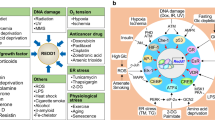
Abstract
Tonicity-responsive enhancer-binding protein (TonEBP), which is also known as nuclear factor of activated T cells 5 (NFAT5), was discovered 20 years ago as a transcriptional regulator of the cellular response to hypertonic (hyperosmotic salinity) stress in the renal medulla. Numerous studies since then have revealed that TonEBP is a pleiotropic stress protein that is involved in a range of immunometabolic diseases. Some of the single-nucleotide polymorphisms (SNPs) in TONEBP introns are cis-expression quantitative trait loci that affect TONEBP transcription. These SNPs are associated with increased risk of type 2 diabetes mellitus, diabetic nephropathy, inflammation, high blood pressure and abnormal plasma osmolality, indicating that variation in TONEBP expression might contribute to these phenotypes. In addition, functional studies have shown that TonEBP is involved in the pathogenesis of rheumatoid arthritis, atherosclerosis, diabetic nephropathy, acute kidney injury, hyperlipidaemia and insulin resistance, autoimmune diseases (including type 1 diabetes mellitus and multiple sclerosis), salt-sensitive hypertension and hepatocellular carcinoma. These pathological activities of TonEBP are in contrast to the protective actions of TonEBP in response to hypertonicity, bacterial infection and DNA damage induced by genotoxins. An emerging theme is that TonEBP is a stress protein that mediates the cellular response to a range of pathological insults, including excess caloric intake, inflammation and oxidative stress.
Key points
-
Tonicity-responsive enhancer-binding protein (TonEBP) is a stress protein involved in the cellular response to hypertonicity, autoimmune reactions, inflammation and metabolic and genotoxic stress.
-
TonEBP-mediated responses to autoimmunity, viral infection and metabolic stresses are pathological.
-
TonEBP dysfunction is implicated in metabolic diseases, such as atherosclerosis, rheumatoid arthritis, obesity and type 2 diabetes mellitus.
-
TonEBP-mediated responses to hypertonicity, bacterial infection and genotoxins are protective.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Sheen, M. R. et al. Interstitial tonicity controls TonEBP expression in the renal medulla. Kidney Int. 75, 518–525 (2009).
Kwon, M. S., Lim, S. W. & Kwon, H. M. Hypertonic stress in the kidney: a necessary evil. Physiology 24, 186–191 (2009).
Miyakawa, H., Woo, S. K., Dahl, S. C., Handler, J. S. & Kwon, H. M. Tonicity-responsive enhancer binding protein, a Rel-like protein that stimulates transcription in response to hypertonicity. Proc. Natl Acad. Sci. USA 96, 2538–2542 (1999).
Lopez-Rodriguez, C. et al. Loss of NFAT5 results in renal atrophy and lack of tonicity-responsive gene expression. Proc. Natl Acad. Sci. USA 101, 2392–2397 (2004).
Nakayama, Y., Peng, T., Sands, J. M. & Bagnasco, S. M. The TonE/TonEBP pathways mediates tonicity-responsive regulation of UT-A urea transporter expression. J. Biol. Chem. 275, 38275–38280 (2000).
Choi, S. Y. et al. Tonicity-responsive enhancer-binding protein mediates hyperglycemia-induced inflammation and vascular and renal injury. J. Am. Soc. Nephrol. 29, 492–504 (2018).
Boeger, C. A. et al. NFAT5 and SLC4A10 loci associate with plasma osmolality. J. Am. Soc. Nephrol. 28, 2311–2321 (2017).
Rosen, E. D. et al. Epigenetics and epigenomics: implications for diabetes and obesity. Diabetes 67, 1923–1931 (2018).
Semenza, G. L. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu. Rev. Pathol. 9, 47–71 (2013).
Maouyo, D. et al. Mouse TonEBP-NFAT5: expression in early development and alternative splicing. Am. J. Physiol. 282, F802–F809 (2002).
Lee, S. D., Colla, E., Sheen, M. R., Na, K. Y. & Kwon, H. M. Multiple domains of TonEBP cooperate to stimulate transcription in response to hypertonicity. J. Biol. Chem. 278, 47571–47577 (2003).
Kwon, M. S. et al. Novel nuclear localization signal regulated by ambient tonicity in vertebrates. J. Biol. Chem. 283, 22400–22409 (2008).
Raat, N. J., van Os, C. H. & Bindels, R. J. Effects of osmotic perturbation on [Ca2+]i and pHi in rabbit proximal tubular cells in primary culture. Am. J. Physiol. 269, F205–F211 (1995).
Stroud, J. C., Lopez-Rodriguez, C., Rao, A. & Chen, L. Structure of a TonEBP-DNA complex reveals DNA encircled by a transcription factor. Nat. Struct. Biol. 9, 90–94 (2002).
Lee, S. D., Woo, S. K. & Kwon, H. M. Dimerization is required for phosphorylation and DNA binding of TonEBP/NFAT5. Biochem. Biophys. Res. Commun. 294, 968–975 (2002).
Kim, J. A. et al. Modulation of TonEBP activity by SUMO modification in response to hypertonicity. Front. Physiol. 5, 200 (2014).
Irarrazabal, C. R., Liu, J. C., Burg, M. B. & Feraris, J. D. ATM, a DNA dmage-inducible kinase, contributes to activation by high NaCl of the transcription factor TonEBP/OREBP. Proc. Natl Acad. Sci. USA 101, 8809–9914 (2004).
Gallazzini, M. et al. High NaCl-induced activation of CDK5 increases phosphorylation of the osmoprotective transcription factor TonEBP/OREBP at threonine 135, which contributes to its rapid nuclear localization. Mol. Biol. Cell 22, 703–714 (2011).
Kang, H. J. et al. TonEBP regulates PCNA polyubiquitination in response to DNA damage through interaction with SHPRH and USP1. iScience 19, 177–190 (2019). This study details the dynamic interactions between TonEBP and enzymes of the ubiquitylation machinery, which lead to changes in PCNA ubiquitylation and DNA repair without affecting gene expression.
Lee, H. H. et al. LPS-induced NF-κB enhanceosome requires TonEBP/NFAT5 without DNA binding. Sci. Rep. 6, 24921 (2016).
Colla, E. et al. TonEBP is inhibited by RNA helicase A via interaction involving the E’F loop. Biochem. J. 393, 411–419 (2006).
Irarrazabal, C. E. et al. Phospholipase C-γ1 is involved in signaling the activation by high NaCl of the osmoprotective transcription factor TonEBP/OREBP. Proc. Natl Acad. Sci. USA 107, 906–911 (2010).
Woo, S. K., Dahl, S. C., Handler, J. S. & Kwon, H. M. Bidirectional regulation of tonicity-responsive enhancer binding protein in response to changes in tonicity. Am. J. Physiol. Renal Physiol. 278, F1006–F1012 (2000).
Cai, Q., Ferraris, J. D. & Burg, M. B. High NaCl increases TonEBP/OREBP mRNA and protein by stabilizing its mRNA. Am. J. Physiol. Renal Physiol. 289, F803–F807 (2005).
Kojima, R. et al. Hypertonicity-induced expression of monocyte chemoattractant protein-1 through a novel cis-acting element and MAPK signaling pathways. J. Immunol. 184, 5253–5262 (2010).
Gallazzini, M., Yu, M. J., Gunaratne, R., Burg, M. B. & Ferraris, J. D. c-Abl medicates high NaCl-induced phosphorylation and activation of the transcription factor TonEBP/OREBP. FASEB J. 24, 4325–4335 (2010).
Zhang, Z. et al. Ataxia telangiectasia-mutated, a DNA damage-inducible kinase, contributes to high NaCl-induced nuclear localization of transcription factor TonEBP/OREBP. Am. J. Physiol. Renal Physiol. 289, F506–F511 (2005).
Zhou, X., Ferraris, J. D. & Burg, M. B. Mitochondrial reactive oxygen species contribute to high NaCl-induced activation of the transcription factor TonEBP/OREBP. Am. J. Physiol. Renal Physiol. 290, F1169–F1176 (2006).
Choi, S. et al. Transcription factor NFAT5 promotes macrophage survival in rheumatoid arthritis. J. Clin. Invest. 127, 954–969 (2017).
Buxade, M. et al. Gene expression induced by Toll-like receptors in macrophages requires the transcription factor NFAT5. J. Exp. Med. 209, 379–393 (2012). This study reveals the role of TonEBP in antimicrobial immunity.
Yoo, E. J. et al. TonEBP suppresses the HO-1 gene by blocking recruitment of Nrf2 to its promoter. Front. Immunol. 10, 850 (2019). This study defines the suppression of anti-inflammatory polarization of macrophages by TonEBP at the molecular level.
Choi, S. Y. et al. TonEBP suppresses IL-10-mediated immunomodulation. Sci. Rep. 6, 25726 (2016).
Jeong, G. R. et al. Inflammatory signals induce the expression of tonicity-responsive enhancer binding protein (TonEBP) in microglia. J. Neuroimmunol. 295–296, 21–29 (2016).
Lee, J. H. et al. Tonicity-responsive enhancer-binding protein promotes hepatocellular carcinogenesis, recurrence and metastasis. Gut 68, 347–358 (2019). This study shows that TonEBP is a strong biomarker of recurrence and mortality in patients with HCC.
Zhai, S., Li, M., Sun, B. & Han, Y. Amelioration of lipopolysaccharide-induced nephrotic proteinuria by NFAT5 depletion involves suppressed NF-κB activity. Inflammation 42, 1326–1335 (2019).
Villanueva, S. et al. NFAT5 is activated by hypoxia: role of ischemia and reperfusion in the rat kidney. PLoS One 7, e39665 (2012).
Zhou, B. et al. Hypertonic induction of aquaporin-5: novel role of hypoxia-inducible factor-1alpha. Am. J. Physiol. Cell Physiol. 292, C1280–C1290 (2007).
Yoo, E. J. et al. Transcriptional regulator TonEBP mediates oxidative damages in ischemic kidney injury. Cell 8, 1284 (2019). This study provides insights into the involvement of the TonEBP pathway in mitochondrial dysfunction and ROS in proximal tubule injury.
Mak, K. M. C. et al. Nuclear factor of activated T cells 5 deficiency increases the severity of neuronal cell death in ischemic injury. Neurosignals 20, 237–251 (2012).
Neubert, P. et al. HIF1A and NFAT5 coordinate Na+-boosted antibacterial defense via enhanced autophagy and autolysosomal targeting. Autophagy 15, 1899–1916 (2019).
Halterman, J. A., Kwon, H. M., Leitinger, N. & Wamhoff, B. R. NFAT5 expression in bone marrow-derived cells enhances atherosclerosis and drives macrophage migration. Front. Physiol. 3, 313 (2012).
Lee, H. H. et al. TonEBP/NFAT5 promotes obesity and insulin resistance by epigenetic suppression of white adipose tissue beiging. Nat. Commun. 10, 3536 (2019). This study defines the role of TonEBP in the risk of obesity and T2DM at the molecular level.
Xie, H., Lim, B. & Lodish, H. F. MicroRNAs induced during adipogenesis that accelerate fat cell development are downregulated in obesity. Diabetes 58, 1050–1057 (2009).
Hosogai, N. et al. Adipose tissue hypoxia in obesity and its impact on adipokine dysregulation. Diabetes 56, 901–911 (2007).
Hinske, L. C. et al. Intronic miRNA-641 controls its host gene’s pathway PI3K/AKT and this relationship is dysfunctional in glioblastoma multiforme. Biochem. Biophys. Res. Commun. 489, 477–483 (2017).
Tao, H. et al. NFAT5 is regulated by p53/miR-27a signal axis and promotes mouse ovarian granulosa cells proliferation. Int. J. Biol. Sci. 15, 287–297 (2019).
Meng, X., Li, Z., Zhou, S., Xiao, S. & Yu, P. miR-194 suppresses high glucose-induced non-small cell lung cancer cell progression by targeting NFAT5. Thorac. Cancer 10, 1051–1059 (2019).
Li, W. et al. MiR-568 inhibits the activation and function of CD4+ T cells and Treg cells by targeting NFAT5. Int. Immunol. 26, 269–281 (2014).
Ying, W. et al. MicroRNA-223 is a crucial mediator of PPARγ-regulated alternative macrophage activation. J. Clin. Invest. 125, 4149–4159 (2015).
Xin, Y. et al. miR-20b inhibits T cell proliferation and activation via NFAT signaling pathway in thymoma-associated myasthenia gravis. Biomed. Res. Int. 2016, 9595718 (2016).
Kästle, M. et al. MicroRNA cluster 106a~363 is involved in T helper 17 cell differentiation. Immunology 152, 402–413 (2017).
Ge, G. et al. miR-10b-5p regulates C2C12 myoblasts proliferation and differentiation. Biosci. Biotechnol. Biochem. 83, 291–299 (2019).
Neuhofer, W. et al. Regulation of TonEBP transcriptional activator in MDCK cells following changes in ambient tonicity. Am. J. Physiol. 283, C1604–C1611 (2002).
Berry, M. R. et al. Renal sodium gradient orchestrates a dynamic antibacterial defense zone. Cell 170, 860–874 (2017). This study details how medullary hypertonicity provides protection from ascending infection through the actions of TonEBP.
Manchnik, A. et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat. Med. 15, 545–552 (2009). This paper describes how TonEBP signalling in macrophages mediates sodium removal and blood pressure homeostasis in the skin.
Jantsch, J. et al. Cutaneous Na+ storage strengthens the antimicrobial barrier function of the skin and boosts macrophage-driven host defense. Cell Metab. 21, 493–501 (2015).
Berga-Bolanos, R., Drews-Elger, K., Aramburu, J. & Lopez-Rodriguez, C. NFAT5 regulates T lymphocyte homeostatis and CD24-dependent T cell expansion under pathologic hypernatremia. J. Immunol. 185, 6624–6635 (2010).
Go, W. Y., Liu, X., Roti, M. A., Liu, F. & Ho, S. N. NFAT5/TonEBP mutant mice define osmotic stress as a critical feature of the lymphoid microenvironment. Proc. Natl Acad. Sci. USA 101, 10673–10678 (2004).
Tellechea, M., Buxade, M., Tejedor, S., Aramburu, J. & Lopez-Rodriguez, C. NFAT5-regulated macrophage polarization supports the proinflammatory function of macrophages and T lymphocytes. J. Immunol. 200, 305–315 (2018).
Buxade, M. et al. Macrophage-specific MHCII expression is regulated by a remote Ciita enhancer controlled by NFAT5. J. Exp. Med. 215, 2901–2918 (2018).
Korn, T., Bettelli, E., Oukka, M. & Kuchroo, V. K. IL-17 and Th17 cells. Annu. Rev. Immunol. 27, 485–517 (2009).
Kleinewiefeld, M. et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 496, 518–522 (2013). This study reports the first connection of TonEBP with pathological CD4 + T cell differentiation and autoimmunity.
Vandenbark, A. A. & Offner, H. Critical evaluation of regulatory T cells in autoimmunity: are the most potent regulatory specificities being ignored? Immunology 125, 1–13 (2008).
Matthias, J. et al. Sodium chloride is an ionic checkpoint for human TH2 cells and shapes the atopic skin microenvironment. Sci. Transl Med. 11, 480 (2019).
Schwartz, L. et al. Is inflammation a consequence of extracellular hyperosmolarity? J. Inflamm. 6, 21 (2009).
Roth, I. et al. Osmoprotective transcription factor NFAT5/TonEBP modulates nuclear factor-κB activity. Mol. Biol. Cell. 21, 3459–3474 (2010).
Farabaugh, K. T. et al. Protein kinase R mediates the inflammatory response induced by hyperosmotic stress. Mol. Cell Biol. 37, e00521-16 (2017).
Serr, I. et al. A miRNA181a/NFAT5 axis links impaired T cell tolerance induction with autoimmune type 1 diabetes. Sci. Transl Med. 10, eaag1782 (2018).
Yoon, H. J. et al. NFAT5 is a critical regulator of inflammatory arthritis. Arthritis Rheum. 63, 1843–1852 (2011).
Lee, S. et al. Transcription factor NFAT5 promotes migration and invasion of rheumatoid synoviocytes via coagulation factor III and CCL2. J. Immunol. 201, 359–370 (2018).
El-Serag, H. B. Hepatocellular carcinoma. N. Eng. J. Med. 365, 1118–1127 (2011).
Fox, C. S. et al. Trends in cardiovascular complication of diabetes. JAMA 292, 2495–2499 (2004).
Goettgen, A. et al. New loci associated with kidney function and chronic kidney disease. Nat. Genet. 42, 376–384 (2010).
Mahajan, A. et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenomic maps. Nat. Genet. 50, 1505–1513 (2018). This study provides the first genetic evidence for an association between TONEBP polymorphisms and risk of T2DM.
Kavanagh, D. H. et al. Haplotype association analysis of genes within the WNT signaling pathways in diabetic nephropathy. BMC Nephrol. 14, 126 (2013).
Tragante, V. et al. Gene-centric meta-analysis in 87,736 individuals of European ancestry identifies multiple blood-pressure-related loci. Am. J. Hum. Genet. 94, 349–360 (2014).
Parsa, A. et al. Genotype-based changes in serum uric acid affect blood pressure. Kidney Int. 81, 502–507 (2012).
Goettgen, A. et al. Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat. Genet. 45, 145–154 (2013).
Acknowledgements
Work in the laboratory of H.M.K. was supported by the National Research Foundation grants 2018R1A5A1024340, 2017R1E1A1A01074673 and NRF-2019R1A2C1089260.
Author information
Authors and Affiliations
Contributions
H.M.K., S.Y.C. and W.L.-K. researched the data for the article, contributed substantially to discussion of the content and reviewed and/or edited the manuscript before submission; H.M.K. and S.Y.C. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Nephrology thanks W. Neuhofer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Choi, S.Y., Lee-Kwon, W. & Kwon, H.M. The evolving role of TonEBP as an immunometabolic stress protein. Nat Rev Nephrol 16, 352–364 (2020). https://doi.org/10.1038/s41581-020-0261-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-020-0261-1
This article is cited by
-
TonEBP/NFAT5 expression is associated with cisplatin resistance and migration in macrophage-induced A549 cells
BMC Molecular and Cell Biology (2024)
-
Long-term health outcomes associated with hydration status
Nature Reviews Nephrology (2024)
-
Lysine methylation promotes NFAT5 activation and determines temozolomide efficacy in glioblastoma
Nature Communications (2023)
-
The role of the osmosensitive transcription factor NFAT5 in corneal edema resorption after injury
Experimental & Molecular Medicine (2023)
-
Loss of RANBP3L leads to transformation of renal epithelial cells towards a renal clear cell carcinoma like phenotype
Journal of Experimental & Clinical Cancer Research (2021)