Abstract
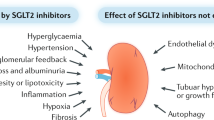
Kidney size and glomerular filtration rate (GFR) often increase with the onset of diabetes, and elevated GFR is a risk factor for the development of diabetic kidney disease. Hyperfiltration mainly occurs in response to signals passed from the tubule to the glomerulus: high levels of glucose in the glomerular filtrate drive increased reabsorption of glucose and sodium by the sodium–glucose cotransporters SGLT2 and SGLT1 in the proximal tubule. Passive reabsorption of chloride and water also increases. The overall capacity for proximal reabsorption is augmented by growth of the proximal tubule, which (alongside sodium–glucose cotransport) further limits urinary glucose loss. Hyperreabsorption of sodium and chloride induces tubuloglomerular feedback from the macula densa to increase GFR. In addition, sodium–glucose cotransport by SGLT1 on macula densa cells triggers the production of nitric oxide, which also contributes to glomerular hyperfiltration. Although hyperfiltration restores sodium and chloride excretion it imposes added physical stress on the filtration barrier and increases the oxygen demand to drive reabsorption. Tubular growth is associated with the development of a senescence-like molecular signature that sets the stage for inflammation and fibrosis. SGLT2 inhibitors attenuate the proximal reabsorption of sodium and glucose, normalize tubuloglomerular feedback signals and mitigate hyperfiltration. This tubule-centred model of diabetic kidney physiology predicts the salutary effect of SGLT2 inhibitors on hard renal outcomes, as shown in large-scale clinical trials.
Key points
-
Rising blood glucose levels drive increased delivery of glucose into the Bowman’s space by glomerular filtration and cause increased reabsorption of glucose and sodium by the proximal tubule sodium–glucose cotransporters, SGLT2 and SGLT1, up to a transport maximum.
-
Diabetes mellitus causes the kidney tubule to grow and increase its capacity for transport; most of this growth occurs in the proximal tubule, which increases glucose reabsorption and contributes to hyperglycaemia, and induces abnormally high reabsorption of filtered sodium chloride (NaCl).
-
The increase in proximal reabsorption reduces NaCl and fluid delivery to the macula densa and induces glomerular hyperfiltration through tubuloglomerular feedback and a decline in tubular back pressure, which helps to normalize renal NaCl and fluid excretion.
-
Glomerular hyperfiltration places physical stress on the filtration barrier and increases the demand for oxygen to reabsorb the filtered load; hypertrophic tubular cells exhibit a senescence-like phenotype, with pro-inflammatory and pro-fibrotic consequences that might promote the development of diabetic kidney disease (DKD).
-
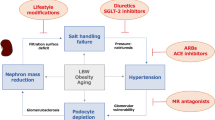
Not all patients with diabetes develop DKD, suggesting that the tubular growth and hyperreabsorption response must be subject to genetic or environmental influences; one such environmental factor is dietary NaCl, which mitigates hyperfiltration in diabetes by suppressing proximal reabsorption and thereby increasing the tubuloglomerular feedback signal.
-
The clinical relevance of increased proximal reabsorption and hyperfiltration in diabetes is demonstrated by the ability of SGLT2 to improve renal outcomes in patients with diabetes, which has been verified in large-scale clinical trials.
-
SGLT1 is a positive regulator of NOS1 in the macula densa, and acts as a glucose sensor to stimulate hyperfiltration and potentially regulate tubule growth in the diabetic kidney.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
United States Renal Data System. 2015 Annual Data Report: Epidemiology of kidney disease in the United States, Bethesda, MD. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases 2015, https://www.usrds.org/2015/view/ (2015).
Thomas, M. C. et al. Diabetic kidney disease. Nat. Rev. Dis. Prim. 1, 15018 (2015).
Jha, V. et al. Chronic kidney disease: global dimension and perspectives. Lancet 382, 260–272 (2013).
Papademetriou, V. et al. Chronic kidney disease and intensive glycemic control increase cardiovascular risk in patients with type 2 diabetes. Kidney Int. 87, 649–659 (2015).
Lanting, L. C., Joung, I. M., Mackenbach, J. P., Lamberts, S. W. & Bootsma, A. H. Ethnic differences in mortality, end-stage complications, and quality of care among diabetic patients: a review. Diabetes Care 28, 2280–2288 (2005).
Salem, R. M. et al. Genome-wide association study of diabetic kidney disease highlights biology involved in glomerular basement membrane collagen. J. Am. Soc. Nephrol. 30, 2000–2016 (2019).
van Zuydam, N. R. et al. A genome-wide association study of diabetic kidney disease in subjects with type 2 diabetes. Diabetes 67, 1414–1427 (2018).
Gheith, O., Farouk, N., Nampoory, N., Halim, M. A. & Al-Otaibi, T. Diabetic kidney disease: world wide difference of prevalence and risk factors. J. Nephropharmacol. 5, 49–56 (2016).
Vallon, V. & Komers, R. Pathophysiology of the diabetic kidney. Compr. Physiol. 1, 1175–1232 (2011).
Reidy, K., Kang, H. M., Hostetter, T. & Susztak, K. Molecular mechanisms of diabetic kidney disease. J. Clin. Invest. 124, 2333–2340 (2014).
Fu, J., Lee, K., Chuang, P. Y., Liu, Z. & He, J. C. Glomerular endothelial cell injury and cross talk in diabetic kidney disease. Am. J. Physiol. Renal Physiol. 308, F287–F297 (2015).
Kanwar, Y. S., Sun, L., Xie, P., Liu, F. Y. & Chen, S. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu. Rev. Pathol. 6, 395–423 (2011).
Ziyadeh, F. N. & Wolf, G. Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Curr. Diabetes Rev. 4, 39–45 (2008).
Hostetter, T. H. Diabetic nephropathy. Metabolic versus hemodynamic considerations. Diabetes Care 15, 1205–1215 (1992).
Vallon, V. & Thomson, S. C. Renal function in diabetic disease models: the tubular system in the pathophysiology of the diabetic kidney. Annu. Rev. Physiol. 74, 351–375 (2012).
Vallon, V., Richter, K., Blantz, R. C., Thomson, S. & Osswald, H. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J. Am. Soc. Nephrol. 10, 2569–2576 (1999).
Thomson, S. C., Vallon, V. & Blantz, R. C. Kidney function in early diabetes: the tubular hypothesis of glomerular filtration. Am. J. Physiol. Renal Physiol. 286, F8–F15 (2004).
Macisaac, R. J. et al. Nonalbuminuric renal insufficiency in type 2 diabetes. Diabetes Care 27, 195–200 (2004).
Mogensen, C. E., Christensen, C. K. & Vittinghus, E. The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes 32, 64–78 (1983).
Kramer, H. J., Nguyen, Q. D., Curhan, G. & Hsu, C. Y. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA 289, 3273–3277 (2003).
Magee, G. M. et al. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia 52, 691–697 (2009).
Tonneijck, L. et al. Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J. Am. Soc. Nephrol. 28, 1023–1039 (2017).
Vallon, V. & Thomson, S. C. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia 60, 215–225 (2017).
Hallow, K. M., Gebremichael, Y., Helmlinger, G. & Vallon, V. Primary proximal tubule hyperreabsorption and impaired tubular transport counterregulation determine glomerular hyperfiltration in diabetes: a modeling analysis. Am. J. Physiol. Renal Physiol. 312, F819–F835 (2017).
Vallon, V. & Osswald, H. Dipyridamole prevents diabetes-induced alterations of kidney function in rats. Naunyn Schmiedebergs Arch. Pharmacol. 349, 217–222 (1994).
Thomson, S., Bao, D., Deng, A. & Vallon, V. Adenosine formed by 5ʹ-nucleotidase mediates tubuloglomerular feedback. J. Clin. Invest. 106, 289–298 (2000).
Pollock, C. A., Lawrence, J. R. & Field, M. J. Tubular sodium handling and tubuloglomerular feedback in experimental diabetes mellitus. Am. J. Physiol. 260, F946–F952 (1991).
Vallon, V. et al. Salt-sensitivity of proximal reabsorption alters macula densa salt and explains the paradoxical effect of dietary salt on glomerular filtration rate in diabetes mellitus. J. Am. Soc. Nephrol. 13, 1865–1871 (2002).
Vallon, V., Blantz, R. C. & Thomson, S. Homeostatic efficiency of tubuloglomerular feedback is reduced in established diabetes mellitus in rats. Am. J. Physiol. 269, F876–F883 (1995).
Ditzel, J., Lervang, H. H. & Brochner-Mortensen, J. Renal sodium metabolism in relation to hypertension in diabetes. Diabete Metab. 15, 292–295 (1989).
Mbanya, J. C., Thomas, T. H., Taylor, R., Alberti, K. G. & Wilkinson, R. Increased proximal tubular sodium reabsorption in hypertensive patients with type 2 diabetes. Diabet. Med. 6, 614–620 (1989).
Brochner-Mortensen, J., Stockel, M., Sorensen, P. J., Nielsen, A. H. & Ditzel, J. Proximal glomerulo-tubular balance in patients with type 1 (insulin-dependent) diabetes mellitus. Diabetologia 27, 189–192 (1984).
Thomson, S. C. et al. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am. J. Physiol. Regul. Integr. Comp. Physiol 302, R75–R83 (2012).
Blantz, R. C., Singh, P., Deng, A., Thomson, S. C. & Vallon, V. Acute saline expansion increases nephron filtration and distal flow rate but maintains tubuloglomerular feedback responsiveness: role of adenosine A(1) receptors. Am. J. Physiol. Renal Physiol. 303, F405–F411 (2012).
Woods, L. L., Mizelle, H. L. & Hall, J. E. Control of renal hemodynamics in hyperglycemia: possible role of tubuloglomerular feedback. Am. J. Physiol. 252, F65–F73 (1987).
Vallon, V. et al. Adenosine A(1) receptors determine glomerular hyperfiltration and the salt paradox in early streptozotocin diabetes mellitus. Nephron Physiol. 111, 30–38 (2009).
Vallon, V., Muhlbauer, B. & Osswald, H. Adenosine and kidney function. Physiol. Rev. 86, 901–940 (2006).
Ren, Y., Garvin, J. L., Liu, R. & Carretero, O. A. Possible mechanism of efferent arteriole (Ef-Art) tubuloglomerular feedback. Kidney Int. 71, 861–866 (2007).
Sallstrom, J. et al. Diabetes-induced hyperfiltration in adenosine A(1)-receptor deficient mice lacking the tubuloglomerular feedback mechanism. Acta Physiol. 190, 253–259 (2007).
Faulhaber-Walter, R. et al. Lack of A1 adenosine receptors augments diabetic hyperfiltration and glomerular injury. J. Am. Soc. Nephrol. 19, 722–730 (2008).
Weinstein, A. M. Osmotic diuresis in a mathematical model of the rat proximal tubule. Am. J. Physiol. 250, F874–F884 (1986).
Skrtic, M. et al. Influence of sex on hyperfiltration in patients with uncomplicated type 1 diabetes. Am. J. Physiol. Renal Physiol. 312, F599–F606 (2017).
Vervoort, G., Veldman, B., Berden, J. H., Smits, P. & Wetzels, J. F. Glomerular hyperfiltration in type 1 diabetes mellitus results from primary changes in proximal tubular sodium handling without changes in volume expansion. Eur. J. Clin. Invest. 35, 330–336 (2005).
Hannedouche, T. P. et al. Renal hemodynamics and segmental tubular reabsorption in early type 1 diabetes. Kidney Int. 37, 1126–1133 (1990).
Pruijm, M. et al. Glomerular hyperfiltration and increased proximal sodium reabsorption in subjects with type 2 diabetes or impaired fasting glucose in a population of the African region. Nephrol. Dial. Transplant. 25, 2225–2231 (2010).
Thomson, S. C. et al. Ornithine decarboxylase, kidney size, and the tubular hypothesis of glomerular hyperfiltration in experimental diabetes. J. Clin. Invest. 107, 217–224 (2001).
Barfuss, D. W. & Schafer, J. A. Differences in active and passive glucose transport along the proximal nephron. Am. J. Physiol. 241, F322–F332 (1981).
Turner, R. J. & Moran, A. Heterogeneity of sodium-dependent D-glucose transport sites along the proximal tubule: evidence from vesicle studies. Am. J. Physiol. 242, F406–F414 (1982).
Wright, E. M., Loo, D. D. & Hirayama, B. A. Biology of human sodium glucose transporters. Physiol. Rev. 91, 733–794 (2011).
Vrhovac, I. et al. Localizations of Na-D-glucose cotransporters SGLT1 and SGLT2 in human kidney and of SGLT1 in human small intestine, liver, lung, and heart. Pflugers Arch. 467, 1881–1898 (2015).
Vallon, V. et al. SGLT2 mediates glucose reabsorption in the early proximal tubule. J. Am. Soc. Nephrol. 22, 104–112 (2011).
Sabolic, I. et al. Expression of Na+-D-glucose cotransporter SGLT2 in rodents is kidney-specific and exhibits sex and species differences. Am. J. Physiol. Cell Physiol. 302, C1174–C1188 (2012).
Balen, D. et al. Revised immunolocalization of the Na+-D-glucose cotransporter SGLT1 in rat organs with an improved antibody. Am. J. Physiol. Cell Physiol. 295, C475–C489 (2008).
Rieg, T. et al. Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am. J. Physiol. Renal Physiol. 306, F188–F193 (2014).
Gorboulev, V. et al. Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 61, 187–196 (2012).
Santer, R. & Calado, J. Familial renal glucosuria and SGLT2: from a Mendelian trait to a therapeutic target. Clin. J. Am. Soc. Nephrol. 5, 133–141 (2010).
Martin, M. G., Turk, E., Lostao, M. P., Kerner, C. & Wright, E. M. Defects in Na+/glucose cotransporter (SGLT1) trafficking and function cause glucose-galactose malabsorption. Nat. Genet. 12, 216–220 (1996).
DeFronzo, R. A. et al. Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes. Diabetes Care 36, 3169–3176 (2013).
Farber, S. J., Berger, E. Y. & Earle, D. P. Effect of diabetes and insulin of the maximum capacity of the renal tubules to reabsorb glucose. J. Clin. Invest. 30, 125–129 (1951).
Mogensen, C. E. Maximum tubular reabsorption capacity for glucose and renal hemodynamcis during rapid hypertonic glucose infusion in normal and diabetic subjects. Scand. J. Clin. Lab. Invest. 28, 101–109 (1971).
Vallon, V., Blantz, R. C. & Thomson, S. Glomerular hyperfiltration and the salt paradox in early type 1 diabetes mellitus: a tubulo-centric view. J. Am. Soc. Nephrol. 14, 530–537 (2003).
Vallon, V. et al. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am. J. Physiol. Renal Physiol. 304, F156–F167 (2013).
Vallon, V. et al. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am. J. Physiol. Renal Physiol. 306, F194–F204 (2014).
Kamran, M., Peterson, R. G. & Dominguez, J. H. Overexpression of GLUT2 gene in renal proximal tubules of diabetic Zucker rats. J. Am. Soc. Nephrol. 8, 943–948 (1997).
Freitas, H. S. et al. SLC2A2 gene expression in kidney of diabetic rats is regulated by HNF-1alpha and HNF-3beta. Mol. Cell Endocrinol. 305, 63–70 (2009).
Chin, E. et al. Changes in facilitative glucose transporter messenger ribonucleic acid levels in the diabetic rat kidney. Endocrinol. 138, 1267–1275 (1997).
Dominguez, J. H., Camp, K., Maianu, L., Feister, H. & Garvey, W. T. Molecular adaptations of GLUT1 and GLUT2 in renal proximal tubules of diabetic rats. Am. J. Physiol. 266, F283–F290 (1994).
Marks, J., Carvou, N. J., Debnam, E. S., Srai, S. K. & Unwin, R. J. Diabetes increases facilitative glucose uptake and GLUT2 expression at the rat proximal tubule brush border membrane. J. Physiol. 553, 137–145 (2003).
Hinden, L. et al. Modulation of renal GLUT2 by the cannabinoid-1 receptor: implications for the treatment of diabetic nephropathy. J. Am. Soc. Nephrol. 29, 434–448 (2018).
Goestemeyer, A. K., Marks, J., Srai, S. K., Debnam, E. S. & Unwin, R. J. GLUT2 protein at the rat proximal tubule brush border membrane correlates with protein kinase C (PKC)-betal and plasma glucose concentration. Diabetologia 50, 2209–2217 (2007).
Song, P. et al. Knockout of Na-glucose-cotransporter SGLT1 mitigates diabetes-induced upregulation of nitric oxide synthase-1 in macula densa and glomerular hyperfiltration. Am. J. Physiol. Renal Physiol. 317, F207–F217 (2019).
Kellett, G. L., Brot-Laroche, E., Mace, O. J. & Leturque, A. Sugar absorption in the intestine: the role of GLUT2. Annu. Rev. Nutr. 28, 35–54 (2008).
Rahmoune, H. et al. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes 54, 3427–3434 (2005).
Wang, X. X. et al. SGLT2 expression is increased in human diabetic nephropathy: SGLT2 inhibition decreases renal lipid accumulation, inflammation and the development of nephropathy in diabetic mice. J. Biol. Chem. 292, 5335–5348 (2017).
Seyer-Hansen, K. Renal hypertrophy in experimental diabetes: some functional aspects. J. Diabet. Complications 1, 7–10 (1987).
Vallon, V. The proximal tubule in the pathophysiology of the diabetic kidney. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R1009–R1022 (2011).
Weinstein, A. M. et al. Flow-dependent transport in a mathematical model of rat proximal tubule. Am. J. Physiol. Renal Physiol. 292, F1164–F1181 (2007).
Fattah, H., Layton, A. & Vallon, V. How do kidneys adapt to a deficit or loss in nephron number? Physiol. 34, 189–197 (2019).
Osorio, H. et al. Effect of treatment with losartan on salt sensitivity and SGLT2 expression in hypertensive diabetic rats. Diabetes Res. Clin. Pract. 86, e46–e49 (2009).
Freitas, H. S. et al. Na(+)-glucose transporter-2 messenger ribonucleic acid expression in kidney of diabetic rats correlates with glycemic levels: involvement of hepatocyte nuclear factor-1alpha expression and activity. Endocrinol. 149, 717–724 (2008).
Ghezzi, C. & Wright, E. M. Regulation of the human Na+dependent glucose cotransporter hSGLT2. Am. J. Physiol. Cell Physiol. 303, C348–C354 (2012).
Tiwari, S., Riazi, S. & Ecelbarger, C. A. Insulin’s impact on renal sodium transport and blood pressure in health, obesity, and diabetes. Am. J. Physiol. Renal Physiol. 293, F974–F984 (2007).
Toyoki, D. et al. Insulin stimulates uric acid reabsorption via regulating urate transporter 1 and ATP-binding cassette subfamily G member 2. Am. J. Physiol. Renal Physiol. 313, F826–F834 (2017).
Novikov, A. et al. SGLT2 inhibition and renal urate excretion: role of luminal glucose, GLUT9, and URAT1. Am. J. Physiol. Renal Physiol. 316, F173–F185 (2019).
Pessoa, T. D., Campos, L. C., Carraro-Lacroix, L., Girardi, A. C. & Malnic, G. Functional role of glucose metabolism, osmotic stress, and sodium-glucose cotransporter isoform-mediated transport on Na+/H+exchanger isoform 3 activity in the renal proximal tubule. J. Am. Soc. Nephrol. 25, 2028–2039 (2014).
Huang, W. et al. Tubular NHE3 is a determinant of the acute natriuretic and chronic blood pressure lowering effect of the SGLT2 inhibitor empagliflozin. FASEB J. 32, (Supplement No 1), 620.17 (2018).
Coady, M. J. et al. MAP17 is a necessary activator of renal Na+/glucose cotransporter SGLT2. J. Am. Soc. Nephrol. 28, 85–93 (2017).
Fu, Y., Gerasimova, M., Mayoux, E., Masuda, T. & Vallon, V. SGLT2 inhibitor empagliflozin increases renal NHE3 phosphorylation in diabetic Akita mice: possible implications for the prevention of glomerular hyperfiltration. Diabetes 63, (supplement 1), A132 (2014).
Onishi, A. et al. Effect of renal tubule-specific knockdown of the Na(+)/H(+) exchanger NHE3 in Akita diabetic mice. Am. J. Physiol. Renal Physiol. 317, F419–F434 (2019).
Lin, R. et al. D-glucose acts via sodium/glucose cotransporter 1 to increase NHE3 in mouse jejunal brush border by a Na+/H+exchange regulatory factor 2-dependent process. Gastroenterology 140, 560–571 (2011).
Masuda, T. et al. Unmasking a sustained negative effect of SGLT2 inhibition on body fluid volume in the rat. Am. J. Physiol. Renal Physiol. 315, F653–F664 (2018).
Vallon, V. Tubular transport in acute kidney injury: relevance for diagnosis, prognosis and intervention. Nephron 134, 160–166 (2016).
Zapata-Morales, J. R., Galicia-Cruz, O. G., Franco, M. & Martinez, Y. M. Hypoxia-inducible factor-1alpha (HIF-1alpha) protein diminishes sodium glucose transport 1 (SGLT1) and SGLT2 protein expression in renal epithelial tubular cells (LLC-PK1) under hypoxia. J. Biol. Chem. 289, 346–357 (2014).
Schmidt, C., Hocherl, K., Schweda, F., Kurtz, A. & Bucher, M. Regulation of renal sodium transporters during severe inflammation. J. Am. Soc. Nephrol. 18, 1072–1083 (2007).
Ghezzi, C. et al. SGLT2 inhibitors act from the extracellular surface of the cell membrane. Physiol. Rep. 2, e12058 (2014).
Fu, Y. et al. The organic anion transporter OAT3 enhances the glucosuric effect of the SGLT2 inhibitor empagliflozin. Am. J. Physiol. Renal Physiol. 315, F386–F394 (2018).
Qiu, H., Novikov, A. & Vallon, V. Ketosis and diabetic ketoacidosis in response to SGLT2 inhibitors: basic mechanisms and therapeutic perspectives. Diabetes Metab. Res. Rev. 33, 5 (2017).
Ferrannini, E., Mark, M. & Mayoux, E. CV Protection in the EMPA-REG OUTCOME trial: a “Thrifty Substrate” hypothesis. Diabetes Care 39, 1108–1114 (2016).
Jurczak, M. J. et al. SGLT2 deletion improves glucose homeostasis and preserves pancreatic beta-cell function. Diabetes 60, 890–898 (2011).
Merovci, A. et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J. Clin. Invest. 124, 509–514 (2014).
Ferrannini, E. et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J. Clin. Invest. 124, 499–508 (2014).
Hansen, H. H. et al. The sodium glucose cotransporter type 2 inhibitor empagliflozin preserves beta-cell mass and restores glucose homeostasis in the male zucker diabetic fatty rat. J. Pharmacol. Exp. Ther. 350, 657–664 (2014).
Macdonald, F. R. et al. The novel sodium glucose transporter 2 inhibitor dapagliflozin sustains pancreatic function and preserves islet morphology in obese, diabetic rats. Diabetes Obes. Metab. 12, 1004–1012 (2010).
Nespoux, J. & Vallon, V. SGLT2 inhibition and kidney protection. Clin. Sci. 132, 1329–1339 (2018).
Kidokoro, K. et al. Evaluation of glomerular hemodynamic function by empagliflozin in diabetic mice using in vivo imaging. Circulation 140, 303–315 (2019).
Cherney, D. Z. et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 129, 587–597 (2014).
van Bommel, E. J., Muskiet, M. H., & van Baar, M. J. B. Dapagliflozin reduces measured GFR by reducing renal efferent arteriolar resistance in type 2 diabetes. Diabetes American Diabetes Association 79th Scientific Sessions, S-157 (Abstract) (2019).
Wanner, C. et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N. Engl. J. Med. 375, 323–334 (2016).
Perkovic, V., Jardine, M., Vijapurkar, U. & Meininger, G. Renal effects of canagliflozin in type 2 diabetes mellitus. Curr. Med. Res. Opin. 31, 2219–2231 (2015).
Heerspink, H. J. et al. Canagliflozin slows progression of renal function decline independently of glycemic effects. J. Am. Soc. Nephrol. 28, 368–375 (2018).
Perkovic, V. et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N. Engl. J. Med. 380, 2295–2306 (2019).
Kohan, D. E. et al. The effect of dapagliflozin on renal function in patients with type 2 diabetes. J. Nephrol. 29, 391–400 (2016).
Yale, J. F. et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes. Metab. 15, 463–473 (2013).
Barnett, A. H. et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2, 369–384 (2014).
Zelniker, T. A. et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 393, 31–39 (2019).
Neal, B. et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 377, 644–657 (2017).
Thomson, S. C. & Vallon, V. Renal effects of sodium-glucose Co-transporter inhibitors. Am. J. Cardiol. 124, S28–S35 (2019).
Foote, C., Perkovic, V. & Neal, B. Effects of SGLT2 inhibitors on cardiovascular outcomes. Diab. Vasc. Dis. Res. 9, 117–123 (2012).
Lambers Heerspink, H. J., de, Z. D., Wie, L., Leslie, B. & List, J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes. Metab. 15, 853–862 (2013).
Zinman, B. et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 373, 2117–2128 (2015).
Kato, E. T. et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation 139, 2528–2536 (2019).
Kanbay, M. et al. Uric acid in metabolic syndrome: from an innocent bystander to a central player. Eur. J. Intern. Med. 29, 3–8 (2016).
Lytvyn, Y. et al. Glycosuria-mediated urinary uric acid excretion in patients with uncomplicated type 1 diabetes mellitus. Am. J. Physiol. Renal Physiol. 308, F77–F83 (2015).
Chino, Y. et al. SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharm. Drug. Dispos. 35, 391–404 (2014).
Vaduganathan, M. & Januzzi, J. L. Jr. Preventing and treating heart failure with sodium-glucose co-transporter 2 inhibitors. Am. J. Cardiol. 124, S20–S27 (2019).
McMurray, J. J. V. et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N. Engl. J. Med. 381, 1995–2008 (2019).
Cannon, C. P. et al. Evaluating the effects of canagliflozin on cardiovascular and renal events in patients with type 2 diabetes and chronic kidney disease according to baseline HbA1c, Including those with HbA1c<7%: results from the CREDENCE trial. Circulation. https://doi.org/10.1161/CIRCULATIONAHA.119.044359 (2019).
Ishizawa, K. et al. Inhibition of sodium glucose cotransporter 2 attenuates the dysregulation of Kelch-Like 3 and NaCl cotransporter in obese diabetic mice. J. Am. Soc. Nephrol. 30, 782–794 (2019).
Monami, M., Nardini, C. & Mannucci, E. Efficacy and safety of sodium glucose co-transport-2 inhibitors in type 2 diabetes: a meta-analysis of randomized clinical trials. Diabetes Obes. Metab. 16, 457–466 (2014).
Layton, A. T., Vallon, V. & Edwards, A. Modeling oxygen consumption in the proximal tubule: effects of NHE and SGLT2 inhibition. Am. J. Physiol. Renal Physiol. 308, F1343–F1357 (2015).
Neill, O. et al. Acute SGLT inhibition normalizes oxygen tension in the renal cortex but causes hypoxia in the renal medulla in anaesthetized control and diabetic rats. Am. J. Physiol. Renal Physiol. 309, F227–F234 (2015).
Layton, A. T., Vallon, V. & Edwards, A. Predicted consequences of diabetes and SGLT inhibition on transport and oxygen consumption along a rat nephron. Am. J. Physiol. Renal Physiol. 310, F1269–F1283 (2016).
Sano, M., Takei, M., Shiraishi, Y. & Suzuki, Y. Increased hematocrit during sodium-glucose cotransporter 2 inhibitor therapy indicates recovery of tubulointerstitial function in diabetic kidneys. J. Clin. Med. Res. 8, 844–847 (2016).
Inzucchi, S. E. et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME trial. Diabetes Care 41, 356–363 (2018).
Layton, A. T. & Vallon, V. SGLT2 inhibition in a kidney with reduced nephron number: modeling and analysis of solute transport and metabolism. Am. J. Physiol. Renal Physiol. 314, F969–F984 (2018).
Nadkarni, G. N. et al. Acute kidney injury in patients on SGLT2 inhibitors: a propensity-matched analysis. Diabetes Care 40, 1479–1485 (2017).
Song, P., Onishi, A., Koepsell, H. & Vallon, V. Sodium glucose cotransporter SGLT1 as a therapeutic target in diabetes mellitus. Expert. Opin. Ther. Targets. 20, 1109–1125 (2016).
Vallon, V. The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annu. Rev. Med. 66, 255–270 (2015).
Powell, D. R. et al. Improved glycemic control in mice lacking Sglt1 and Sglt2. Am. J. Physiol. Endocrinol. Metab. 304, E117–E130 (2013).
Gallo, L. A., Wright, E. M. & Vallon, V. Probing SGLT2 as a therapeutic target for diabetes: Basic physiology and consequences. Diab. Vasc. Dis. Res. 12, 78–89 (2015).
Gembardt, F. et al. The SGLT2 inhibitor empagliflozin ameliorates early features of diabetic nephropathy in BTBR ob/ob type 2 diabetic mice with and without hypertension. Am. J. Physiol. Renal Physiol. 307, F317–F325 (2014).
Han, H. J., Lee, Y. J., Park, S. H., Lee, J. H. & Taub, M. High glucose-induced oxidative stress inhibits Na+/glucose cotransporter activity in renal proximal tubule cells. Am. J. Physiol. Renal Physiol. 288, F988–F996 (2005).
Nespoux, J. et al. Gene deletion of the Na-glucose cotransporter SGLT1 ameliorates kidney recovery in a murine model of acute kidney injury induced by ischemia-reperfusion. Am. J. Physiol. Renal Physiol. 316, F1201–F1210 (2019).
Wilcox, C. S. et al. Nitric oxide synthase in macula densa regulates glomerular capillary pressure. Proc. Natl Acad. Sci. USA 89, 11993–11997 (1992).
Liu, R., Carretero, O. A., Ren, Y. & Garvin, J. L. Increased intracellular pH at the macula densa activates nNOS during tubuloglomerular feedback. Kidney Int. 67, 1837–1843 (2005).
Vallon, V. et al. Feedback control of glomerular vascular tone in neuronal nitric oxide synthase knockout mice. J. Am. Soc. Nephrol. 12, 1599–1606 (2001).
Komers, R., Lindsley, J. N., Oyama, T. T., Allison, K. M. & Anderson, S. Role of neuronal nitric oxide synthase (NOS1) in the pathogenesis of renal hemodynamic changes in diabetes. Am. J. Physiol. Renal Physiol. 279, F573–F583 (2000).
Tolins, J. P., Shultz, P. J., Raij, L., Brown, D. M. & Mauer, S. M. Abnormal renal hemodynamic response to reduced renal perfusion pressure in diabetic rats: role of NO. Am. J. Physiol. 265, F886–F895 (1993).
Zhang, J. et al. Macula densa SGLT1-NOS1-TGF pathway — a new mechanism for glomerular hyperfiltration during hyperglycemia. J. Am. Soc. Nephrol. 30, 578–593 (2019).
Thomson, S. C. et al. Early diabetes as a model for testing the regulation of juxtaglomerular NOS I. Am. J. Physiol. Renal Physiol. 287, F732–F738 (2004).
Guyton, A. C. Blood pressure control — special role of the kidneys and body fluids. Science 252, 1813–1816 (1991).
Lu, D. et al. Salt-sensitive splice variant of nNOS expressed in the macula densa cells. Am. J. Physiol. Renal Physiol 298, F1465–F1471 (2010).
Lu, Y. et al. Macula Densa nitric oxide synthase 1beta protects against salt-sensitive hypertension. J. Am. Soc. Nephrol. 27, 2346–2356 (2016).
Wang, X. et al. Inhibition of nitric oxide synthase 1 induces salt-sensitive hypertension in nitric oxide synthase 1alpha knockout and wild-type mice. Hypertension 67, 792–799 (2016).
Welch, W. J. & Wilcox, C. S. Role of nitric oxide in tubuloglomerular feedback: effects of dietary salt. Clin. Exp. Pharmacol. Physiol. 24, 582–586 (1997).
Thomson, S. C. Nitric oxide mediates anomalous tubuloglomerular feedback in rats fed high-NaCl diet after subtotal nephrectomy. Am. J. Physiol. Renal Physiol. 316, F223–F230 (2019).
Thomson, S. C. et al. Temporal adjustment of the juxtaglomerular apparatus during sustained inhibition of proximal reabsorption. J. Clin. Invest. 104, 1149–1158 (1999).
Rieg, T. & Vallon, V. Development of SGLT1 and SGLT2 inhibitors. Diabetologia 61, 2079–2086 (2018).
Sims, H., Smith, K. H., Bramlage, P. & Minguet, J. Sotagliflozin: a dual sodium-glucose co-transporter-1 and -2 inhibitor for the management of Type 1 and Type 2 diabetes mellitus. Diabet. Med. 35, 1037–1048 (2018).
Cefalo, C. M. A. et al. Sotagliflozin, the first dual SGLT inhibitor: current outlook and perspectives. Cardiovasc. Diabetol. 18, 20 (2019).
Zerbini, G. et al. Persistent renal hypertrophy and faster decline of glomerular filtration rate precede the development of microalbuminuria in type 1 diabetes. Diabetes 55, 2620–2625 (2006).
Rigalleau, V. et al. Large kidneys predict poor renal outcome in subjects with diabetes and chronic kidney disease. BMC. Nephrol. 11, 3 (2010).
Lawson, M. L., Sochett, E. B., Chait, P. G., Balfe, J. W. & Daneman, D. Effect of puberty on markers of glomerular hypertrophy and hypertension in IDDM. Diabetes 45, 51–55 (1996).
Bognetti, E., Zoja, A., Meschi, F., Paesano, P. L. & Chiumello, G. Relationship between kidney volume, microalbuminuria and duration of diabetes mellitus. Diabetologia 39, 1409 (1996).
Baumgartl, H. J., Sigl, G., Banholzer, P., Haslbeck, M. & Standl, E. On the prognosis of IDDM patients with large kidneys. Nephrol. Dial. Transplant. 13, 630–634 (1998).
Wolf, G. & Ziyadeh, F. N. Molecular mechanisms of diabetic renal hypertrophy. Kidney Int. 56, 393–405 (1999).
Chiarelli, F., Gaspari, S. & Marcovecchio, M. L. Role of growth factors in diabetic kidney disease. Horm. Metab. Res. 41, 585–593 (2009).
Kamesaki, H., Nishizawa, K., Michaud, G. Y., Cossman, J. & Kiyono, T. TGF-beta 1 induces the cyclin-dependent kinase inhibitor p27Kip1 mRNA and protein in murine B cells. J. Immunol. 160, 770–777 (1998).
Wolf, G. et al. High glucose stimulates expression of p27Kip1 in cultured mouse mesangial cells: relationship to hypertrophy. Am. J. Physiol. 273, F348–F356 (1997).
Huang, J. S., Chuang, L. Y., Guh, J. Y. & Huang, Y. J. Effects of nitric oxide and antioxidants on advanced glycation end products-induced hypertrophic growth in human renal tubular cells. Toxicol. Sci. 111, 109–119 (2009).
Chen, J. K., Chen, J., Thomas, G., Kozma, S. C. & Harris, R. C. S6 kinase 1 knockout inhibits uninephrectomy- or diabetes-induced renal hypertrophy. Am. J. Physiol. Renal Physiol 297, F585–F593 (2009).
Yang, D. et al. Autophagy in diabetic kidney disease: regulation, pathological role and therapeutic potential. Cell Mol. Life Sci. 75, 669–688 (2018).
Satriano, J. et al. Transition of kidney tubule cells to a senescent phenotype in early experimental diabetes. Am. J. Physiol. Cell Physiol. 299, C374–C380 (2010).
Verzola, D. et al. Accelerated senescence in the kidneys of patients with type 2 diabetic nephropathy. Am. J. Physiol. Renal Physiol 295, F1563–F1573 (2008).
Phillips, A. O., Steadman, R., Morrisey, K. & Williams, J. D. Polarity of stimulation and secretion of transforming growth factor-beta 1 by cultured proximal tubular cells. Am. J. Pathol. 150, 1101–1111 (1997).
Phillips, A. O. & Steadman, R. Diabetic nephropathy: the central role of renal proximal tubular cells in tubulointerstitial injury. Histol. Histopathol. 17, 247–252 (2002).
Sokolovska, J. et al. Influence of metformin on GLUT1 gene and protein expression in rat streptozotocin diabetes mellitus model. Arch. Physiol. Biochem. 116, 137–145 (2010).
Lee, M. J. et al. A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am. J. Physiol. Renal Physiol. 292, F617–F627 (2007).
Lan, R. et al. Mitochondrial pathology and glycolytic shift during proximal tubule atrophy after ischemic AKI. J. Am. Soc. Nephrol. 27, 3356–3367 (2016).
Farsijani, N. M. et al. Renal epithelium regulates erythropoiesis via HIF-dependent suppression of erythropoietin. J. Clin. Invest. 126, 1425–1437 (2016).
Heilig, C. W. et al. GLUT1 regulation of the pro-sclerotic mediators of diabetic nephropathy. Am. J. Nephrol. 38, 39–49 (2013).
Pedersen, S. B., Flyvbjerg, A. & Richelsen, B. Inhibition of renal ornithine decarboxylase activity prevents kidney hypertrophy in experimental diabetes. Am. J. Physiol. 264, C453–C456 (1993).
Noh, H. & King, G. L. The role of protein kinase C activation in diabetic nephropathy. Kidney Int. Suppl. S49-S53 https://doi.org/10.1038/sj.ki.5002386 (2007).
Meier, M. et al. Deletion of protein kinase C-beta isoform in vivo reduces renal hypertrophy but not albuminuria in the streptozotocin-induced diabetic mouse model. Diabetes 56, 346–354 (2007).
Efendiev, R., Bertorello, A. M. & Pedemonte, C. H. PKC-beta and PKC-zeta mediate opposing effects on proximal tubule Na+,K+-ATPase activity. FEBS Lett. 456, 45–48 (1999).
Vallon, V., Wead, L. M. & Blantz, R. C. Renal hemodynamics and plasma and kidney angiotensin II in established diabetes mellitus in rats: effect of sodium and salt restriction. J. Am. Soc. Nephrol. 5, 1761–1767 (1995).
Vallon, V. et al. Effect of chronic salt loading on kidney function in early and established diabetes mellitus in rats. J. Lab. Clin. Med. 130, 76–82 (1997).
Lau, C. et al. Salt-resistant blood pressure and salt-sensitive renal autoregulation in chronic streptozotocin diabetes. Am. J. Physiol. Regul. Integr. Comp. Physiol 296, R1761–R1770 (2009).
Miller, J. A. Renal responses to sodium restriction in patients with early diabetes mellitus. J. Am. Soc. Nephrol. 8, 749–755 (1997).
Miracle, C. M., Rieg, T., Mansoury, H., Vallon, V. & Thomson, S. C. Ornithine decarboxylase inhibitor eliminates hyperresponsiveness of the early diabetic proximal tubule to dietary salt. Am. J. Physiol. Renal Physiol 295, F995–F1002 (2008).
Birk, C. et al. The salt paradox of the early diabetic kidney is independent of renal innervation. Kidney Blood Press. Res. 26, 344–350 (2003).
Ekinci, E. I. et al. Dietary salt intake and mortality in patients with type 2 diabetes. Diabetes Care 34, 703–709 (2011).
Thomas, M. C. et al. The association between dietary sodium intake, ESRD, and all-cause mortality in patients with type 1 diabetes. Diabetes Care 34, 861–866 (2011).
Holtkamp, F. A. et al. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int. 80, 282–287 (2011).
O’Bryan, G. T. & Hostetter, T. H. The renal hemodynamic basis of diabetic nephropathy. Semin. Nephrol. 17, 93–100 (1997).
Pruijm, M. et al. Reduced cortical oxygenation predicts a progressive decline of renal function in patients with chronic kidney disease. Kidney Int. 93, 932–940 (2018).
Singh, D. K., Winocour, P. & Farrington, K. Mechanisms of disease: the hypoxic tubular hypothesis of diabetic nephropathy. Nat. Clin. Pract. Nephrol. 4, 216–226 (2008).
Magri, C. J. & Fava, S. The role of tubular injury in diabetic nephropathy. Eur. J. Intern. Med. 20, 551–555 (2009).
Thomas, M. C., Burns, W. C. & Cooper, M. E. Tubular changes in early diabetic nephropathy. Adv. Chronic. Kidney Dis. 12, 177–186 (2005).
Ren, J. L., Pan, J. S., Lu, Y. P., Sun, P. & Han, J. Inflammatory signaling and cellular senescence. Cell Signal. 21, 378–383 (2009).
Ziyadeh, F. N. et al. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proc. Natl Acad. Sci. USA 97, 8015–8020 (2000).
Brosius, F. C., Tuttle, K. R. & Kretzler, M. JAK inhibition in the treatment of diabetic kidney disease. Diabetologia 59, 1624–1627 (2016).
Velagapudi, C. et al. The tuberin/mTOR pathway promotes apoptosis of tubular epithelial cells in diabetes. J. Am. Soc. Nephrol. 22, 262–273 (2011).
Lee, Y. H. et al. Empagliflozin attenuates diabetic tubulopathy by improving mitochondrial fragmentation and autophagy. Am. J. Physiol. Renal Physiol 317, F767–F780 (2019).
Vallon, V. Do tubular changes in the diabetic kidney affect the susceptibility to acute kidney injury? Nephron Clin. Pract. 127, 133–138 (2014).
Acknowledgements
The authors are supported by NIH grants R01DK112042 (V.V., S.C.T.), R01DK106102, R01HL142814, RF1AG061296 (V.V.), the UAB/UCSD O’Brien Center of Acute Kidney Injury NIH-P30DK079337 (V.V., S.C.T.) and the Department of Veterans Affairs (V.V., S.C.T.).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
V.V. declares that he has served as a consultant and received honoraria from Astra-Zeneca, Bayer, Boehringer Ingelheim, Janssen Pharmaceutical, Eli Lilly, Merck and Retrophin, and has received grant support for investigator-initiated research from Astra-Zeneca, Bayer, Boehringer Ingelheim, Fresenius and Janssen. S.C.T. declares that he has received grant support for investigator-initiated research from Merck.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Glomerular ultrafiltration coefficient
-
A measure of the glomerular capillary membrane’s permeability to water; specifically, the volume of fluid filtered in unit time through a unit area of glomerular capillary membrane per unit pressure difference.
- Tubuloglomerular feedback
-
A mechanism that inversely relates single nephron glomerular filtration rate to the NaCl concentration at the macula densa of the same nephron.
- Tubular back pressure
-
The hydrostatic pressure in the Bowman’s space.
- Filtration fraction
-
The fraction of renal plasma flow that is filtered by the glomeruli.
- Gibbs free energy
-
The thermodynamic potential (or available energy) associated with a chemical reaction that can be used to do reversible work at a constant temperature and pressure.
- Electrogenic
-
A type of transport process that leads to the translocation of net charge across the membrane and produces a change in the electrical potential of a cell.
- Facilitated diffusion
-
The process of spontaneous passive transport of molecules or ions across a biological membrane along their electrochemical gradient, usually via a transmembrane integral protein.
Rights and permissions
About this article
Cite this article
Vallon, V., Thomson, S.C. The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat Rev Nephrol 16, 317–336 (2020). https://doi.org/10.1038/s41581-020-0256-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-020-0256-y
This article is cited by
-
YME1L-mediated mitophagy protects renal tubular cells against cellular senescence under diabetic conditions
Biological Research (2024)
-
Acetyl-CoA synthetase 2 promotes diabetic renal tubular injury in mice by rewiring fatty acid metabolism through SIRT1/ChREBP pathway
Acta Pharmacologica Sinica (2024)
-
The clinical benefits of sodium–glucose cotransporter type 2 inhibitors in people with gout
Nature Reviews Rheumatology (2024)
-
Obesity and the kidney: mechanistic links and therapeutic advances
Nature Reviews Endocrinology (2024)
-
Multifunctional nanoparticle-mediated combining therapy for human diseases
Signal Transduction and Targeted Therapy (2024)