al. Metabolomic adaptations and correlates of survival to immune checkpoint blockade. Nat. Commun. 10, 4346 (2019).
Lameirinhas, A., Miranda-Goncalves, V., Henrique, R. & Jeronimo, C. The complex interplay between metabolic reprogramming and epigenetic alterations in renal cell carcinoma. Genes 10, 264 (2019).
Wettersten, H. I. et al. Grade-dependent metabolic reprogramming in kidney cancer revealed by combined proteomics and metabolomics analysis. Cancer Res. 75, 2541–2552 (2015).
Lucarelli, G. et al. Activation of the kynurenine pathway predicts poor outcome in patients with clear cell renal cell carcinoma. Urol. Oncol. 35, 461.e15–461.e27 (2017).
Li, L. & Kaelin, W. G. Jr New insights into the biology of renal cell carcinoma. Hematol. Oncol. Clin. North. Am. 25, 667–686 (2011).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Robert, C. et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 364, 2517–2526 (2011).
Yang, J. C. et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J. Immunother. 30, 825–830 (2007).
Hammers, H. J. et al. Safety and efficacy of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma: the CheckMate 016 study. J. Clin. Oncol. 35, 3851–3858 (2017).
Motzer, R. J. et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 378, 1277–1290 (2018).
Cella, D. et al. Patient-reported outcomes of patients with advanced renal cell carcinoma treated with nivolumab plus ipilimumab versus sunitinib (CheckMate 214): a randomised, phase 3 trial. Lancet Oncol. 20, 297–310 (2019).
Brahmer, J. R. et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 28, 3167–3175 (2010).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Motzer, R. J. et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J. Clin. Oncol. 33, 1430–1437 (2015).
Motzer, R. J. et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 373, 1803–1813 (2015).
Choueiri, T. K. et al. Immunomodulatory activity of nivolumab in metastatic renal cell carcinoma. Clin. Cancer Res. 22, 5461–5471 (2016).
McDermott, D. F. et al. Pembrolizumab monotherapy as first-line therapy in advanced clear cell renal cell carcinoma (accRCC): results from cohort A of KEYNOTE-427 [abstract]. J. Clin. Oncol. 36 (Suppl. 15), 4500 (2018).
McDermott, D. F. et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat. Med. 24, 749–757 (2018).
Vaishampayan, U. et al. Avelumab monotherapy as first-line or second-line treatment in patients with metastatic renal cell carcinoma: phase Ib results from the JAVELIN Solid Tumor trial. J. Immunother. Cancer 7, 275 (2019).
Takyar, S., Diaz, J., Sehgal, M., Sapunar, F. & Pandha, H. First-line therapy for treatment-naive patients with advanced/metastatic renal cell carcinoma: a systematic review of published randomized controlled trials. Anticancer Drugs 27, 383–397 (2016).
Duran, I. et al. Resistance to targeted therapies in renal cancer: the importance of changing the mechanism of action. Target. Oncol. 12, 19–35 (2017).
Almand, B. et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J. Immunol. 166, 678–689 (2001).
Yang, J., Yan, J. & Liu, B. Targeting VEGF/VEGFR to modulate antitumor immunity. Front. Immunol. 9, 978 (2018).
Escudier, B. et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 370, 2103–2111 (2007).
Rini, B. I. et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. J. Clin. Oncol. 28, 2137–2143 (2010).
Considine, B. & Hurwitz, M. E. Current status and future directions of immunotherapy in renal cell carcinoma. Curr. Oncol. Rep. 21, 34 (2019).
Motzer, R. J. et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 380, 1103–1115 (2019).
Rini, B. I. et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 380, 1116–1127 (2019).
de Velasco, G. et al. Comprehensive analysis of survival outcomes in non-clear cell renal cell carcinoma patients treated in clinical trials. Clin. Genitourin. Cancer 15, 652–660.e1 (2017).
Koshkin, V. S. et al. Clinical activity of nivolumab in patients with non-clear cell renal cell carcinoma. J. Immunother. Cancer 6, 9 (2018).
McKay, R. R. et al. The clinical activity of PD-1/PD-L1 inhibitors in metastatic non-clear cell renal cell carcinoma. Cancer Immunol. Res. 6, 758–765 (2018).
Lee, J.-L. et al. KEYNOTE-427 cohort B: first-line pembrolizumab (pembro) monotherapy for advanced non‒clear cell renal cell carcinoma (NCC-RCC) [abstract]. J. Clin. Oncol. 37(Suppl. 15), 4569 (2019).
Ravaud, A. et al. Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N. Engl. J. Med. 375, 2246–2254 (2016).
Wang, J. et al. Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nat. Med. 25, 656–666 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03665285 (2019).
Patel, S. P. & Kurzrock, R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol. Cancer Ther. 14, 847–856 (2015).
Basu, A., Yearley, J. H., Annamalai, L., Pryzbycin, C. & Rini, B. Association of PD-L1, PD-L2, and immune response markers in matched renal clear cell carcinoma primary and metastatic tissue specimens. Am. J. Clin. Pathol. 151, 217–225 (2019).
Snyder, A. et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 371, 2189–2199 (2014).
Rizvi, N. A. et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–128 (2015).
Alexandrov, L. B. et al. Signatures of mutational processes in human cancer. Nature 500, 415–421 (2013).
Le, D. T. et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357, 409–413 (2017).
Lemery, S., Keegan, P., Pazdur, R. & First, F. D. A. Approval agnostic of cancer site – when a biomarker defines the indication. N. Engl. J. Med. 377, 1409–1412 (2017).
Turajlic, S. et al. Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: a pan-cancer analysis. Lancet Oncol. 18, 1009–1021 (2017).
Zaretsky, J. M. et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N. Engl. J. Med. 375, 819–829 (2016).
Shin, D. S. et al. Primary resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Discov. 7, 188–201 (2017).
Benci, J. L. et al. Tumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockade. Cell 167, 1540–1554.e12 (2016).
Rini., B. et al. Molecular correlates differentiate response to atezolizumab (atezo) + bevacizumab (bev) vs sunitinib (sun): results from a phase III study (IMmotion151) in untreated metastatic renal cell carcinoma (mRCC) [abstract]. Ann. Oncol. 29, viii724–viii725 (2018).
Kim, H. Y. et al. Discovery of potential biomarkers in human melanoma cells with different metastatic potential by metabolic and lipidomic profiling. Sci. Rep. 7, 8864 (2017).
Tanimine, N., Turka, L. A. & Priyadharshini, B. Navigating T-cell immunometabolism in transplant. Transplantation 102, 230–239 (2018).
Mock, A. et al. Serum very long-chain fatty acid-containing lipids predict response to immune checkpoint inhibitors in urological cancers. Cancer Immunol. Immunother. 68, 2005–2014 (2019).
Routy, B. et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97 (2018).
Rosenberg, S. A., Restifo, N. P., Yang, J. C., Morgan, R. A. & Dudley, M. E. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat. Rev. Cancer 8, 299–308 (2008).
Rosenberg, S. A. et al. Prospective randomized trial of high-dose interleukin-2 alone or in conjunction with lymphokine-activated killer cells for the treatment of patients with advanced cancer. J. Natl Cancer Inst. 85, 622–632 (1993).
Figlin, R. A. et al. Multicenter, randomized, phase III trial of CD8(+) tumor-infiltrating lymphocytes in combination with recombinant interleukin-2 in metastatic renal cell carcinoma. J. Clin. Oncol. 17, 2521–2529 (1999).
Andersen, R. et al. T-cell responses in the microenvironment of primary renal cell carcinoma–implications for adoptive cell therapy. Cancer Immunol. Res. 6, 222–235 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02830724 (2020).
Shaffer, D. R. et al. T cells redirected against CD70 for the immunotherapy of CD70-positive malignancies. Blood 117, 4304–4314 (2011).
Li, H. et al. Antitumor activity of EGFR-specific CAR T cells against non-small-cell lung cancer cells in vitro and in mice. Cell Death Dis. 9, 177 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03618381 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03696030 (2020).
Zhang, J. & Wang, L. The emerging world of TCR-T cell trials against cancer: a systematic review. Technol. Cancer Res. Treat. 18, 1533033819831068 (2019).
Habif, G., Crinier, A., Andre, P., Vivier, E. & Narni-Mancinelli, E. Targeting natural killer cells in solid tumors. Cell Mol. Immunol. 16, 415–422 (2019).
Burger, M. C. et al. CAR-engineered NK cells for the treatment of glioblastoma: turning innate effectors into precision tools for cancer immunotherapy. Front. Immunol. 10, 2683 (2019).
Zhang, Q. et al. Synergistic effects of cabozantinib and EGFR-specific CAR-NK-92 cells in renal cell carcinoma. J. Immunol. Res. 2017, 6915912 (2017).
Boissel, L. et al. Retargeting NK-92 cells by means of CD19- and CD20-specific chimeric antigen receptors compares favorably with antibody-dependent cellular cytotoxicity. Oncoimmunology 2, e26527 (2013).
Romanski, A. et al. CD19-CAR engineered NK-92 cells are sufficient to overcome NK cell resistance in B-cell malignancies. J. Cell Mol. Med. 20, 1287–1294 (2016).
Rini, B. I. et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet 393, 2404–2415 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02811861 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03141177 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03055013 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03138512 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03142334 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03024996 (2020).
Acknowledgements
The work of J.H.F. is supported by NIH grant R01 CA168488. The work of C.M.D.-M. is supported by NIH grant R21 CA188767.
Author information
Authors and Affiliations
Contributions
C.M.D.-M. and J.H.F researched the data for the article. C.M.D.-M. and B.I.R. made substantial contributions to discussions of the content. All authors wrote the article and edited or reviewed the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Nephrology thanks J. Bedke, W. Fridman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Type 2 T helper
-
A subset of CD4+ T cells that promotes innate immune responses, particularly antibody production.
- Type 1 T helper
-
A subset of CD4+ T cells that mediates cellular immune responses.
- Objective response rate
-
The proportion of patients with a reduction in tumour size of a predefined amount and for a minimum time period.
- Microsatellite instability
-
(MSI). A predisposition to mutation owing to deficient mismatch repair.
- Deficient mismatch repair
-
(dMMR). Loss of function of the mismatch repair pathway, which corrects DNA mismatches generated during DNA replication and thus prevents mutations.
Rights and permissions
About this article
Cite this article
Díaz-Montero, C.M., Rini, B.I. & Finke, J.H. The immunology of renal cell carcinoma. Nat Rev Nephrol 16, 721–735 (2020). https://doi.org/10.1038/s41581-020-0316-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-020-0316-3
This article is cited by
-
Single-cell combined bioinformatics analysis: construction of immune cluster and risk prognostic model in kidney renal clear cells based on CD8+ T cell-associated genes
European Journal of Medical Research (2024)
-
Identification of PANoptosis-related signature reveals immune infiltration characteristics and immunotherapy responses for renal cell carcinoma
BMC Cancer (2024)
-
Single-cell analysis reveals ADGRL4+ renal tubule cells as a highly aggressive cell type in clear cell renal cell carcinoma
Scientific Reports (2024)
-
Targeting STING elicits GSDMD-dependent pyroptosis and boosts anti-tumor immunity in renal cell carcinoma
Oncogene (2024)
-
STING agonist, SMA-2, inhibits clear cell renal cell carcinoma through improving tumor microenvironment
Molecular and Cellular Biochemistry (2024)